Abstract
Background
Alcoholic hepatitis is a cause of major morbidity and mortality that lacks effective therapies. Both experimental and clinical evidence indicate that the multifunctional cytokine tumor necrosis factor-α (TNFα) contributes to pathogenesis and clinical sequelae of alcoholic hepatitis. A pilot study demonstrated that the TNFα-neutralizing molecule, etanercept, could be an effective treatment for patients with alcoholic hepatitis.
Methods
Forty-eight patients with moderate to severe alcoholic hepatitis (MELD score ≥15) were enrolled and randomized to groups that were given up to 6 subcutaneous injections of either etanercept or placebo for three weeks. Primary study endpoints included mortality at 1- and 6-month timepoints.
Results
There were no significant baseline differences between the placebo and etanercept groups in demographics or disease severity parameters including age, gender, and MELD score. The 1-month mortality rates of patients receiving placebo and etanercept were similar on an intention-to-treat basis (22.7% versus 36.4%, respectively; OR and 95% CI: 1.8 and 0.5–6.5). The 6-month mortality rate was significantly higher in the etanercept group, compared with the placebo group (57.7% versus 22.7%, respectively; OR and 95% CI: 4.6 and 1.3–16.4, p=0.017). Rates of infectious serious adverse events were significantly higher in the etanercept group, compared with the placebo group (34.6% versus 9.1%, p=0.04).
Conclusion
In patients with moderate to severe alcoholic hepatitis, etanercept was associated with a significantly higher mortality rate after 6 months, indicating that etanercept is not effective for the treatment of patients with alcoholic hepatitis.
Keywords: tumor necrosis factor, alcoholic hepatitis, MELD, clinical trial
Introduction
Alcohol-related liver disease is a major cause of world-wide morbidity and mortality, with the acute syndrome of alcoholic hepatitis carrying a particularly poor prognosis 1–3. Although an array of potential treatments have been studied, including corticosteroids, nutritional supplementation, anabolic steroids, insulin/glucagon, colchicine, propylthiouracil, d-penicillamine, and pentoxifylline 4–14, the optimum treatment regimen is controversial and varies between medical centers.
Data from animal models of alcohol feeding demonstrate that tumor necrosis factor-alpha (TNFα) contributes to pathogenesis of alcoholic hepatitis and that inhibition of TNFα attenuates alcoholic liver injury 15–18. Studies in humans demonstrate that TNFα correlates with disease severity in alcoholic hepatitis, providing clinical evidence that TNFα may contribute to the clinical sequelae of alcoholic hepatitis 19, 20. Furthermore, a recent study suggested that pentoxifylline, an agent which putatively functions by inhibiting TNFα release, may be of benefit in alcoholic hepatitis 10.
Etanercept is a soluble TNFα receptor:FC fusion protein consisting of the extracellular ligand binding portion of the human p75 TNF receptor and the FC portion of IgG, which binds and neutralizes unbound serum TNFα, thereby blocking cytokine signals downstream from TNFα. Etanercept is presently approved for treatment of inflammatory arthritis and under investigation for efficacy in other inflammatory conditions 21–25. On the other hand, agents which neutralize TNFα such as etanercept, have been implicated in infections and other adverse events 26. Nonetheless, a recent open-label pilot study of etanercept in patients with alcoholic hepatitis demonstrated encouraging results 27, and therefore the present study was designed to test the efficacy of etanercept in patients with alcoholic hepatitis, in a double-blind randomized, placebo-controlled fashion.
Patients and Methods
Patients
Patients with alcoholic hepatitis were eligible for recruitment from seven academic medical centers between June 2004 and June 2007. Patients were eligible for enrollment if they were greater than 18 years of age at entry with clinical evaluation and testing supporting a diagnosis of alcoholic hepatitis including jaundice, hepatomegaly, leukocytosis, fever, and elevations in transaminase levels, as well as exclusion of other causes of hepatitis including viral (negative HbsAg and anti-HCV), autoimmune (antinuclear antibody titer < 1:40, negative anti-mitochondrial antibody and smooth muscle antibody), drugs, or metabolic disorders (normal ceruloplasmin levels), in the setting of compatible alcohol consumption. Significant alcohol consumption was defined as >40 grams per day for a minimum of 6 months and within the 3 months prior to study enrollment. In the course of evaluation, all patients received ultrasonographic or cross-sectioning imaging of liver to assist in exclusion of concomitant or alternative diagnoses. In patients in whom the diagnosis remained uncertain, liver biopsy was performed for histologic confirmation prior to enrollment. Histologic or ultrasonographic evidence of cirrhosis did not exclude enrollment. Enrollment included patients with moderate to severe disease as evidenced by a calculated Model for End-Stage Liver Disease score (MELD) of >15 28–31. Although the Maddrey discriminant function (DF) has been used to stratify patients for prior alcoholic hepatitis trials, we chose a MELD-based entry criteria since INR values that are used for MELD calculations, are less variable between multiple sites/laboratories than the prothrombin time that is used for DF, and MELD is equivalent to DF for selecting risk of death in patients with alcoholic hepatitis. 28
Exclusion criteria included hypersensitivity to etanercept, presence of infection including pneumonitis or sepsis documented by chest x-ray or blood, urine, ascites cultures, and history of autoimmune disease. Patients receiving corticosteroids, pentoxifylline, propylthiouracil, or thalidomide in the preceding 4 weeks prior to evaluation did not qualify for enrollment. In women, a negative pregnancy test, surgical sterility, or post menopausal state was a requirement for enrollment and breast-feeding women were not eligible.
Study design and randomization
The study was designed as a randomized, double blind, placebo controlled trial. Eligible and enrolled patients were randomized to receive either etanercept or placebo. Randomization was conducted through the use of log books in the study pharmacy at each individual site, in which randomly generated numbers (blocks of 4) for each strata were recorded. Enrolled patients were entered sequentially to receive the assigned treatment. Patient, coordinator, and physician were blinded to randomization group. Randomization was conducted separately at each center in absence of a stratification scheme. Patients were examined at baseline prior to enrollment and underwent serologic evaluation that included serum electrolytes, liver tests, laboratory parameters required for MELD score calculation, and coagulation parameters.
Treatment
The placebo group received subcutaneous injections of placebo on days 1, 4, 8, 11, 15, and 18 (+/−2 days for each dosing date), whereas the etanercept group received subcutaneous injections of etanercept (25 mg) at identical time points. This is the FDA-approved dosage for rheumatoid arthritis 21. Injections were discontinued in patients who developed serious infectious complications (e.g. pneumonia, sepsis, spontaneous bacterial peritonitis) based on clinical evaluation and testing results. In addition to receiving placebo or etanercept, all patients received supportive care which consisted of aggressive treatment of complications of portal hypertension, appropriate antibiotics in response to development of infection, standard fluid hydration, and enteral/parenteral nutritional management as deemed appropriate by the managing team. Treatment with steroids, pentoxifylline, propylthiouracil, or thalidomide was not allowed during the course of active treatment or follow up. Patients were evaluated in person or by phone visit by a nurse coordinator or co-investigator on days 1, 4, 8, 11, 15, and 18, as well as at 1, 3, and 6 months.
Cytokine assays
Serum samples were collected at time of blood collection prior to administration of the first dose of etanercept/placebo and stored at −80 °C. A control group of serum samples was collected concurrently from healthy patients undergoing screening colonoscopy that corresponded to patients by gender and age ± two years. Serum levels of interleukin 6 (IL-6), and interleukin 8 (IL-8) were quantified using commercially available human ELISA assays (Invitrogen Corporation - Carlsbad, California).
Endpoints
Patient mortality at one and six months was the primary endpoint for this study. Clinical secondary endpoints included prevalence of infections and cause of death. Multiple causes of death were recorded if the attending physician felt that there were multiple, equally contributory factors for death.
Statistical analysis and Sample Size Calculation
The primary endpoint of this two arm study was mortality at 1 month and 6 months. Univariate logistic regression was used to assess the difference in 1-month and 6-month mortality rates for the study arm effect, as well as for other baseline patient variables. In addition, the study arm effect was assessed using logistic regression adjusting for each of the other baseline covariates of interest one at a time in separate models. Odds ratios (OR) and 95% confidence intervals (CI) based on the logistic regression model estimates were computed. A secondary analysis of mortality involved estimation of survival probability as a function of time using Kaplan-Meier estimation for the two treatment groups, right censoring patients at 180 days. Another secondary analysis compared the proportion of patients in the two treatment arms having one or more adverse events, including infections, using the Fisher’s exact test. All analyses are shown as “intention to treat”. The alpha-level was set at 0.05 for statistical significance.
The estimated survival probability of 0.40 for the control group was based on prior data, which demonstrated that patients with alcoholic hepatitis and the entry MELD score have an estimated mortality of 60% 28. The estimated effect size was based on improvement in mortality observed in a prior open-label etanercept study compared to a historical control cohort, in which survival improved from an expected 53 % to an observed 92% 27. In calculating our sample size we chose a more conservative effect size of 35%. Based on a 35% effect size, a two group chi-square test with a 0.025 one-sided significance level would have 80% power to detect the difference between the null hypothesis proportion of 0.40 and the alternative proportion of 0.75 when the sample size is 60 (30 in each arm). This sample size was consistent with prior high quality treatment trials in alcoholic hepatitis 32. The smaller final enrollment figure of 48 patients in this trial increased our minimally detectable effect size by an additional 5%.
As the study was funded by NIH as only a three year study, and had already undergone a one-year no-cost extension, recruitment was terminated at the completion of the fourth year due to lack of further funding, and analysis was performed with the 48 recruited patients.
Ethical issues
Informed consent was obtained from all participants and approval from the Institutional Review Board was obtained at all enrollment sites. An independent data safety board reviewed interim data and analyses, which were blinded to all co-investigators.
Results
Patient demographics
Patient screening and recruitment at each of the 7 study sites are shown in Table 1. During the recruitment period, 174 patients were evaluated for enrollment. 126 of the screened patients either declined to participate or did not meet enrollment criteria, while 48 patients met the inclusion criteria for the study and agreed to participate. These 48 patients were randomized to treatment with either placebo or etanercept and constituted the “intention to treat” analysis cohort (Figure 1). Within each treatment group, two patients were enrolled but never received study drug due to withdrawal of consent (n=3) or inadvertently receiving corticosteroids (n=1). Exclusion of these patients constituted the “per protocol” analysis cohort. All ensuing analyses were performed and reported as “intention to treat,” with “per protocol” analysis performed as specifically indicated.
Table 1.
Patient enrollment by recruitment site
| Site | Patients screened (n) | Patients enrolled (n) |
|---|---|---|
| Mayo – Rochester | 84 | 26 |
| Hennepin County | 24 | 9 |
| University of Indiana | 9 | 7 |
| University of Alabama | 5 | 3 |
| Scott & White/Texas A&M | 12 | 2 |
| University of Minnesota | 1 | 1 |
| University of Louisville | 39 | 0 |
Figure 1. Flow diagram.
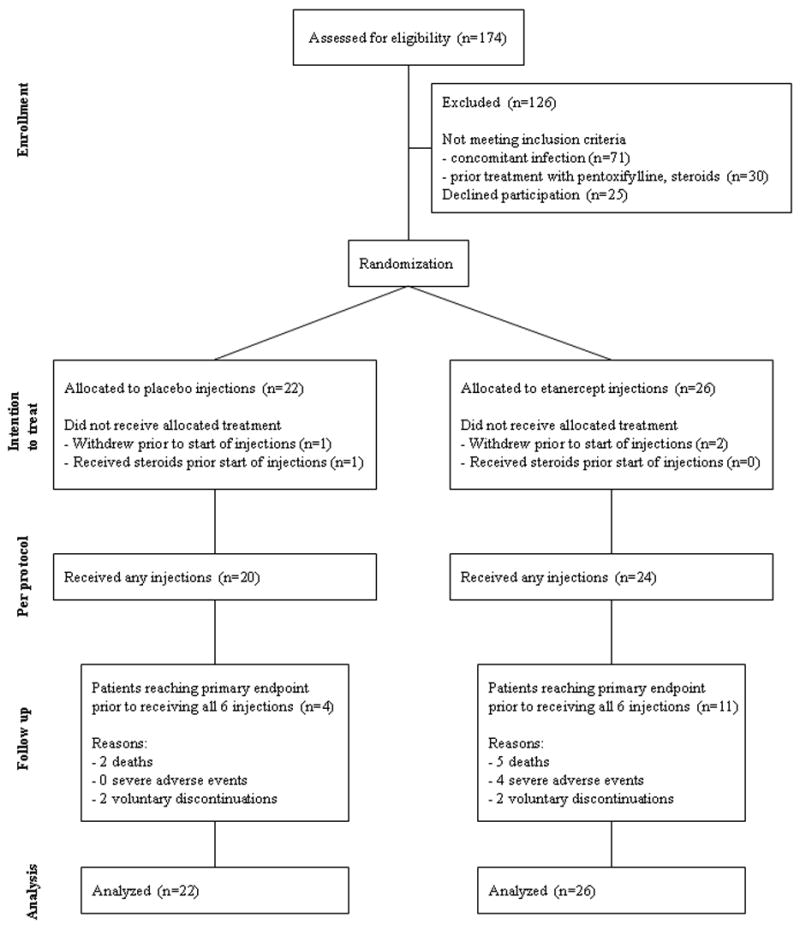
Flow of participants through each stage of the trial is shown.
The two groups were similar with regards to baseline demographic, clinical, and laboratory parameters (Table 2). A majority of patients were male, and predominantly Caucasian. Alcohol consumption history recorded a similar number of drinks per day and number of drinks per “drinking” day, between the two arms, although the self-reported “years of drinking” was significantly greater in the etanercept group than in the placebo group (18.9±11.3 versus 11.6±7.6 years respectively, p=0.02). There was also a trend toward a higher incidence of ascites in the etanercept group than in the placebo group, although this was not statistically significant (58% versus 36% respectively, p=0.14).
Table 2.
Baseline patient characteristics
| Placebo (n=22) | Etanercept (n=26) | p-value | |
|---|---|---|---|
| Age at enrollment, mean (sd) | 49.1 (9.2) | 52.8 (9.3) | 0.17 a |
| Male gender, n (%) | 17 (77%) | 18 (69%) | 0.53 c |
| Caucasian, n (%) | 18 (82%) | 22 (85%) | 0.94 a |
| Number of standard drinks per drinking day, mean (sd) | 12.1 (8.9) | 10.3 (6.8) | 0.46 a |
| Number of standard drinks per day, mean (sd) | 11.4 (9.0) | 9.6 (6.7) | 0.46 a |
| Number of years of reported drinking pattern, mean (sd) | 11.6 (7.6) | 18.9 (11.3) | 0.02 a |
| Total Bilirubin, mean (sd) | 18.5 (10.9) | 20.8 (9.6) | 0.44 a |
| median (range) | 14.9 (2.6,42.8) | 22.1 (4.5,35.1) | 0.40 b |
| Creatinine, mean (sd) | 1.2 (0.8) | 1.6 (1.6) | 0.29 a |
| median (range) | 0.8 (0.5,3.0) | 0.9 (0.2,7.6) | 0.94 b |
| INR, mean (sd) | 1.7 (0.5) | 1.6 (0.4) | 0.31 a |
| median (range) | 1.6 (1.0,2.6) | 1.5 (0.9,2.4) | 0.45 b |
| Enrollment MELD, mean (sd) | 24.6 (6.0) | 25.4 (7.4) | 0.68 a |
| AST, mean (sd) | 142 (95) | 161 (110) | 0.53 a |
| median (range) | 114 (42,418) | 114 (33,450) | 0.69 b |
| Albumin, mean (sd) | 2.7 (0.5) | 2.7 (0.6) | 0.74 a |
| median (range) | 2.6 (1.9,3.7) | 2.8 (1.5,4.0) | 0.62 b |
| Ascites at enrollment, n (%) | 8 (36%) | 15 (58%) | 0.14 c |
| Hepatic Encephalopathy, n (%) | 1 (5%) | 4 (15%) | 0.36 c |
| IL-6 (pg/mL) (sd) | 74.0±49.6 | 84.05±52.1 | 0.64 a |
| IL-8 (pg/mL) (sd) | 43.9±29.8 | 58.5±24.3 | 0.24 a |
Significance test with a two-sample t-test assuming unequal variances
Significance test with Wilcoxon rank sum test
Significance test with chi-square test
As IL-6 and IL-8 are key cytokines downstream of TNFα, baseline serum levels of IL-6, and IL-8 were assayed and are shown in Table 2. No significant differences between active treatment and placebo groups were seen. Comparison of study patients with their age and gender matched healthy controls showed that study patients had significantly higher levels of IL-6 (79.7 ± 50.1 pg/mL versus 24.0 ± 8.4 pg/mL, p<0.001) and IL-8 (52.6 ± 27.0 pg/mL versus 15.5 ± 26.9 pg/mL, p<0.001) as has been previously reported 27.
Patient Survival
1 and 6-month survival rates constituted the primary end-point of this study. The 1-month mortality rate (Table 3) was not significantly different between the two groups (placebo: 22.7% versus etanercept: 34.6%; OR: 1.8 and 95% CI: 0.5–6.5). However, mortality at 6 months was significantly higher in the etanercept group (Table 4; placebo: 22.7% versus etanercept: 57.7%; OR 4.6 and 95% CI: 1.3–16.4). Even with “per protocol” analysis, that is only analyzing patients who received any injections, etanercept continued to be significantly associated with mortality. Univariate analysis of 6-month data revealed that baseline MELD score, presence of ascites, and enrollment in the etanercept arm were significantly associated with increased mortality (Table 4). Since the number of death events precluded multivariate analysis, the specific variables of baseline MELD and presence of ascites were investigated to determine if they influenced the effect of etanercept as a predictor of death at 6 months. Despite correction for any baseline variable, etanercept continued to be significantly associated with mortality (Table 4, right columns).
Table 3.
Patient survival and predictors of death at 30 days
| Deaths at 30 days n (%) | Odds Ratio* (95% CI) | p-value* | Odds Ratio+ (95% CI) | p-value+ | |
|---|---|---|---|---|---|
| Etanercept (n=26) | 9 (34.6%) | 1.8 (0.5,6.5) | 0.37 | ||
| Placebo (n=22) | 5 (22.7%) | 1.0 (reference) | |||
| Age at enrollment | 1.6 (0.8,3.4) | 0.21 | 1.6 (0.4,5.9) | 0.50 | |
| Male gender | 0.9 (0.2,3.6) | 0.88 | 1.8 (0.5,6.5) | 0.37 | |
| Drinks/drinking day | 1.0 (0.9,1.1) | 0.78 | 1.8 (0.5,6.5) | 0.39 | |
| Years of reported alcohol use | 1.0 (0.95,1.1) | 0.69 | 1.7 (0.4,6.8) | 0.48 | |
| Enrollment MELD | 1.1 (0.97,1.2) | 0.17 | 1.7 (0.5,6.4) | 0.41 | |
| Albumin | 0.4 (0.1,1.8) | 0.22 | 1.2 (0.3,5.8) | 0.81 | |
| Ascites | 2.6 (0.7,9.3) | 0.15 | 1.5 (0.4,5.7) | 0.53 | |
| Hepatic Encephalopathy | 0.6 (0.1,5.7) | 0.64 | 1.9 (0.5,7.1) | 0.32 |
Univariate model, odds ratio for death (<=30 days).
Odds Ratio for death (<=30 days) in patient receiving etanercept (relative to Placebo), adjusting for indicated variable (age, gender, etc).
Table 4.
Patient survival and predictors of death at 180 days
| Deaths at 180 days n (%) | Odds Ratio* (95% CI) | p-value* | Odds Ratio+ (95% CI) | p-value+ | |
|---|---|---|---|---|---|
| Etanercept, n=26 | 15 (57.7%) | 4.6 (1.3,16.4) | 0.017 | ||
| Placebo, n=22 | 5 (22.7%) | 1.0 (reference) | |||
| Male gender | 1.2 (0.3,4.4) | 0.78 | 4.8 (1.3,17.3) | 0.026 | |
| Age at enrollment | 1.5 (0.8,3.0) | 0.21 | 4.3 (1.2,15.4) | 0.037 | |
| Drinks/drinking day | 1.0 (0.9,1.1) | 0.50 | 4.0 (1.1,14.3) | 0.03 | |
| Years of reported alcohol use | 1.0 (0.95,1.1) | 0.72 | 5.8 (1.4,24.3) | 0.02 | |
| Ascites | 3.3 (1.01,11.1) | 0.049 | 4.0 (1.1,14.9) | 0.04 | |
| Enrollment MELD | 1.2 (1.04,1.3) | 0.01 | 5.8 (1.4,24.5) | 0.02 | |
| Albumin | 0.4 (0.1,1.7) | 0.22 | 5.7 (1.2,27.4) | 0.03 | |
| Hepatic Encephalopathy | 2.3 (0.3,15.2) | 0.39 | 4.4 (1.2,15.9) | 0.02 |
Univariate model, odds ratio for death (<=180 days).
Odds Ratio for death (<=180 days) in patient receiving etanercept (relative to Placebo), adjusting for indicated variable (age, gender, etc).
Mortality data are depicted in Figure 2 in the form of Kaplan Meier survival analysis (Figure 2); multiple deaths occurred in the etanercept arm after 30 days, in contradistinction to the lack of mortality in the placebo arm after 30 days, thus accounting for the significant mortality difference observed at 6-month analysis. This observation is expanded in Table 5, which displays causes of deaths which occurred prior to 30 days, and after 30 days in the two study arms. The major causes of death in both groups included renal failure and hepatic encephalopathy.
Figure 2. Kaplan Meier Survival Analysis.
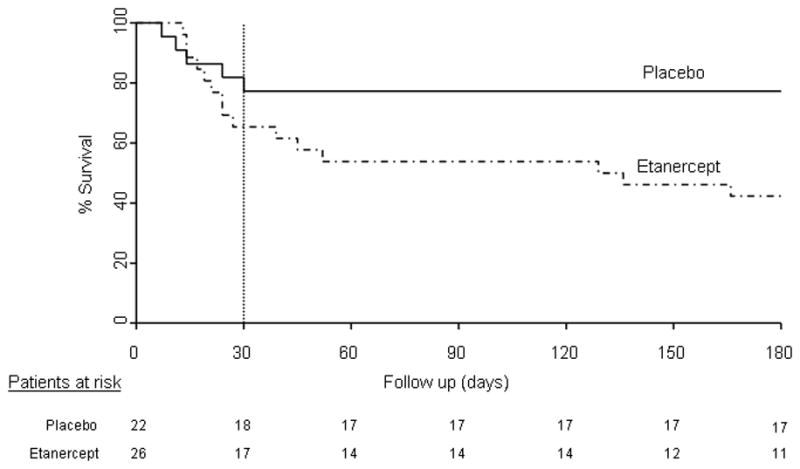
30-day mortality (vertical hashed line) was not significantly different between placebo (solid line) and etanercept (hashed line) groups. 6-month mortality rate was significantly higher in the etanercept arm compared to the placebo arm (p<0.05).
Table 5.
Causes of death
| Placebo (n=5) | Etanercept (n=15) | |||
|---|---|---|---|---|
| Death within one month (n=5) | Death one through six months (n=0) | Death within one month (n=9) | Death one through six months (n=6) | |
| Renal Failure | 3 | 0 | 8 | 5 |
| Sepsis | 0 | 0 | 3 | 0 |
| Hepatic encephalopathy | 4 | 0 | 4 | 2 |
| GI bleeding | 2 | 0 | 2 | 1 |
| Cardiopulmonary† | 1 | 0 | 1 | 0 |
Includes congestive heart failure and respiratory failure
Note that some patients have multiple listed causes of death.
Adverse Events
Severe adverse events (SAEs) were tabulated for each group and are presented in Table 6. SAE were reported significantly more frequently in the etanercept group than placebo group (18 versus 9, respectively; p=0.049), particularly infectious SAE (34.6% versus 9.1%, p=0.04).
Table 6.
Severe adverse events (SAE)
| Serious Adverse Events (SAE) | Placebo (n=22) | Etanercept (n=26) | p-value* |
|---|---|---|---|
| Patients with any SAE, n (%) | 9 (41%) | 18 (69%) | 0.049 |
| GI bleeding, n (%) | 2 (9.1%) | 3 (11.5%) | 1.0 |
| Renal failure, n (%) | 4 (18.2%) | 11 (42.3%) | 0.12 |
| Cardiopulmonary failure, n (%) | 1 (4.5%) | 1 (3.8%) | 1.0 |
| Hepatic encephalopathy, n (%) | 6 (27.3%) | 9 (34.6%) | 0.76 |
| Infection, n (%) | 2 (9.1%) | 9 (34.6%) | 0.04 |
| -Pneumonia, n | 2 | 2 | |
| -Urinary tract infection, n | 0 | 2 | |
| -SBP, n | 0 | 3 | |
| -Sepsis, n | 0 | 3 | |
| -Catheter infection, n | 0 | 1 |
Significance test by Fisher’s exact test.
Each SAE represents one patient experiencing that particular event.
Each patient may have more than one SAE or infection, resulting in totals that exceed 100%.
Discussion
Multiple lines of experimental evidence indicate a central role for TNFα in the pathogenesis of alcoholic hepatitis, including correlations of cytokine levels with mortality in humans. Furthermore, prior human trials have suggested that inhibition of TNFα may be beneficial in patients with alcoholic hepatitis 13, 27, 33. However, the present double blinded, placebo controlled trial demonstrates a significantly higher rate of 6 month mortality in patients with moderate to severe alcoholic hepatitis who received the soluble TNFα receptor:FC fusion protein, etanercept. The six month mortality rate was more than double that of the placebo group; a robust effect which persisted in post-hoc analyses despite adjustments for baseline patient characteristics, and regardless of “intention to treat” or “per protocol” analysis. Although the present study was discontinued prior to completion of target enrollment, it is highly unlikely that further patient recruitment would have revealed a survival benefit of etanercept; at least 88 additional patients per treatment arm would have been needed for this effect to be observed, indicating a very low likelihood of a type II error.
Some study design aspects of this study are also worth mention as they differ from prior trials conducted in patients with alcoholic hepatitis. First, the present study utilized a MELD based entry criteria for disease severity rather than the traditional DF. We and others have recently demonstrated that MELD is at least equivalent to the DF for prediction of death in patients with alcoholic hepatitis 28, 29. Furthermore, MELD maintains some logistic advantages over the DF, owing to use of INR rather than prothrombin time in MELD calculation 28, 29. Indeed, the placebo mortality rate in our study with MELD-based entry criteria was 22.7%, which was in line with previously reported placebo mortality in randomized trials that utilized a DF-based inclusion criteria 14, 33–35. Secondly, the present study was a placebo-controlled design which did not include a corticosteroid arm. Although many may favor a corticosteroid arm rather than a placebo arm in treatment trials of alcoholic hepatitis given the positive corticosteroid trials in the literature; the generalizability of corticosteroid use in practice varies widely. Indeed, none of the sites participating in this trial utilize corticosteroids on a routine basis in patients with alcoholic hepatitis, thus providing a clear rationale for a placebo arm in place of a corticosteroid arm.
Why was six month mortality increased in the etanercept arm but not one month mortality despite a treatment protocol administration length of only three weeks? The most common cause of this late death was renal failure which occurs frequently in response to infectious sequelae concomitant with hepatic deterioration. TNFα functions not only in pathways that lead to hepatocyte apoptosis and liver injury but also in pathways of liver regeneration and innate immunity 36–42. Thus, one possibility to account for the increased late mortality may relate to impaired liver regeneration in patients receiving etanercept, especially since liver regeneration is likely important for recovery from alcohol induced liver injury 43. Another possibility relates to effects of etanercept on immune function, since TNFα contributes importantly to immune cell functions necessary for combating mycobacteria and atypical organisms 44. This may be particularly relevant in patients with advanced liver disease and cirrhosis, since these patients already evidence impaired immune function. Indeed, a recent treatment trial demonstrated increased infection related mortality in patients with alcoholic hepatitis that received the TNFα neutralizing antibody, infliximab, in combination with corticosteroids, which led to early discontinuation of that study 45. A higher rate of infection was also observed in the etanercept arm of the present study, suggesting that neutralization of serum TNFα in patients with cirrhosis, who are prone to hepatic deterioration and infectious sequelae, may be unsafe.
Pentoxifylline, a putative inhibitor of TNFα release, was demonstrated to be effective in patients with alcoholic hepatitis 10. Thus, it is possible that approaches that target TNFα generation rather than serum neutralization (as occurred in our study), may demonstrate therapeutic benefit in alcoholic hepatitis. However, in the aforementioned trial, pentoxifylline was not actually demonstrated to reduce serum TNFα levels. Indeed, there are diverse alternative mechanisms independent of TNFα by which this compound may confer beneficial effect 11. Regardless, the present study demonstrates that, despite the dominant cytokine derangements in patients with alcoholic hepatitis, approaches to neutralize TNFα in serum are not warranted in patients with alcoholic hepatitis.
Supplementary Material
Acknowledgments
The authors thank the lead research coordinator at Mayo, Stephanie M. Johnson, and Michele Hooper (Amgen) for assistance, as well as the following members of the study DSMB: William Sandborn, Russell Wiesner, and Walter Kremers.
Footnotes
Author Disclosure: This study was funded through the NIH (R01 AA013933 to VS), the NIH funded Mayo Clinical Research Unit (CTSA), and Amgen (to VS), which provided study drug, and partially defrayed costs for cytokine analyses that exceeded the NIH budget. All analyses and writing were conducted at Mayo Clinic. Authors have no other conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCullough A, O’Connor J. Alcoholic liver disease: proposed recommendations for the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2022–2036. doi: 10.1111/j.1572-0241.1998.00587.x. [DOI] [PubMed] [Google Scholar]
- 2.Lieber C. Alcoholic liver disease: new insights in pathogenesis lead to new treatments. J Hepatol. 2000;32:113–128. doi: 10.1016/s0168-8278(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 3.Menon K, Gores G, Shah V. Pathogenesis, diagnosis, and treatment of alcoholic liver disease. Mayo Clin Proc. 2001;76:1021–1029. doi: 10.4065/76.10.1021. [DOI] [PubMed] [Google Scholar]
- 4.Baker AL, Jaspan JB, Haines NW, Hatfield GE, Krager PS, Schneider JF. A randomized clinical trial of insulin and glucagon infusion for treatment of alcoholic hepatitis: progress report in 50 patients. Gastroenterology. 1981;80:1410–4. [PubMed] [Google Scholar]
- 5.Halle P, Pare P, Kaptein E, Kanel G, Redeker A, Reynolds T. Double-blind, controlled trial of propylthiouracil in patients with severe acute alcoholic hepatitis. Gastroenterology. 1982;82:925–31. [PubMed] [Google Scholar]
- 6.Akriviadis E, Steindel H, Pinto P, Fong T, Kanel G, Reynolds T, Gupta S. Failure of colchicine to improve short-term survival in patients with alcoholic hepatitis. Gastroenterology. 1990;99:811–8. doi: 10.1016/0016-5085(90)90973-5. [DOI] [PubMed] [Google Scholar]
- 7.Morgan TR, Moritz T, Mendenhall C. Nutritional therapy for alcoholic hepatitis. VA Cooperative 275 Study Group [letter; comment] Gastroenterology. 1992;103:357–9. doi: 10.1016/0016-5085(92)91156-x. [DOI] [PubMed] [Google Scholar]
- 8.Mendenhall CL, Moritz TE, Roselle GA, Morgan TR, Nemchausky BA, Tamburro CH, Schiff ER, McClain CJ, Marsano LS, Allen JI, et al. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: results of a Department of Veterans Affairs cooperative study. Hepatology. 1993;17:564–76. doi: 10.1002/hep.1840170407. [DOI] [PubMed] [Google Scholar]
- 9.Cabre E, Rodriguez-Iglesias P, Caballeria J, Quer J, Sanchez-Lombrana J, Pares A, Papo M, Planas R, Gassull M. Short and long term outcome of severe alcohol nduced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology. 2000;32:36–42. doi: 10.1053/jhep.2000.8627. [DOI] [PubMed] [Google Scholar]
- 10.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–48. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 11.Morgan T, McClain C. Pentoxifylline and alcoholic hepatitis. Gastroenterology. 2000;119:1787–91. doi: 10.1053/gast.2000.20826. [DOI] [PubMed] [Google Scholar]
- 12.Mathurin P, Mendenhall C, Carithers R, Jr, Ramond M, Maddrey W, Garstide P, Rueff B, Naveau S, Chaput J, Poynard T. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36:547–8. doi: 10.1016/s0168-8278(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 13.Tilg H, Jalan R, Kaser A, Davies NA, Offner FA, Hodges SJ, Ludwiczek O, Shawcross D, Zoller H, Alisa A, Mookerjee RP, Graziadei I, Datz C, Trauner M, Schuppan D, Obrist P, Vogel W, Williams R. Anti-tumor necrosis factor-alpha monoclonal antibody therapy in severe alcoholic hepatitis. J Hepatol. 2003;38:419–25. doi: 10.1016/s0168-8278(02)00442-7. [DOI] [PubMed] [Google Scholar]
- 14.Morgan TR, Weiss DG, Nemchausky B, Schiff ER, Anand B, Simon F, Kidao J, Cecil B, Mendenhall CL, Nelson D, Lieber C, Pedrosa M, Jeffers L, Bloor J, Lumeng L, Marsano L, McClain C, Mishra G, Myers B, Leo M, Ponomarenko Y, Taylor D, Chedid A, French S, Kanel G, Murray N, Pinto P, Fong TL, Sather MR. Colchicine treatment of alcoholic cirrhosis: a randomized, placebo-controlled clinical trial of patient survival. Gastroenterology. 2005;128:882–90. doi: 10.1053/j.gastro.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 15.Neuman MG. Cytokines--central factors in alcoholic liver disease. Alcohol Research & Health: the Journal of the National Institute on Alcohol Abuse & Alcoholism. 2003;27:307–16. [PMC free article] [PubMed] [Google Scholar]
- 16.Hoek JB, Pastorino JG. Cellular signaling mechanisms in alcohol-induced liver damage. Seminars in Liver Disease. 2004;24:257–72. doi: 10.1055/s-2004-832939. [DOI] [PubMed] [Google Scholar]
- 17.Latvala J, Hietala J, Koivisto H, Jarvi K, Anttila P, Niemela O. Immune Responses to Ethanol Metabolites and Cytokine Profiles Differentiate Alcoholics with or without Liver Disease. American Journal of Gastroenterology. 2005;100:1303–10. doi: 10.1111/j.1572-0241.2005.41509.x. [DOI] [PubMed] [Google Scholar]
- 18.McClain C, Barve S, Joshi-Barve S, Song Z, Deaciuc I, Chen T, Hill D. Dysregulated cytokine metabolism, altered hepatic methionine metabolism and proteasome dysfunction in alcoholic liver disease. Alcoholism: Clinical & Experimental Research. 2005;29:180S–8S. doi: 10.1097/01.alc.0000189276.34230.f5. [DOI] [PubMed] [Google Scholar]
- 19.Tilg H, Diehl A. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 20.Jalan R, Williams R, Kaser A, Davies N, Zoller H, Hodges S, Graziedei I. Clinical and cytokine response to anti-TNF antibody therapy in severe alcoholic hepatitis. Hepatology. 2001;34:441A. [Google Scholar]
- 21.Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, Weaver AL, Keystone EC, Furst DE, Mease PJ, Ruderman EM, Horwitz DA, Arkfeld DG, Garrison L, Burge DJ, Blosch CM, Lange ML, McDonnell ND, Weinblatt ME. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Annals of Internal Medicine. 1999;130:478–86. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- 22.Sandborn W, Hanauer S, Katz S, Safdi M, Wolf D, Baerg R, Tremaine W, Johnson T, Diehl N, Zinsmeister A. Etanercept for active Crohn’s disease: A randomized double-blind, placebo-controlled trial. Gastroenterology. 2001;121:1088–94. doi: 10.1053/gast.2001.28674. [DOI] [PubMed] [Google Scholar]
- 23.ten Cate R, van Suijlekom-Smit L, Brinkman D, Bekkering W, Jansen-van Wijngaarden C. Etanercept in four children with therapy-resistant systemic juvenile idiopathic arthritis. Rheumatology. 2002;41:228–229. doi: 10.1093/rheumatology/41.2.228. [DOI] [PubMed] [Google Scholar]
- 24.Gorman J, Sack K, Davis JJ. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Eng J Med. 2002;346:1349–1356. doi: 10.1056/NEJMoa012664. [DOI] [PubMed] [Google Scholar]
- 25.Steensma D, Mesa R, Li C, Gray L, Tefferi A. Etanercept, a soluble tumor necrosis factor receptor, palliates constitutional symptoms in patients with myelofibrosis with myeloid metaplasia: results of a pilot study. Blood. 2002;99:2252–2254. doi: 10.1182/blood.v99.6.2252. [DOI] [PubMed] [Google Scholar]
- 26.Phillips K, Husni M, Karlson E, Coblyn J. Experience with etanercept in an academic medical center: are infection rates increased? Arthritis Rheum. 2002;47:17–21. doi: 10.1002/art1.10243. [DOI] [PubMed] [Google Scholar]
- 27.Menon K, Stadheim L, Kamath P, Gores G, Peine C, Shah V. Safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol. 2004;99:255–60. doi: 10.1111/j.1572-0241.2004.04034.x. [DOI] [PubMed] [Google Scholar]
- 28.Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, Malinchoc M, Kamath PS, Shah V. MELD accurately predicts mortality in patients with alcoholic hepatitis. [see comment] Hepatology. 2005;41:353–8. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 29.Sheth M, Riggs M, Patel T. Utility of the Mayo end-stage liver disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol. 2001;2:2. doi: 10.1186/1471-230X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srikureja W, Kyulo NL, Runyon BA, Hu K-Q. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. [see comment] Journal of Hepatology. 2005;42:700–6. doi: 10.1016/j.jhep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Said A, Williams J, Holden J, Remington P, Gangnon R, Musat A, Lucey M. Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease. J Hepatol. 2004;40:897–903. doi: 10.1016/j.jhep.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Carithers RJ, Herlong H, Diehl A, Shaw E, Combes B, Fallon H, Maddrey W. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial [see comments] Annals of Internal Medicine. 1989;110:685–90. doi: 10.7326/0003-4819-110-9-685. [DOI] [PubMed] [Google Scholar]
- 33.Spahr L, Rubbia-Brandt L, Frossard J, Giostra E, Rougemont A, Pugin J, Fischer M, Egger H, Hadengue A. Combination of steroids with infliximab or placebo in severe alcoholic hepatitis: a randomized controlled pilot study. J Hepatol. 2002;37:448–455. doi: 10.1016/s0168-8278(02)00230-1. [DOI] [PubMed] [Google Scholar]
- 34.Bird GL, Prach AT, McMahon AD, Forrest JA, Mills PR, Danesh BJ. Randomised controlled double-blind trial of the calcium channel antagonist amlodipine in the treatment of acute alcoholic hepatitis. J Hepatol. 1998;28:194–8. doi: 10.1016/0168-8278(88)80005-9. [DOI] [PubMed] [Google Scholar]
- 35.Mato J, Camara J, Fernandez de Paz J, Caballeria L, Coll S, Caballero A, Garcia-Buey L, Beltran J, Venita V, Caballeri J, Sola R, Moreno-Otero R, Barrao F, Martin-Duce A, Correa J, Pares A, Barrao E, Garcia-Magaz I, Puerta J, Moreno J, Boissard G, Ortiz P, Rodes J. S-Adenosylmethionine in alcoholic liver cirrhosis: a randomized placebo-controlled, double-blind, multicenter clinical trial. Journal of Hepatology. 1999;30:1081–1089. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 36.Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. American Journal of Pathology. 1995;146:1220–34. [PMC free article] [PubMed] [Google Scholar]
- 37.Schafer C, Schips I, Landig J, Bode JC, Bode C. Tumor-necrosis-factor and interleukin-6 response of peripheral blood monocytes to low concentrations of lipopolysaccharide in patients with alcoholic liver disease. Zeitschrift fur Gastroenterologie. 1995;33:503–8. [PubMed] [Google Scholar]
- 38.Lechner AJ, Velasquez A, Knudsen KR, Johanns CA, Tracy TF, Jr, Matuschak GM. Cholestatic liver injury increases circulating TNF-alpha and IL-6 and mortality after Escherichia coli endotoxemia. American Journal of Respiratory & Critical Care Medicine. 1998;157:1550–8. doi: 10.1164/ajrccm.157.5.9709067. [DOI] [PubMed] [Google Scholar]
- 39.Yamada Y, Fausto N. Deficient liver regeneration after carbon tetrachloride injury in mice lacking type 1 but not type 2 tumor necrosis factor receptor. American Journal of Pathology. 1998;152:1577–89. [PMC free article] [PubMed] [Google Scholar]
- 40.Yin M, Wheeler M, Kono H, Bradford B, Gallucci R, Luster M, Thurman R. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–52. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 41.Gallucci R, Simeonova P, Toriumi W, Luster M. TNF-alpha regulates TGF alpha expression in regenerating murine liver and isolated hepatocytes. J Immunol. 2000;164:872–8. doi: 10.4049/jimmunol.164.2.872. [DOI] [PubMed] [Google Scholar]
- 42.Hajeer A, Hutchinson I. TNF-α gene polymorphism: clinical and biological implications. Microsc Res Tech. 2000;50:216–228. doi: 10.1002/1097-0029(20000801)50:3<216::AID-JEMT5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 43.Akerman P, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, Diehl AM. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992;263:G579–85. doi: 10.1152/ajpgi.1992.263.4.G579. [DOI] [PubMed] [Google Scholar]
- 44.Roach D, Bean A, Demangel C, France M, Briscoe H, Britton W. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–4627. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 45.Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, Davion T, Oberti F, Broet P, Emilie D. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390–7. doi: 10.1002/hep.20206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


