Abstract
The exposure of cells to several metal ions stabilizes HIF-1α protein. However, the molecular mechanisms are not completely understood. They may involve inhibition of hydroxylation by either substitution of iron by metal ions or by iron oxidation in the hydroxylases. Here we provide evidence supporting the latter mechanism. We show that HIF-1α stabilization in human lung epithelial cells occurred following exposure to various metal and metalloid ions, including those that cannot substitute for iron in the hydroxylases. In each case addition of the reducing agent ascorbic acid (AA)* abolished HIF-1α protein stabilization. To better understand the role of iron oxidation in hydroxylase inhibition and to define the role of AA in the enzyme recovery we applied molecular modeling techniques. Our results indicate that the energy required for iron substitution by Ni(II) in the enzyme is high and unlikely to be achieved in a biological system. Additionally, computer modeling allowed us to identify a tridentate coordination of AA with the enzyme-bound iron, which explains the specific demand for AA as the iron reductant. Thus, the stabilization of HIF-1α by numerous metal ions that cannot substitute for iron in the enzyme, the alleviation of this effect by AA, and our computer modeling data support the hypothesis of iron oxidation in the hydroxylases following exposure to metal ions.
Keywords: antioxidants, metals, transcription factors
Metals are widely used in modern societies and sources of exposure are numerous. Major sources of occupational exposure are smelters, mining activities, hazardous waste sites, and even natural sources. Burning fossil fuel is one of the sources of environmental exposure of general population. It results in the emission of residual oil fly ash, which contains metal ions, such as Fe(II/III), Mn(II), Ni(II), V(V), Ca(II), Mg(II), and Zn(II) (Dreher et al., 1997). Epidemiological data show associations between particulate matter concentrations and adverse health effects in exposed populations (EPA, 1996). It is becoming clear that the metals contained in these particles may stimulate inflammatory responses and contribute to lung injury. The activation of a number of transcription factors, such as AP-1, ATF-2, CREB, HIF-1, and NF-κB, by metal ions plays an important role in these processes (Ghio et al., 2002; Kaczmarek et al., 2007; McNeilly et al., 2005; O'Hara et al., 2007). In this work we focus our attention on HIF-1 activation. HIF-1 activation can damage lung tissues in many ways. It can cause apoptosis through activating expression of the proapoptotic protein Bnip3L (Krick et al., 2005), or through the activation of interleukin (IL)-8, which leads to inflammatory responses (Kim et al., 2006). The HIF-1 transcription factor is composed of two subunits: HIF-1α and HIF-1β. HIF-1α is a short-lived protein with a regulatory function and exposure of human cells to Ni(II) or Co(II) has been shown to result in HIF-1α protein stabilization (Salnikow et al., 1999; Wang and Semenza, 1995).
Previous studies indicate that the molecular mechanism of HIF-1 transcription factor activation by Ni(II) or Co(II) involves inhibition of HIF-1α hydroxylation (Salnikow et al., 2004). The hydroxylation of HIF-1α proline residues 402 and 564 facilitates the interaction of this protein with the von Hippel-Lindau protein and targets it for degradation. The hydroxylation of the asparagine 803 residue inhibits the transcriptional response by preventing interaction of the HIF-1α protein with acetyltransferase p300 (Maxwell and Salnikow, 2004). Thus, inhibition of hydroxylation results in HIF-1α stabilization and HIF-1 transactivation. The hydroxylases, which are involved in HIF-1α modification, are iron-containing enzymes. Because divalent metal ions such as Ni(II) or Co(II) resemble iron, it has been suggested that these metals could replace the iron in the enzymes but cannot efficiently carry out the reaction, or, alternatively, they could interfere with cellular iron uptake, thus depleting the intracellular iron (Davidson et al., 2005). In addition, metals can directly, catalytically, or indirectly, through oxidative stress, oxidize ascorbic acid (AA), which is essential for maintaining reduced iron in asparaginyl and prolyl hydroxylases. The latter hypothesis is supported by the demonstration that intracellular ascorbate is depleted by metals and the addition of ascorbate to Co(II)- or Ni(II)-exposed cells reactivates HIF-1α hydroxylation (Karaczyn et al., 2006; Knowles et al., 2003; Salnikow et al., 2004). Recently we have shown that Cr(VI), which cannot replace iron, but can directly oxidize AA stabilizes HIF-1α in human lung cells (Kaczmarek et al., 2007). In this study, we show that numerous metal ions can stabilize HIF-1α protein. They mediate AA oxidation in a cell-free system and the supplementation of cells exposed to metal ions with AA in all cases abolished HIF-1α stabilization.
To better understand the role of AA in the hydroxylase reaction, we did molecular modeling of the iron-AA interaction in the reaction center of hydroxylases. We found that AA is a unique molecule that fits perfectly into the enzyme's active center and cannot be replaced by other reducing molecules. The computer modeling data also suggest that the enzyme's iron is unlikely to be replaced in living cells due to high activation barriers.
MATERIALS AND METHODS
Reagents.
NiSO4·6H2O, was obtained from Alfa Aesar (Ward Hill, MA). NaAsO2, KH2AsO4, CuCl2, MnCl2·4H2O, FeSO4·7H2O, FeCl3·6H2O, Na3VO4, L-ascorbic acid, dehydroascorbic acid, and other chemicals were obtained from Sigma-Aldrich (St Louis, MO). Complete Protease inhibitor cocktail was purchased from Roche (Indianapolis, IN).
Cell lines and culture conditions.
The 1HAEo- human cell line was obtained from Dr D. C. Gruenert (Gruenert et al., 1995). Cells were grown on plastic plates coated with a mixture of bovine serum albumin (Invitrogen Corporation, Carlsbad, CA) and collagen (Cohesion Technologies, Inc., Palo Alto, CA) in Minimum Essential Medium with Earle's modified salts (Invitrogen) containing 10% fetal calf serum, 2mM L-glutamine, 100 μg/ml streptomycin, and 100 U/ml penicillin. For exposure, cells were plated at a density 25–30·103 cells/cm2 in complete medium under 95% air, 5% CO2, at 37°C. Cells were exposed to metals 30–36 h later.
HPLC determination of AA oxidation.
Determination of AA oxidation by metals was done in HEPES buffer as described in (Kaczmarek et al., 2007).
Nuclear magnetic resonance studies of dehydroascorbate and 2,3-diketogulonic acid oxidation.
The 1H NMR spectra were recorded on a Varian UNITYINOVA spectrometer at 400 MHz immediately after the dehydroascorbate (DHA) (Sigma) was dissolved in 50mM HEPES buffer, pH 7.4 (Sigma). Additional spectra were then recorded every hour. HEPES buffer was prepared in D2O and contained 0.75 wt% sodium-2,2,3,3-tetradeuterio-3-trimethylsilylpropionate as an internal standard (Sigma). The pH was adjusted using DCl (Sigma). Ni(II) stock solution was prepared by dissolving NiSO4 (Sigma-Aldrich) in D2O.
Metal determination by inductively coupled plasma optical emission spectroscopy.
After the treatments, the cells were washed three times, counted and collected in PBS buffer. Cell pellets were dissolved in 3 N HCl/10% trichloroacetic acid by heating overnight at 70°C. They were then centrifuged for 5 min at 20,700 × g, and the supernatants were diluted 1:10 with H2O. The samples analyzed by inductively coupled plasma optical emission spectroscopy on a PerkinElmer 4300DV spectrometer. Samples were analyzed for Fe and Ni at 238.204 and 231.604 nm, respectively, with confirmation at 259.939 nm for Fe and 221.648 nm for Ni. Their concentrations were determined based on a standard curve prepared by gravimetric dilution of a multi-element standard obtained from SPEX CertiPrep, Inc. (Metuchen, NJ) and verified with standards prepared by gravimetric dilution of a multi-element standard obtained from VHG Labs, Inc. (Manchester, NH). The measurements were done in duplicate and the experiments were repeated twice.
Western blotting.
Total cell protein extracts were obtained after lysing cells in lysis buffer (Cell Signaling Technology, Inc., Danvers, MA) for 15 min at 4°C. Equal protein loading was assured by prior quantitation using the Bradford assay. Forty-microgram samples of each protein extract were separated by gel electrophoresis in 4–12% NuPAGE Bis-Tris minigels (Invitrogen, Carlsbad, CA) in MOPS buffer, pH 7.7, and transferred onto PVDF membranes (Invitrogen). Nuclear extracts were obtained as previously described (Salnikow et al., 2004). Fifteen-microgram samples of each protein were separated by 4–12% gradient NuPAGE sodium dodecyl sulfate (SDS) Bis-Tris gels in MOPS-SDS running buffer (Invitrogen), under reducing conditions. Proteins were transferred onto PVDF membranes. The membranes were blocked using a 5% nonfat dry milk solution (Immun-Blot Blotting Grade Blocker nonfat dry milk, Bio-Rad Laboratories) in the presence of 5% goat serum (Sigma, Sigma-Aldrich). The protein bands were visualized on the membranes using anti-HIF-1α (BD Biosciences Pharmingen, San Jose, CA), or anti-β-actin antibodies (Novus Biologicals, Littleton, CO). The secondary antibodies were goat anti-rabbit HRP-conjugated antibodies, or goat anti-mouse HRP-conjugated antibodies (Cell Signaling Technology, Inc). The chemiluminescent signal was detected using the Super Signal kit (Pierce Biotechnology Inc., Rockford, IL).
ODD-Luc reporter assay.
The transient transfection experiments were carried out as previously described (Salnikow et al., 2004). The transfection experiments were repeated at least twice and each condition was tested in quadruplicate. The results shown are the mean values ± SD.
Molecular modeling of iron substitution and AA-iron interaction.
Molecular mechanics (MM)–based molecular dynamics (MD) studies were carried out using the X-PLOR program Version 3.1 (Brünger, 1993). Trajectories were computed using the CHARMM 27 parameter set with periodic boundary conditions and standard cutoffs for coulombic (12 Å) and van der Waals interactions (8 Å). Thermalization was done using the standard Berendsen thermostat as implemented in X-PLOR using parameters Fβ = 100 and T = 298K. Integration of the equations of motion was performed using a Verlet algorithm with a 1-fs step. Trajectories were stabilized for 250 ps before production runs were collected (500 ps). Ion pulling calculations were performed using a type of steered dynamics called feedback restrain molecular dynamics (FRMD) (Cachau et al., 1994). FRMD affords a self-consistent method for the computation of restrains unlike similar procedures that rely on guessed values. Although the computational cost is higher, the unbiased nature of the FRMD scan helps remove uncertainty from the complex estimation of kinetic and thermodynamic constants in macromolecular calculations.
Dynamics trajectories using hybrid quantum mechanics/molecular mechanics methods (QM/MM) were calculated using the DYNGA program (Parker et al., 2003) and Gaussian 03 as a slave program (Frisch et al., 2003) in the framework of the PBE/4-31G* approach (Hehre et al., 1972).
Trajectory calculations were performed for a cookie cutter model of the reaction center region anchored in the surrounding protein using either fixed of anchor points or semiharmonic restraints (see Supplemental Data). No significant differences were observed using either technique. The presented results are those from using fixed anchor points.
The integration of the equations of motion in QM/MM calculations was done using a Verlet algorithm coupled with a Nose thermostat (thermostat mass = 1000) and an integration step of 0.5 fs. Initial velocities were obtained from MM calculations followed by 5 ps of stabilization using quasi-harmonically restrained hydrogens. The hydrogens were subsequently freed and the trajectories were collected for 5–15 ps. Pople's 4-31G* atomic basis was used to describe the atoms in the QM region. For a control, larger basis sets (6-31G*) were used to study single points and the corresponding results were similar to those based on 4-31G* calculations (data not shown).
RESULTS
Stabilization of HIF-1α Protein by Metalloid or Metal Ions in Human Lung Cells
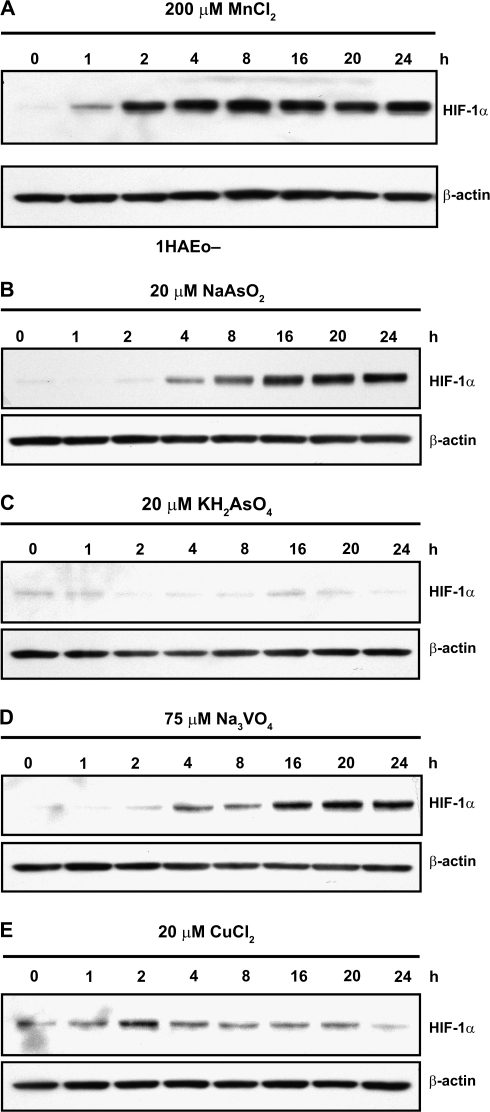
In previous studies it was demonstrated that HIF-1α protein stabilization could be caused by exposure to Co(II), Ni(II), or Cr(VI) and that AA diminished this effect (Kaczmarek et al., 2007; Knowles et al., 2003; Salnikow et al., 2004). Here we investigated the role of AA in the negating the HIF-1α protein stabilization by As(III), Mn(II), V(V), and Cu(II) in cultured cells. Metal concentrations were selected based on minimal toxicity during the time of exposure. Figure 1A shows the time course of HIF-1α protein stabilization by Mn(II). The protein accumulation began at 1 h of 1HAEo- cells exposure and it reached plateau level by 2 h. The amount of protein remained at the same level thereafter up to 24 h. The time course of HIF-1α protein stabilization by As(III), As(V), and V(V) is shown in Figures 1B–D. The selected dose of arsenate did not induce HIF-1α during a 24-h exposure. The stabilization of HIF-1α by As(III) and V(V) was delayed compared with previously observed time courses for Cr(VI), or Ni(II) (Kaczmarek et al., 2007) and in present work for Mn(II), and showed strong stabilization by 8–16 h. Twenty micromolars of Cu(II) slightly stabilized HIF-1α protein at 2- to 4-h exposure (Fig. 1E).
FIG. 1.
Effects of metals exposure on HIF-1α levels in 1HAEo- cells. Cells were exposed to a metal for the indicated time periods. Nuclear protein extracts (15 μg) were prepared for immunoblotting as described in Materials and Methods and probed with antibodies against HIF-1α. The same membrane was probed with antibodies against β-actin to provide a loading control. 1HAEo- cells were exposed to 200μM MnCl2 (A), to 20μM NaAsO2 (B), or to 20μM KH2AsO4 (C), or to 75μM Na3VO4 (D), or to 20μM CuCl2 (E).
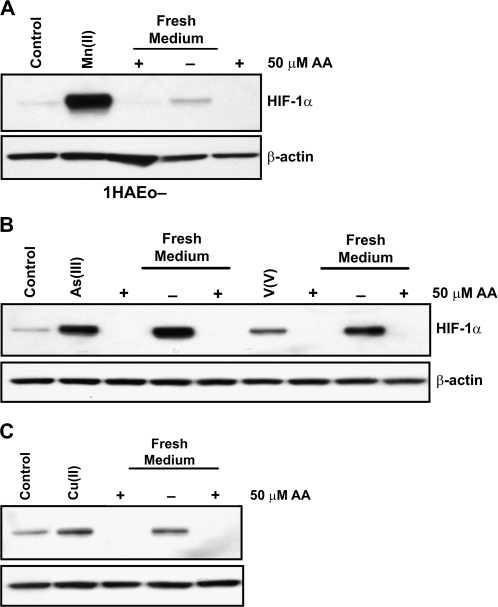
Based on the time course data we decided to use 4-h time point to examine the effect of AA on HIF-1α degradation in metal-exposed cells. The HIF-1α protein was stabilized by exposure to Mn(II), As(III), V(V), or Cu(II) for 4 h, then cells were washed and incubated in fresh complete medium, with or without supplementation with 50μM AA, for an additional 4 h. The addition of AA stimulated HIF-1α protein degradation in all cases, whereas the medium change did not work or worked partially in 1HAEo- cells (Figs. 2A–C).
FIG. 2.
AA destabilizes HIF-1α in 1HAEo- cells exposed to Mn(II), As(III), V(V), or Cu(II). 1HAEo- were exposed to a metal for 4 h to induce HIF-1α protein, the medium was exchanged for fresh medium with or without 50μM AA and cells were incubated for an additional 4 h. Nuclear protein extracts (15 μg) were prepared for immunoblotting as described in Figure 1. 1HAEo- cells were exposed to 200μM MnCl2 (A), or to 20μM NaAsO2 or to 75μM Na3VO4 (B), or to 20μM CuCl2 (C).
Effect of metalloid or metal ions on AA oxidation in a cell-free system.
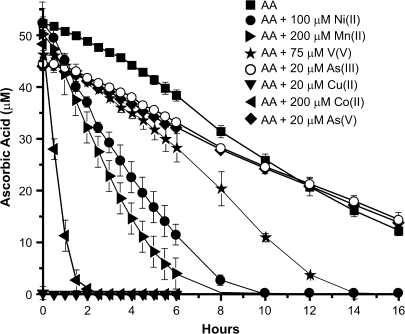
To compare the effects of the metal ions on the course of AA oxidation, we incubated 50μM AA in HEPES buffer, pH 7.4, in the absence or presence of 20μM As(III), 20μM As(V), 200μM Co(II), 200μM Mn(II), 20μM Cu(II), or 75μM V(V) under ambient air at 37°C up to 16 h (Fig. 3). The kinetics of AA oxidation by the oxygen in the air was similar to that described earlier by our group with T1/2 of approximately 10 h (Kaczmarek et al., 2007). As(II) and As(V) had practically no effect on AA half-life. Exposure to V(V) decreased AA half-life to approximately 7 h, whereas Ni(II) and Mn(II) had stronger effect with AA half-life of 3.5 and 3.0 h, respectively. Co(II) was much more efficient with T1/2 of approximately 1 h. Cu(II) oxidized all available AA in a few minutes (Fig. 3). Thus, all of the metal ions tested, except for As(III) and As(V) increased AA oxidation in a cell-free system. AA can be oxidized to DHA, which can be recycled by living cells back to AA, or oxidized even further to 2,3-diketogulonic acid (DKG) or other small degradation products, which the cell cannot recycle (Linster and Van Schaftingen, 2007).
FIG. 3.
Kinetics of AA oxidation by metals in cell-free system. Fifty micromolars of AA in HEPES buffer was incubated at 37°C alone or in the presence of 20μM As(III), 20μM As(V), 200μM Co(II), 20μM Cu(II), 200μM Mn(II), 100μM Ni(II), and 75μM V(V) for the indicated time periods. Samples were analyzed using HPLC as described in “Materials and Methods.” The data are presented as mean values ± the SD.
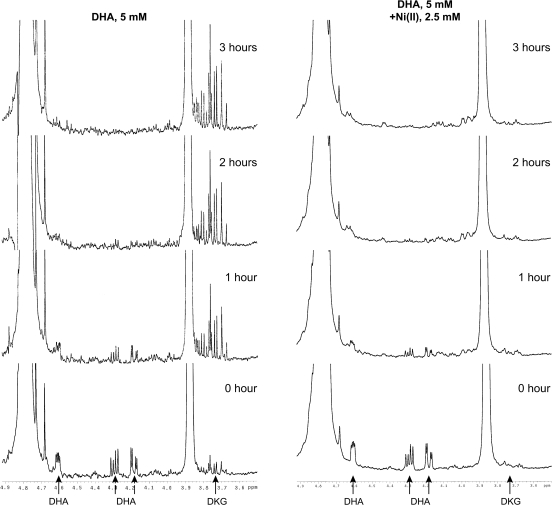
To determine whether exposure to Ni(II) results in irreversible degradation of DHA to DKG or other smaller products, we measured the time course of DHA degradation and the appearance of DKG using 1H NMR. In the 1H NMR spectra. DHA produced three signals at δH = 4.2, 4.3, and 4.6 ppm and DKG produced one signal at δH = 3.75 ppm in agreement with previously published data (Nishikawa and Kurata, 2000; Nishikawa et al., 2001). Because DHA is unstable at neutral or basic pH, the signals disappeared after 1 h (Fig. 4). At the same time DKG signal grew, indicating that it was the major product of DHA degradation. In the control, DKG signal remained stable following 3 h of incubation. In the presence of Ni(II) ions, the DKG signal was not observed after a similar incubation, suggesting a nickel-catalyzed degradation of DKG to smaller products, which cannot be seen under our experimental conditions (Fig. 4).
FIG. 4.
Proton NMR spectra of the incubated DHA solutions with or without Ni(II). Five millimolar DHA was dissolved alone (left) or in the presence 2.5mM Ni(II) (right) in HEPES buffer (pH 7.4) prepared in D2O. Spectra were recorded at a zero time point and then every hour up to 3 h. The positions of the DHA and DKG protons are shown with arrows.
Inhibition of HIF-1α hydroxylation is decreased in the presence of AA.
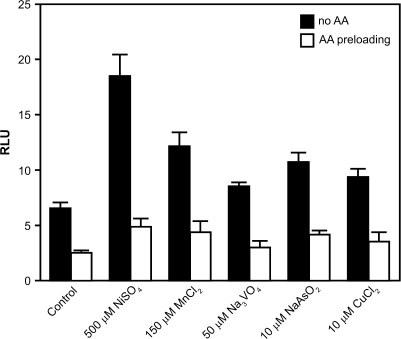
Because the addition of AA to cells resulted in HIF-1α protein destabilization, we decided to determine whether HIF-1α protein hydroxylation was involved in this process. We utilized an ODD-Luc reporter plasmid (Salnikow et al., 2004) that represents an oxygen-dependent HIF-1α degradation domain fused in frame to a luciferase reporter. This chimeric protein behaves like HIF-1α in living cells. The intensity of the luciferase signal is directly proportional to the degree of the proline hydroxylation inhibition in the HIF-1α ODD domain. Figure 5 shows that preloading cells with AA significantly alleviated the inhibition of HIF hydroxylation by all tested metals.
FIG. 5.
Preloading with AA alleviates inhibition of hydroxylation by metals. 1HAEo- cells were transfected with an ODD-Luc reporter plasmid. After expressing the reporter for 24 h, cells were either preloaded with 50μM of AA for 2 h or left alone for an additional 4 h following exposure to metals at the indicated concentrations.
Level of intracellular iron does not affect HIF-1α stabilization by nickel.
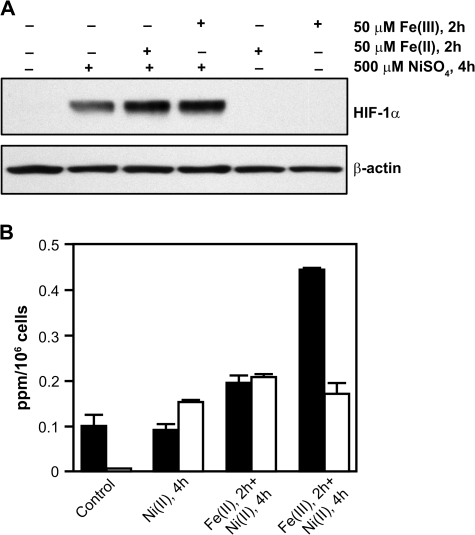
In the hydroxylases, the iron coordinates with nitrogens of two histidines (His) and a carboxyl group of aspartic acid (Asp) (Hegg and Que, 1997). If the strength of the iron-binding motif in the enzyme allowed for exchange with other metals then iron supplementation should prevent the effect of other metals on HIF-1α protein stabilization. Because it has been suggested that Ni(II) can inhibit iron transport by competing with iron for DMTI (Davidson et al., 2005; Kang et al., 2006), we decided to first preload cells with iron for 2 h and then expose the cells to Ni(II). We also used Fe(II) or Fe(III) ions, which are delivered to cells through different pathways. The pretreatment resulted in an increase of intracellular iron, most significantly for Fe(III) (Fig. 6B), but did not change HIF-1α protein stabilization following Ni(II) exposure (Fig. 6A). Thus, no correlation was found between iron level and HIF-1α protein upregulation even when the iron level exceeded the Ni(II) level (Fig. 6B). In agreement with these data, only a weak effect of iron pretreatment on HIF-1α protein stabilization by Ni(II) was recently reported by others (Kang et al., 2006).
FIG. 6.
Changes in iron levels do not affect HIF-1α protein. (A) 1HAEo- cells were preloaded with 50μM of FeSO4 or 50μM of FeCl3 for 2 h and then exposed to 500μM NiSO4 for an additional 4 h. Nuclear protein extracts (15 μg) were prepared for immunoblotting as described in Materials and Methods and probed with antibodies against HIF-1α. The same membrane was probed with antibodies against β-actin to provide a loading control. (B) Levels of intracellular iron (black bars) and nickel (open bars) following the exposure described in (A).
Modeling of Fe(II)/Ni(II) replacement in prolyl hydroxylases.
The possible catalytic role of metals other than Fe(II) in the active center of the enzyme was tested before using density functional theory calculations (Topol et al., 2006). We modeled the reaction route from the reaction components to the high-spin metal-oxide intermediate in the activation of the oxygen molecule by 2-oxoglutarate (2OG)–dependent enzymes for three metal ions—Fe(II), Ni(II), and Co(II)—in the active site (Topol et al., 2006). The results of these calculations allowed us to postulate that, if the Fe(II) in the active site could be substituted by Co(II) or Ni(II), the enzyme activity would be considerably diminished due to the presence of high activation barriers. Using the previously built computer model, we used a combination of MM, dynamics, and quantum chemistry to address the possibility of exchanging Fe(II) for Ni(II) in the hydroxylases. Our initial model was mostly based on two X-ray structures of the asparagine hydroxylase (FIH) that processes Asn803 in HIF-1α (monomer: PDBID 1H2N 2.80 Å resolution; dimer: PDBID 1IZ3 2.84 Å resolution) that were available at the time. The models were built by first relaxing the structures to a local minimum close to the observed structure and subsequently annealing this structure over several cycles of MD calculations (Supplemental Data I). MD calculations reveal a very large FIH binding pocket flexibility, with an RMSD of 6 Å for a trajectory of 500 ps and an average RMSD for the entire structure of 4 Å. This flexibility may explain the large amount of disorder observed in the experimental structure around this region. Further examination of the trajectory revealed that the reaction center remained largely unchanged along the trajectory, with an average RMSD of only 1.2 Å when compared with the experimental structure. Furthermore, there was no evidence of loosening of the coordination of ion at any point along the trajectory. The recent release of a PHD2 crystal structure (PDB ID codes 2G19 and 2G1M) (McDonough et al., 2006) allowed for a comparison of our model with the PHD2 structure reaction center, which agreed with that structure within 1.0 Å RMSD. A comparison of the PHD2 and FIH active centers reveals that their iron-binding sites use similar ligands, two His and a carboxyl group Asp, and are entirely equivalent to those present in other canonical hydroxylases (Hegg and Que, 1997; McDonough et al., 2006). We further explored the properties of the iron-binding center of the active site using activated MD trajectories pulling the ion away from the reaction center. The only successful attempt resulted in an apparent barrier of 37 kcal/mol for the removal of the Fe(II) from the reaction center. When an ion replacement was attempted by pushing Ni(II) into the reaction center while pulling the Fe(II) away from it (steered dynamics), the result was an even larger (52 kcal/mol barrier). Similar experiments with Fe(III) resulted in barriers of 55 and 73 kcal/mol, respectively. Accurate estimates of the reaction center differential binding affinity for several ions, including Co(II) and Ni(II), using quantum chemistry methods and appropriately corrected by the ion solvation energy, show a very similar preference of the enzyme for all of them (all binding energies are within 4 kcal/mol). Thus, the results of our computational modeling experiments suggest that the direct exchange of ions, for example, Fe(II)/Ni(II), in hydroxylases is a highly unlikely event in the cell due to high activation barriers.
Modeling of AA interaction with the hydroxylase.
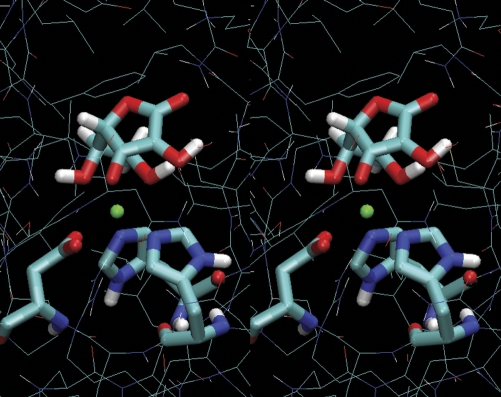
It has been suggested that maintaining reduced iron in the enzyme active center by AA keeps the enzyme active. AA is not stoichiometrically consumed in the reactions catalyzed by the hydroxylases (Nietfeld and Kemp, 1981), but its absence results in the inactivation of the enzymes (de Jong et al., 1982). Current literature shows a scarcity of information on the detailed nature of AA-iron in-solvent interactions. Additionally, the frail nature of AA has resulted in a paucity of structural data on the AA interaction with the hydroxylase reaction center in AA-hydroxylase crystal structures. Thus, we resorted to molecular modeling as a suitable procedure to understand AA-hydroxylase interactions. To study the nature of the AA-enzyme interaction, we took advantage of the abundance of hydroxylase structures in the PDB, all of which are known to use AA as the reducing agent in vivo (de Jong et al., 1982). We used these structures (Supplemental Data, Table S1) in combination with FRMD (Cachau et al., 1994) to model a common form of binding that satisfies all structures simultaneously. FRMD affords an entirely automated procedure for the fitting of restraints and can simultaneously deal with multiple sequences (Cachau et al., 1994). The FRMD search for the AA bound conformer resulted in a tridentate form of AA-iron interaction in the active center (as one of the main contributors to the AA-reaction center binding) (Fig. 7). Furthermore, our molecular modeling studies showed that the relative preference of the enzyme for the tridentate vs. the bidentate forms was enhanced when Fe(III), rather than Fe(II), was considered. The prevalence of a conformer was computed during FRMD trajectories by counting all instances when a bidentate form was encountered (Nb) and all instances when a tridentate form was encountered (Nt) and then computing the ratio (R = Nt/Nb). The relative value of R when Fe(III) or Fe(II) is considered (Rrel = RFe(III)/RFe(II)) reveals the higher prevalence of the tridentate form when AA is transiently bound to the Fe(III) enzyme (Rrel = 23). This result further suggests that the tridentate-bound form may be a critical step in the enzyme recovery mechanism, because following reduction of Fe(III) to Fe(II) by AA, the decreased bidentate semi-DHA binding will initiate the process of semi-DHA release. This will open the reaction center to other ligands, such as 2OG. Regretfully, the delicate nature of the AA-Fe(III) or semi-DHA-Fe(III) complexes makes it difficult to track these events by experimental means. We are currently exploring hybrid quantum mechanical/molecular tools to explore the behavior of the enzyme reaction center during the reduction step.
FIG. 7.
Stereo view of tridentate interaction of AA with iron in active center of hydroxylases. The structure represents predominant form of interaction between AA and the iron in the hydroxylase active center obtained in molecular modeling experiments. The iron cation is indicated in green. It is coordinated with oxygen at third, fifth and sixth carbons. The enzyme iron coordinating triad is indicated in liquorice style.
DISCUSSION
The induction of hypoxia-like conditions in cells exposed to transition metals has been initially described by Bunn and coworkers (Goldberg et al., 1988). They showed an increase in erythropoietin gene expression following exposure to Co(II), Ni(II), and Mn(II). The investigation of the mechanisms of regulation of erythropoietin gene expression resulted in discovery of the HIF-1 transcription factor (Wang and Semenza, 1995). The HIF-1α subunit, which is constantly produced, is rapidly degraded under normoxic conditions, via the von Hippel-Lindau tumor suppressor gene product (pVHL)–mediated ubiquitin-proteasome pathway (Ivan et al., 2001; Jaakkola et al., 2001). In spite of the significant progress in understanding HIF-1α regulation under hypoxic and normoxic conditions, the mechanism of HIF-1α stabilization by metals under ambient oxygen conditions is still not well understood. Because HIF-1α stabilization depends on the inhibition of the hydroxylases’ activity, two main hypotheses have been proposed to explain the stabilization of HIF-1α protein by metals: the “replacement hypothesis” and the “iron oxidation hypothesis.” In both cases, the hydroxylase activity could be inhibited. The “replacement hypothesis” postulates the substitution of the iron in the enzyme by divalent metal ions, such as Co(II), Ni(II), Cu(II), Mn(II), or Zn(II) (Davidson et al., 2006; Schofield and Ratcliffe, 2004). It also proposes that the ligands in the hydroxylase active center, that is, the two His and a the Asp carboxyl group, are weak iron binders (Schofield and Ratcliffe, 2004). This assumption is based on spectroscopic and structural analyses of isolated hydroxylases. If the iron-binding motif in the enzyme is weak in holding iron, then an excessive amount of intracellular iron should out-compete any other ion and thus prevent HIF-1α protein stabilization. However, the experimental data, including those presented in this paper, suggest that this is not the case (Martin et al., 2005; Salnikow et al., 2004).
Using computer modeling, our work shows that the energy required for the removal of iron from the enzyme or replacement by Ni(II) is too high to be achieved in a living cell. These calculations show that removal of metal ions from the enzymes could only take place following protein denaturation and renaturation, which is unlikely to occur in a living cell. Additionally, in this and other papers, it has been shown that HIF-1α can be stabilized by metal anions, such as those of As(III), Cr(VI), and V(V), that cannot substitute for iron in the hydroxylases (Kaczmarek et al., 2007; Li et al., 2006). These data impose significant limitations on the “replacement hypothesis” leaving the “iron oxidation hypothesis” as a reasonable alternative.
HIF protein hydroxylation is carried out by nonheme, iron-containing dioxygenases (Ryle and Hausinger, 2002). Among them are the FIH-1 asparaginyl hydroxylase, and the PHD1, PHD2, and PHD3 prolyl hydroxylases. They utilize molecular oxygen and 2OG as substrates. In the course of the hydroxylation reaction, the enzyme splits dioxygen into two atoms, one of which is converted into a hydroxyl group and the other is involved in the decarboxylation of 2OG. The reaction itself does not requires AA, but AA is essential for maintaining iron as Fe(II) (de Jong et al., 1982).
In this paper, we compare the effect of metals such as As(III), Co(II), Mn(II), Ni(II), V(V), and Cu(II) on AA oxidation in a cell-free system. These results show that, in the presence of ambient oxygen, Co(II), Cu(II), Mn(II), and Ni(II) strongly promote AA oxidation, with V(V) being less efficient, and As(III) and As(V) having no detectable effect. These data are in line with previously published observation of accelerated degradation of AA in solution in the presence of certain metals (Satoh and Sakagami, 1997).
The effect of metals on AA in cells is distinct from that observed in cell-free system. First, cellular AA is better protected against oxidation and can be maintained in a reduced state through constant recycling using cellular reducing potential (Linster and Van Schaftingen, 2007). Moreover, slow intracellular delivery and binding of metals to the storage proteins should attenuate cellular AA oxidation. Also, certain natural ligands can modulate (either increase or decrease) direct reactivity of a metal with AA and/or change the metal's redox potential and thus affect its capacity to generate ROS. The latter will eventually oxidize other molecules, AA in particular. All these factors plus the time dependence of induction of certain metal-binding ligands (e.g., metallothionein), or destruction of others, make it difficult to understand the temporal and mechanistic aspects of the observed AA oxidation in cells treated with metals. In our experiments, HIF-1α protein was stabilized by all tested metal ions including As(III) or V(V), although As(III) or V(V) stabilized HIF-1α protein with a delay. These data are consistent with previously reported upregulation of HIF-1α protein by both As(III) or V(V) ions in vitro and in vivo (Duyndam et al., 2003; Gao et al., 2002; Hwang et al., 2004; Kamat et al., 2005). The stabilization of HIF-1α in cells by As(III) or V(V) suggests that they may work in cells by initially producing ROS, which then oxidize AA. Thus, ROS could be produced by V(V) reacting with metabolic hydrogen peroxide or through activation of NADH oxidase (Carmichael, 1990; Shi and Dalal, 1992). Similarly, As(III) can stimulate intracellular ROS production through activation of NADH oxidase (Chou et al., 2004).
Changes in cellular homeostasis caused by direct interactions of metals with biomolecules and oxygen, including ROS generation, could also result in the modulation of proteins involved in cellular signaling. Reversible oxidation of critical cysteines in numerous protein kinases and phosphatases could serve as switches for cellular signaling (Harris and Shi, 2003). Because HIF-1α is known to be also regulated by protein modifications other than hydroxylation, this introduces additional complexity in the response to metal exposure. For example, disappearance of HIF-1α after prolonged exposure with Cu(II) or Zn(II) (data not shown) may be explained by the inactivation of PI3 kinase or AKT signaling pathways leading to HIF-1α stabilization (Jiang et al., 2001; Zundel et al., 2000). The critical role of AA in maintaining enzymatic activity of prolyl hydroxylases, on one hand, and the high sensitivity of AA to metal-mediated oxidation, on the other, may greatly obscure but do not eliminate possible roles of signaling pathways in the HIF regulation by metals. Undoubtedly, more studies are needed to better understand this process.
AA is extremely sensitive to the metal ions assisted oxidation. Complexes of Ni(II), Co(II), and other metals with AA are known, but none has been described with DHA. Thus, after AA is oxidized, the bound metal is released and ready to interact with another AA molecule. Therefore, even a trace of strong catalytic metal contamination in AA solutions may facilitate the rapid oxidation of large amounts of AA by ambient oxygen. DHA, if not reduced, undergoes relatively fast, spontaneous hydrolysis to DKG. In this paper we show that Ni(II) ions can promote DKG degradation.
To further understand the role and specificity of AA in hydroxylase activity, we performed molecular modeling of the iron-AA interaction at the active center of the enzyme. The ability of AA to produce a tridentate interaction narrows down the list of potential reducing molecules and provides a model for inactivated enzyme recovery. The tridentate coordination have been previously found in AA-Ca(II) complexes (Hearn and Bugg, 1974). Our data suggest that in order to reduce iron in the hydroxylases antioxidants should both fit to the active center of the enzyme and possess tridentate coordination geometry. These requirements exclude NADH, NADPH, lipoic acid, and glutathione from the list of hydroxylase reducing molecules. Additionally, AA's preference to interact with Fe(III), but not Fe(II), explains AA's effectiveness in hydroxylase recovery. The spontaneous oxidation of iron in the hydroxylase to an inactive, Fe(III)-form requires AA for the rapid recovery of the enzyme to the active Fe(II)-form. AA binding to the iron reaction center is necessary for the single electron exchange reaction that reduces Fe(III) to Fe(II). The product of this exchange is semi-DHA, which can be reduced to AA through a series of rapid enzymatic processes (Linster and Van Schaftingen, 2007). The ability of AA to create the tridentate coordination may help to explain the unique specificity of this molecule, which cannot be replaced by other abundant reducing agents present in the cell, including glutathione (manuscript in preparation).
In conclusion, the data presented here support the hypothesis of iron oxidation in the hydroxylases, rather than its substitution with other ions in metal-exposed tissues. The presented model may be useful in drug-design efforts to modulate the activity of hydroxylases.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/
FUNDING
Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research; and National Cancer Institute, National Institutes of Health, under contract no. (N01-CO-12400).
Acknowledgments
We thank R. Bare for excellent technical assistance. Critical comments from Dr J. Phang were greatly appreciated. We thank the staff and administration of the Advanced Biomedical Computing Center for their support. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- Brünger A. X-PLOR Version 3.1 Manual. New Haven, CT: Yale University Press; 1993. [Google Scholar]
- Cachau RE, Erickson JW, Villar HO. Novel procedure for structure refinement in homology modeling and its application to the human class mu-glutathione S-transferases. Protein Eng. 1994;7:831–839. doi: 10.1093/protein/7.7.831. [DOI] [PubMed] [Google Scholar]
- Carmichael AJ. Reaction of vanadyl with hydrogen peroxide. An ESR and spin trapping study. Free Radic. Res. Commun. 1990;10:37–45. doi: 10.3109/10715769009145931. [DOI] [PubMed] [Google Scholar]
- Chou WC, Jie C, Kenedy AA, Jones RJ, Trush MA, Dang CV. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4578–4583. doi: 10.1073/pnas.0306687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson T, Chen H, Garrick MD, D'Angelo G, Costa M. Soluble nickel interferes with cellular iron homeostasis. Mol. Cell. Biochem. 2005;279:157–162. doi: 10.1007/s11010-005-8288-y. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Chen H, Di Toro DM, D'Angelo G, Costa M. Soluble nickel inhibits HIF-prolyl-hydroxylases creating persistent hypoxic signaling in A549 cells. Mol. Carcinog. 2006;45:479–489. doi: 10.1002/mc.20176. [DOI] [PubMed] [Google Scholar]
- de Jong L, Albracht SP, Kemp A. Prolyl 4-hydroxylase activity in relation to the oxidation state of enzyme-bound iron. The role of ascorbate in peptidyl proline hydroxylation. Biochim. Biophys. Acta. 1982;704:326–332. doi: 10.1016/0167-4838(82)90162-5. [DOI] [PubMed] [Google Scholar]
- Dreher KL, Jaskot RH, Lehmann JR, Richards JH, McGee JK, Ghio AJ, Costa DL. Soluble transition metals mediate residual oil fly ash induced acute lung injury. J. Toxicol. Environ. Health. 1997;50:285–305. [PubMed] [Google Scholar]
- Duyndam MC, Hulscher ST, van der Wall E, Pinedo HM, Boven E. Evidence for a role of p38 kinase in hypoxia-inducible factor 1-independent induction of vascular endothelial growth factor expression by sodium arsenite. J. Biol. Chem. 2003;278:6885–6895. doi: 10.1074/jbc.M206320200. [DOI] [PubMed] [Google Scholar]
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery, JA, Vreven T, Kudin KN, Burant JC, et al. Revision B.04. Pittsburgh, PA: Gaussian, Inc; 2003. Gaussian 03. [Google Scholar]
- Gao N, Ding M, Zheng JZ, Zhang Z, Leonard SS, Liu KJ, Shi X, Jiang BH. Vanadate-induced expression of hypoxia-inducible factor 1 alpha and vascular endothelial growth factor through phosphatidylinositol 3-kinase/Akt pathway and reactive oxygen species. J. Biol. Chem. 2002;277:31963–31971. doi: 10.1074/jbc.M200082200. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Silbajoris R, Carson JL, Samet JM. Biologic effects of oil fly ash. Environ. Health Perspect. 2002;110(Suppl. 1):89–94. doi: 10.1289/ehp.02110s1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MA, Dunning SP, Bunn HF. Regulation of the erythropoietin gene: Evidence that the oxygen sensor is a heme protein. Science. 1988;242:1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- Gruenert DC, Finkbeiner WE, Widdicombe JH. Culture and transformation of human airway epithelial cells. Am. J. Physiol. 1995;268:L347–L360. doi: 10.1152/ajplung.1995.268.3.L347. [DOI] [PubMed] [Google Scholar]
- Harris GK, Shi X. Signaling by carcinogenic metals and metal-induced reactive oxygen species. Mutat. Res. 2003;533:183–200. doi: 10.1016/j.mrfmmm.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Hearn RA, Bugg CE. Calcium-binding to carbohydrates—Crystal-structure of calcium ascorbate dihydrate. Acta Crystallogr. B Struct. Sci. 1974;B30:2705–2711. [Google Scholar]
- Hegg EL, Que L., Jr The 2-His-1-carboxylate facial triad—An emerging structural motif in mononuclear non-heme iron(II) enzymes. Eur. J. Biochem. 1997;250:625–629. doi: 10.1111/j.1432-1033.1997.t01-1-00625.x. [DOI] [PubMed] [Google Scholar]
- Hehre WJ, Ditchfield R, Pople JA. Self-consistent molecular orbital methods XII: Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972;56:2257. [Google Scholar]
- Hwang JT, Lee M, Jung SN, Lee HJ, Kang I, Kim SS, Ha J. AMP-activated protein kinase activity is required for vanadate-induced hypoxia-inducible factor 1alpha expression in DU145 cells. Carcinogenesis. 2004;25:2497–2507. doi: 10.1093/carcin/bgh253. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 2001;12:363–369. [PubMed] [Google Scholar]
- Kaczmarek M, Timofeeva OA, Karaczyn A, Malyguine A, Kasprzak KS, Salnikow K. The role of ascorbate in the modulation of HIF-1alpha protein and HIF-dependent transcription by chromium(VI) and nickel(II) Free Radic. Biol. Med. 2007;42:1246–1257. doi: 10.1016/j.freeradbiomed.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat CD, Green DE, Curilla S, Warnke L, Hamilton JW, Sturup S, Clark C, Ihnat MA. Role of HIF signaling on tumorigenesis in response to chronic low-dose arsenic administration. Toxicol. Sci. 2005;86:248–257. doi: 10.1093/toxsci/kfi190. [DOI] [PubMed] [Google Scholar]
- Kang GS, Li Q, Chen H, Costa M. Effect of metal ions on HIF-1alpha and Fe homeostasis in human A549 cells. Mutat. Res. 2006;610:48–55. doi: 10.1016/j.mrgentox.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Karaczyn A, Ivanov S, Reynolds M, Zhitkovich A, Kasprzak KS, Salnikow K. Ascorbate depletion mediates up-regulation of hypoxia-associated proteins by cell density and nickel. J. Cell. Biochem. 2006;97:1025–1035. doi: 10.1002/jcb.20705. [DOI] [PubMed] [Google Scholar]
- Kim KS, Rajagopal V, Gonsalves C, Johnson C, Kalra VK. A novel role of hypoxia-inducible factor in cobalt chloride- and hypoxia-mediated expression of IL-8 chemokine in human endothelial cells. J. Immunol. 2006;177:7211–7224. doi: 10.4049/jimmunol.177.10.7211. [DOI] [PubMed] [Google Scholar]
- Knowles HJ, Raval RR, Harris AL, Ratcliffe PJ. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003;63:1764–1768. [PubMed] [Google Scholar]
- Krick S, Eul BG, Hanze J, Savai R, Grimminger F, Seeger W, Rose F. Role of hypoxia-inducible factor-1alpha in hypoxia-induced apoptosis of primary alveolar epithelial type II cells. Am. J. Respir. Cell Mol. Biol. 2005;32:395–403. doi: 10.1165/rcmb.2004-0314OC. [DOI] [PubMed] [Google Scholar]
- Li Q, Chen H, Huang X, Costa M. Effects of 12 metal ions on iron regulatory protein 1 (IRP-1) and hypoxia-inducible factor-1 alpha (HIF-1[alpha]) and HIF-regulated genes. Toxicol. Appl. Pharmacol. 2006;213:245–255. doi: 10.1016/j.taap.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS. J. 2007;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- Martin F, Linden T, Katschinski DM, Oehme F, Flamme I, Mukhopadhyay CK, Eckhardt K, Troger J, Barth S, Camenisch G, et al. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: Implications for ceruloplasmin regulation. Blood. 2005;105:4613–4619. doi: 10.1182/blood-2004-10-3980. [DOI] [PubMed] [Google Scholar]
- Maxwell P, Salnikow K. HIF-1: An oxygen and metal responsive transcription factor. Cancer Biol. Ther. 2004;3:29–35. doi: 10.4161/cbt.3.1.547. [DOI] [PubMed] [Google Scholar]
- McDonough MA, Li V, Flashman E, Chowdhury R, Mohr C, Lienard BM, Zondlo J, Oldham NJ, Clifton IJ, Lewis J, et al. Cellular oxygen sensing: Crystal structure of hypoxia-inducible factor prolyl hydroxylase (PHD2) Proc. Natl. Acad. Sci. U. S. A. 2006;103:9814–9819. doi: 10.1073/pnas.0601283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly JD, Jimenez LA, Clay MF, MacNee W, Howe A, Heal MR, Beverland IJ, Donaldson K. Soluble transition metals in welding fumes cause inflammation via activation of NF-kappaB and AP-1. Toxicol. Lett. 2005;158:152–157. doi: 10.1016/j.toxlet.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Nietfeld JJ, Kemp A. The function of ascorbate with respect to prolyl 4-hydroxylase activity. Biochim. Biophys. Acta. 1981;657:159–167. doi: 10.1016/0005-2744(81)90139-x. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Kurata T. Interconversion between dehydro-L-ascorbic acid and L-ascorbic acid. Biosci. Biotechnol. Biochem. 2000;64:476–483. doi: 10.1271/bbb.64.476. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Toyoshima Y, Kurata T. Identification of 3,4-dihydroxy-2-oxo-butanal (L-threosone) as an intermediate compound in oxidative degradation of dehydro-L-ascorbic acid and 2,3-diketo-L-gulonic acid in a deuterium oxide phosphate buffer. Biosci. Biotechnol. Biochem. 2001;65:1707–1712. doi: 10.1271/bbb.65.1707. [DOI] [PubMed] [Google Scholar]
- O'Hara KA, Vaghjiani RJ, Nemec AA, Klei LR, Barchowsky A. Cr(VI)-stimulated STAT3 tyrosine phosphorylation and nuclear translocation in human airway epithelial cells requires Lck. Biochem. J. 2007;402:261–269. doi: 10.1042/BJ20061427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CL, Ventura ON, Burt SK, Cachau RE. DYNGA: A general purpose QM-MM-MD program I: Application to water. Mol. Phys. 2003;101:2659–2668. [Google Scholar]
- Ryle MJ, Hausinger RP. Non-heme iron oxygenases. Curr. Opin. Chem. Biol. 2002;6:193–201. doi: 10.1016/s1367-5931(02)00302-2. [DOI] [PubMed] [Google Scholar]
- Salnikow K, An WG, Melillo G, Blagosklonny MV, Costa M. Nickel-induced transformation shifts the balance between HIF-1 and p53 transcription factors. Carcinogenesis. 1999;20:1819–1823. doi: 10.1093/carcin/20.9.1819. [DOI] [PubMed] [Google Scholar]
- Salnikow K, Donald SP, Bruick RK, Zhitkovich A, Phang JM, Kasprzak KS. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J. Biol. Chem. 2004;279:40337–40344. doi: 10.1074/jbc.M403057200. [DOI] [PubMed] [Google Scholar]
- Satoh K, Sakagami H. Effect of metal ions on radical intensity and cytotoxic activity of ascorbate. Anticancer Res. 1997;17:1125–1129. [PubMed] [Google Scholar]
- Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- Shi X, Dalal NS. Hydroxyl radical generation in the NADH/microsomal reduction of vanadate. Free Radic. Res. Commun. 1992;17:369–376. doi: 10.3109/10715769209083141. [DOI] [PubMed] [Google Scholar]
- Topol IA, Nemukhin AV, Salnikow K, Cachau RE, Abashkin YG, Kasprzak KS, Burt SK. Quantum chemical modeling of reaction mechanism for 2-oxoglutarate dependent enzymes: Effect of substitution of iron by nickel and cobalt. J. Phys. Chem. A Mol. Spectrosc. Kinet. Environ. Gen. Theory. 2006;110:4223–4228. doi: 10.1021/jp055633k. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. National Ambient Air Quality Standards for particulate matter: Proposed rule. U.S. Environmental Protection Agency. Fed. Reg. 1996;61:65638–65713. [Google Scholar]
- Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–396. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.