Abstract
Neuroprotein changes in the spinal cord of rodents with aliphatic γ-diketone axonopathy induced by 2,5-hexanedione (2,5-HD) are compared with those reported previously in aromatic γ-diketone–like axonopathy induced by 1,2-diacetylbenzene (1,2-DAB). Sprague-Dawley rats were treated intraperitoneally with 500 mg/kg/day 2,5-HD, equimolar doses of 2,3-hexanedione (negative control), or an equivalent amount of saline containing 50% dimethyl sulfoxide (vehicle), 5 days a week, for 3 weeks. Analysis of the lumbosacral proteome by 2-dimensional differential in-gel electrophoresis and matrix-assisted laser desorption ionization time-of-flight/tandem mass spectrometry revealed 34 proteins markedly modified by 2,5-HD of which neurofilament triplet L, gelsolin, protein disulfide isomerase, glutathione S-transferase, nicotinamide adenine dinucleotide (reduced) dehydrogenase 1α, pyruvate kinase, and fatty acid synthase were also modified by 1,2-DAB. The expression of proteins involved in maintaining the physical integrity of the cytoskeleton or controlling the redox and protein-folding mechanisms was reduced, whereas that of proteins supporting energy metabolism was mainly increased. The similarity of the neuroproteomic patterns of 2,5-HD and 1,2-DAB axonopathy suggests common biomarkers and/or mechanisms of neurotoxicity associated with exposure to their parent chemicals, namely the industrial solvents n-hexane and 1,2-diethylbenzene, respectively.
Keywords: axonopathy, biomarkers, γ-diketones, proteomics, solvent neurotoxicity, neurodegeneration
Hydrocarbon solvents are of significant interest in occupational and environmental health because they are widely used in industries and may be found as pollutants at hazardous waste sites. The aliphatic solvent n-hexane has been associated with outbreaks of neuropathy among workers in laminating industries, shoe manufacturing, spray painting, and furniture finishing, and among those who deliberately inhale solvent vapors for their euphoric or narcotizing properties (Spencer et al., 1980). In the early 1990′s, the aromatic solvent 1,2-diethylbenzene (1,2-DEB) was reported to induce peripheral neuropathy in rodents (Gagnaire et al., 1990). DEB isomers are byproducts of styrene monomer production and trace components of jet fuel (Spencer et al., 2002). Mechanisms by which these aromatic (1,2-DEB) and aliphatic (n-hexane) solvents induce neuropathies are unclear. Previous studies have shown that their neurotoxic properties are mediated by their structurally similar (γ-diketone-like) and (neuro)protein-reactive metabolites, namely 2,5-hexanedione (2,5-HD, n-hexane metabolite) and 1,2-diacetylbenzene (1,2-DAB, 1,2-DEB metabolite) (DeCaprio, 2000; Gagnaire et al., 1991).
2,5-HD and 1,2-DAB induce proximal (1,2-DAB) or distal (2,5-HD) axonal swellings filled with 10-nm neurofilaments (NFs) and/or a Wallerian-like degeneration in elongate axons in the peripheral and central nervous system (CNS) (Kim et al., 2001; Spencer and Schaumburg, 1977; Tshala-Katumbay et al., 2005). We recently exploited the protein-reactive and axonopathic properties of 1,2-DAB versus the nonprotein reactive and nonaxonopathic properties of its 1,3-DAB isomer to unveil the in vivo (neuro)protein modifications associated with 1,2-DAB axonopathy (Tshala-Katumbay et al., 2008). In the present study, we used a similar approach to determine whether 2,5-HD axonopathy shares similarities with the CNS neuroproteomic changes associated with 1,2-DAB neurotoxicity. Adult male Sprague-Dawley rats were systemically treated over the course of three weeks with 2,5-HD, its 2,3-HD isomer (negative control), or vehicle (saline containing 50% DMSO). The lumbosacral spinal cord proteome was analyzed by 2-dimensional differential in-gel electrophoresis (2D-DIGE) and matrix-assisted laser desorption ionization time-of-flight/tandem mass spectrometry (MALDI-TOF/MS-MS).
We show that 2,5-HD induces a 1,2-DAB–like proteomic signature by altering the expression levels of proteins involved in maintaining the physical integrity of axons (reduced), in controlling redox and protein-folding mechanisms (reduced), and in supporting energy metabolism (increased). These findings suggest common biomarkers and/or mechanisms underlying the pathogenetic mechanisms of 2,5-HD- and 1,2-DAB axonopathy, the respective active metabolites of n-hexane and 1,2-DEB.
METHODS
Chemicals.
2,5-HD (99%) and its isomer 2,3-DAB (96.5%) were purchased from Aldrich Chemical Co. (Madison, WI) and stored at room temperature. Stock samples were checked by gas chromatography-mass spectrometry to confirm the purity of the chemicals. 2,5-HD was found free of 2,3-HD and vice versa.
Animals.
Experiments were conducted with male Sprague-Dawley rats (Charles Rivers, CA) weighing 175–225 g upon arrival. Animals were individually housed and given rodent chow (PMI Nutrition International, NJ) and water ad libitum. They were acclimated for 5 days in a room maintained at 20°C on a 12-h/12-h light-dark cycle prior to commencement of the experimental treatment.
Treatment of animals.
Our previous study indicated that 2,5-HD has a lower neurotoxic potency than 1,2-DAB, such that a dose of ∼3 orders of magnitude that of 1,2-DAB was required to induce comparable neuromuscular weakness in rodents (unpublished data). Animals treated with 20 mg 1,2-DAB/kg/day, 5 days a week, for 3 weeks, exhibited severe neuromuscular weakness and axonal pathology (Tshala-Katumbay et al., 2008). In the present study, rats were treated with 500 mg 2,5-HD/kg/day in a similar dosing schedule. Control animals were treated with an equimolar dose of 2,3-HD or equivalent amount of vehicle (1μl/g body weight), which consisted of saline containing 50% DMSO. Five animals were used for each of the three treatment conditions. Test articles were administered intraperitoneally with a 1-ml disposable plastic syringe equipped with a 27-gauge needle. Injection sites were rotated around the abdomen and care was taken to minimize leakage and discomfort. Animals were observed daily for changes in behavior, physical appearance and locomotion in an open field before and after treatment. They were also weighed before treatment to track changes in body weight and adjust the daily dose of the test article accordingly.
At study termination, three rats were anesthetized with 4% isofluorane (0.7 l oxygen/min), their blood collected via cardiac puncture, and decapitated prior to the removal of the spinal cord as previously described (Tshala-Katumbay et al., 2008). The lumbosacral spinal cord was flash frozen in liquid nitrogen and stored at −80°C prior to proteomic studies. The remaining animals were anesthetized and terminated by intracardiac perfusion with cold 4% paraformaldehyde followed by 5% glutaraldehyde, each in 0.2M sodium cacodylate buffer (pH 7.4). The lumbar spinal cord and sciatic nerve were sampled and post-fixed in excess cacodylate-buffered 1% osmium tetroxide, dehydrated, and embedded in epoxy resin. Cross-sections (∼900 nm) were stained with 1% toluidine blue and screened by bright-field microscopy. Thin sections (∼90 nm) of regions of interest were stained with 2% uranyl acetate followed by 1% lead citrate for examination by transmission electron microscopy.
Proteomic studies.
Flash-frozen rat lumbosacral spinal cords were shipped on dry ice to Applied Biomics (Hayward, CA) for proteomic studies. Total protein was extracted, labeled with Cy2, or Cy3, or Cy5 dyes (GE Healthcare, Piscataway, NJ), and subjected to isoelectric focusing (pH 3–10) and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Gel scanning was carried out immediately after SDS-PAGE using Typhoon TRIO (GE Healthcare, Piscataway, NJ). Scanned images were analyzed by Image Quant software (version 5.0, GE Healthcare) and subjected to in-gel analysis and cross-gel analysis using the DeCyder software (version 6.0, GE Healthcare) with a detection limit of 0.2 ng of protein per spot. The ratio change for differentially expressed protein spots was obtained from the in-gel DeCyder analysis. Protein spots of interest that were consistently differentially expressed in 2,5-HD- versus 2,3-HD- and vehicle-treated samples across a minimum of two SDS gels were picked up by Ettan Spot Picker (GE Healthcare) and subjected to in-gel trypsin digestion, peptide extraction, and desalting prior to MALDI-TOF/MS-MS (ABI 4700, Applied Biosystems, CA) as previously described (Tshala-Katumbay et al., 2008). Peptide fingerprints and partial amino acid sequence information were used for protein identification in the nrNCBI (nonredundant National Center for Biotechnology) databases. Searches were performed without constraining protein molecular weight (MW) or isoelectric point (IP), with variable carbamidomethylation of cysteine and oxidation of methionine residues, and with one missed cleavage allowed in the search parameters. Candidates with protein and ion scores greater than 95% were considered significant.
Statistical analysis.
The body weight of each animal was summarized by fitting a linear trend to the series of body weight measurements. The estimated slope from each fits reflects the change in body weight per day. Nonparametric tests (Kruskall-Wallis, Wilcoxon rank-sum) were used to determine whether the slope coefficients differed among the three groups of animals. All computations were performed using R version 2.6.1 (R Development Core Team, 2007).
Changes in the number of differentially expressed protein spots were analyzed using log-linear models with an allowance made for overdispersion. We also determined the Spearman's correlation (Sr) between the fold change in protein expression and selected physicochemical properties of proteins, including the total protein lysine content (LC), cysteine content (CC), the protein IP, and MW. These various physicochemical properties protein were computed using the ProtParam tool available on the ExPASy server (http://www.expasy.ch/tools/protparam.html).
RESULTS
Physical and Neuropathological Changes
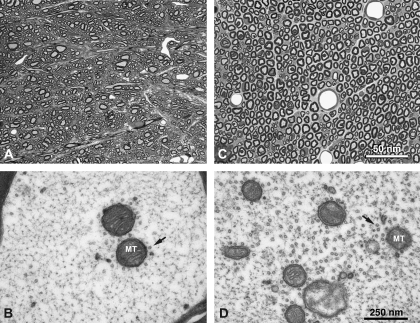
Neuromuscular signs were evident almost a week after beginning of treatment with 2,5-HD. By the end of study-week 2, animals treated with 2,5-HD, but not with 2,3-HD or vehicle, developed advanced neuromuscular weakness, postural and gait abnormalities. Daily changes in body weight significantly differed among the three groups of rats (X2 (2df) = 11.18, p = 0.004; Kruskal-Wallis test). Vehicle-treated animals typically gained an additional 6 g of weight per day compared with 2,3-HD-treated animals (95% CI: 0.1 g less up to 8.8 g more per day), though this was only marginally significant (W = 22, p = 0.055) given the small sample size. Animals given 2,5-HD lost an additional 10.6 g of body weight per day (95% CI: 4.4 to 15 g additional weight lost per day) compared with vehicle-treated animals (W = 25, p = 0.008) and lost an additional 4.5 g of body weight per day (95% CI: 1.7 to 10.9 g additional weight lost per day) compared with 2,3-HD–treated animals (W = 0, p = 0.008). At study termination, only animals treated with 2,5-HD showed nerve fiber (axon) damage in lumbar spinal cord and sciatic nerve (Fig. 1).
FIG. 1.
Cross-sections of lumbar spinal cord (A, light micrograph and B, corresponding electron micrograph) and sciatic nerve (C, light micrograph and D, corresponding electron micrograph) of animals treated with 2,5-HD. Despite nonvisible changes at light microscopy (A and C), electron microscopy revealed cytoskeletal changes in the form of clamping of NFs, clustering of microtubules (arrows) and mitochondria (MT), a key pathological feature of γ-diketone axonopathy. Tissues from animals treated with 2,3-HD or vehicle remain unremarkable (not shown).
Proteomic Changes
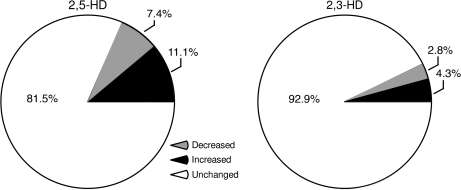
The Decyder analysis of 2D-DIGE protein expression revealed specific protein changes associated with 2,5-HD treatment. Animals treated with axonopathic and protein-reactive 2,5-HD had more changes in protein expression in comparison with those treated with nonaxonopathic 2,3-HD or vehicle. For example, relative to 2,3-HD, the effect of 2,5-HD was to significantly increase the number of detectable protein spots for both “increased” (t(12) = 4.5, p < 0.001) and “decreased” (t(12) = 3.71, p < 0.001) protein expression. This effect was similar for “increased” and “decreased” protein expression (t(12) = 0.045, p = 0.96). The number of modified protein spots was 2.9 times higher in 2,5-HD–treated animals compared with 2,3-HD–treated animals (95% CI: 1.5–4.6 times higher) (Table 1 and Fig. 2). The effects due to chemical (2,5 vs. 2,3-HD) and protein modulation (decrease, increase, or unchanged) were consistent across the three gels (F8,4 = 2.71, p = 0.18; test of interactions with gel) indicating reliable experimental conditions and results across the proteomic experiments.
TABLE 1.
Number of Protein Spots Identified by the Decyder Software as being Differentially Expressed in 2,5-HD- or 2,3-HD- versus Vehicle-Treated Samples
| 2,5-HD versus vehicle |
2,3-HD versus vehicle |
|||||
| Protein expression | Gel 1 | Gel 2 | Gel 3 | Gel 1 | Gel 2 | Gel 3 |
| Decreased | 115 | 150 | 97 | 56 | 36 | 47 |
| Increased | 155 | 136 | 253 | 60 | 62 | 90 |
| Unchanged | 1281 | 1328 | 1383 | 1435 | 1516 | 1596 |
FIG. 2.
Average proportion of protein spots differentially expressed in 2,5-HD versus vehicle-treated samples (left) and 2,3-HD versus vehicle-treated samples (right). Relative to 2,3-HD, 2,5-HD significantly increased the number of detectable proteins with either increased or decreased expression.
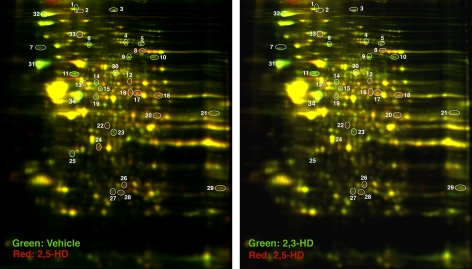
The analysis of superimposed 2D-DIGE gel images conducted independently by two experienced neuroscientists blind to the experimental conditions revealed 34 spots as being markedly modified by treatment with 2,5-HD. MALDI-TOF/MS-MS identification of these spots revealed proteins and/or enzymes that are mainly involved in maintaining the physical integrity of the cytoskeleton and mediating membrane fusion events, in controlling redox and protein-folding mechanisms, and in supporting energy metabolism (spots 1–34 in Fig. 3 and Tables 2 and 3).
FIG. 3.
Representative 2D-DIGE patterns in lumbosacral spinal cords from rats treated with 2,5-HD versus 2,3-HD. The 34 differentially expressed proteins (circles) were subjected to MALDI-TOF/MS-MS for protein identification (Tables 2 and 3).
TABLE 2.
List of Proteins with Low Expression Levels in the Lumbosacral Spinal Cord of Animals Treated with Axonopathic 2,5-HD
| Protein name | Spot number | Acronym | Accession number | MW | IP | Matched peptides | Functional category | Fold changea |
| Spna2 protein | 1 | Spna2 | 47477769 | 282,206.0 | 5.19 | 22 | Protein binding/structural protein | −1.37 |
| Neurofilament triplet L proteinb | 31 | NFL | 13929098 | 61,298.0 | 4.63 | 17 | Protein binding/structural protein | −2.30 |
| Neurofilament triplet M protein | 19 | NFM | 128150 | 95,733.6 | 4.77 | 15 | Protein binding/structural protein | −1.89 |
| Neurofilament triplet H protein | 32 | NFH | 29789026 | 115,279.3 | 5.81 | 12 | Protein binding/structural protein | −1.9 |
| Glial fibrillary acidic protein δ | 34 | GFAPδ | 5030428 | 48,752.2 | 5.72 | 23 | Protein binding/structural protein | −2.95 |
| Gelsolinb | 6 | Gsn | 51854227 | 86,014.1 | 5.76 | 12 | Protein binding/structural protein | −1.98 |
| Internexin α | 11 | Intα | 9506811 | 56,081.6 | 5.2 | 22 | Structural protein | −2.12 |
| PDI related 3b | 14 | PDI | 38382858 | 56,587.7 | 5.88 | 15 | Redox mechanisms/protein folding | −2.48 |
| Pdlim 3 protein | 21 | Pdlim 3 | 51858557 | 34,396.2 | 8.09 | 7 | Protein binding/structural protein | −1.32 |
| Fatty acid synthaseb | 3 | FASN | 57890 | 272,477.9 | 5.96 | 23 | Metabolism of fatty acids | −1.48 |
| Glycogen phosphorylase (EC 2.4.1.1) | 4 | GLPH | 480806 | 96,680.4 | 6.24 | 19 | Metabolism of glycogen | −2.39 |
| Similar to muscle glycogen phosphorylase | 5 | sGLPH | 34861509 | 97,226.7 | 6.65 | 21 | Metabolism of glycogen | −3.39 |
| Similar to α glucosidase II, β subunit | 7 | sαGlucdase | 34860445 | 59,205.1 | 4.39 | 4 | Unknownc | −2.20 |
| N-ethylmaleimide sensitive fusion protein | 9 | NSF | 13489067 | 82,600.0 | 6.55 | 10 | Intracellular membrane fusion protein | −1.91 |
| Hypothetical protein XP_238433d | 10 | HpoP | 27662474 | 9,913.7 | 8.45 | 2 | Unknownc | −2.0 |
| Similar to Sept8 protein | 15 | sSept8 | 34870727 | 52,969.8 | 5.82 | 9 | GTP binding protein | −1.52 |
| NADH dehydrogenase (ubiquinone) 1 αb | 23 | NADH | 46391108 | 31,680.8 | 5.44 | 7 | Energy metabolism | −2.62 |
| N-ethylmaleimide sensitive fusion protein attachment protein α | 25 | NSFα | 18034791 | 33,171.2 | 5.3 | 12 | Intracellular membrane fusion protein | −1.64 |
| Glutathione-S-transferase μ5b | 27 | Gstμ5 | 25282395 | 26,611.1 | 6.33 | 7 | Glutathione conjugation | −1.55 |
| Similar to Zinc finger protein 169d | 29 | sZnfp | 34874068 | 61,085.1 | 9.31 | 6 | Unknown | −1.89 |
| Dihydropyrimidinase-like 2 | 30 | Dhpl2 | 40254595 | 62,238.6 | 5.95 | 19 | Axonal growth and pathfinding | −1.81 |
Maximum fold change values observed after cross-gel analysis of proteomic expression.
Proteins or isoforms similarly modified by 1,2-DAB (Tshala-Katumbay et al., 2008).
Record predicted by automated computational analysis.
Proteins with low confidence scores (< 95%) at mass spectrometry identification.
TABLE 3.
List of Proteins with High Expression Levels in the Lumbosacral Spinal Cord of Animals Treated with Axonopathic 2,5-HD
| Protein name | Spot number | Acronym | Accession number | MW | IP | Peptides matched | Functional category | Fold changea |
| Glial fibrillary acidic protein delta | 2 | GFAPδ | 5030428 | 48,752.2 | 5.72 | 5 | Protein binding/structural protein | + 2.41 |
| Ubiquitin-like modifier activating enzyme 1 | 33 | UbmE1 | 55250575 | 117,713.0 | 5.36 | 18 | Ubiquitin activation | + 1.27 |
| Aconitase 2, mitochondrial | 8 | Aco2 | 40538860 | 85,380.0 | 7.87 | 8 | Energy metabolism | + 1.99 |
| Pyruvate kinase muscleb | 12 | Pkm | 56929 | 57,780.9 | 6.63 | 4 | Energy metabolism | + 3.85 |
| Glutamate dehydrogenase 1 | 16 | GDH | 6980956 | 61,377.3 | 8.05 | 9 | Glutamate metabolism | + 2.02 |
| Glutamate dehydrogenase 1 | 17 | GDH | 6980956 | 61,377.3 | 8.05 | 15 | Glutamate metabolism | + 2.00 |
| Creatine kinase, mitochondrial 1, ubiquitous | 20 | CRK | 60678254 | 46,932.2 | 8.58 | 14 | Energy metabolism | + 1.52 |
| NADH dehydrogenaseb (ubiquinone) 1 alpha | 22 | NADH | 46391108 | 31,680.8 | 5.44 | 7 | Energy metabolism | + 3.53 |
| Malate dehydrogenase 1, NAD (soluble) | 24 | MADH | 37590235 | 36,461.0 | 5.93 | 7 | Energy metabolism | + 1.73 |
| Triose phosphate isomerase 1 protein | 28 | Tpi1 | 38512111 | 26,700.7 | 7.07 | 7 | Energy metabolism | + 1.43 |
| Phosphoglycerate mutase 1 protein | 26 | Pgam1 | 12805529 | 28,813.9 | 6.67 | 10 | Energy metabolism | + 1.67 |
| Similar to blood group A glycosyltransferase 1c | 18 | sGlyct1 | 34853121 | 82,896.2 | 8.35 | 3 | Unknownd | + 1.80 |
| Similar to dihydropyrimidinase related protein-2 | 13 | sDRP-2 | 34874349 | 81,308.3 | 6.34 | 14 | Removed from NCBI database | + 1.62 |
Maximum fold-change values observed after cross-gel analysis of proteomic expression.
Proteins or isoforms similarly modified by 1,2-DAB (Tshala-Katumbay et al., 2008).
Proteins with low confidence scores (< 95%) at mass spectrometry identification.
Record predicted by automated computational analysis.
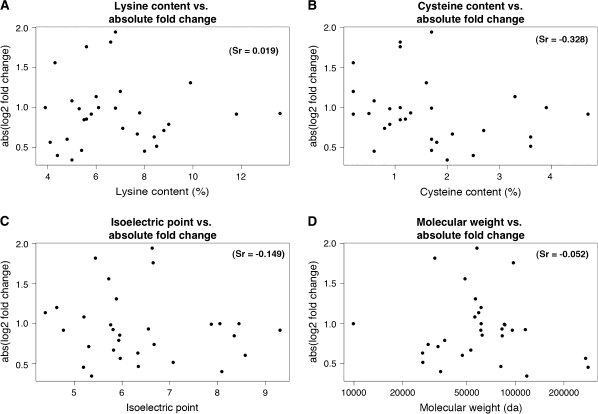
No significant correlation was found between the change in expression levels and the LC (Sr = 0.019, p = 0.92), CC (Sr = −0.328, p = 0.08), the IP (Sr = −0.149, p = 0.43), or the MW (Sr = −0.052, p = 0.78) of the 34 proteins (Fig. 4).
FIG. 4.
Dot plots depicting correlation between protein fold-changes and selected physicochemical properties of individual proteins. No significant correlation was observed between the changes in protein expression and the total LC, CC, IP, or MW.
Most proteins involved in maintaining the axonal shape and physical integrity and those involved in membrane fusion events or in control of redox and protein-folding mechanisms showed lower levels of expression in samples from animals treated with axonopathic 2,5-HD. The expression of proteins that are associated with energy metabolism mainly increased with 2,5-HD treatment (Fig. 5 and Tables 2 and 3).
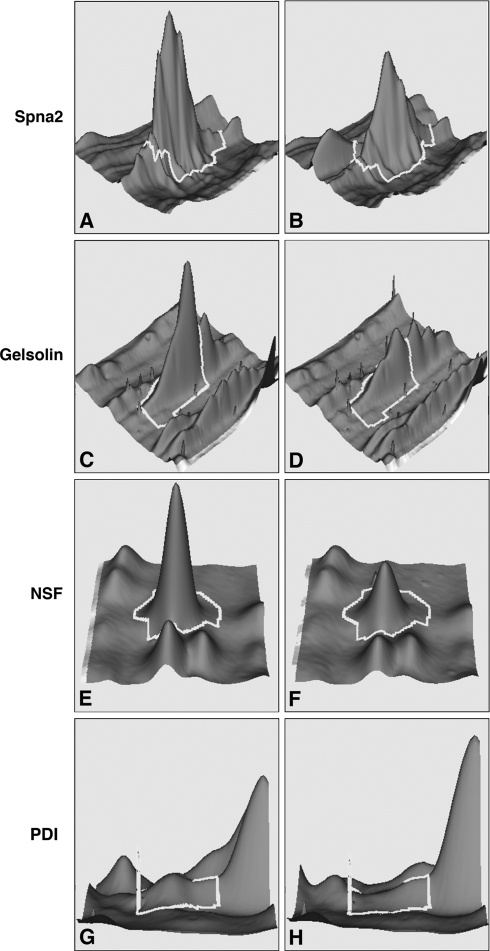
FIG. 5.
Representative DeCyder display of relative abundance of proteins reduced in samples treated with 2,5-HD versus vehicle (fold change in parentheses). Left or right column displays protein abundance in samples treated with vehicle or 2,5-HD, respectively. (A and B) Spna 2 (−1.37); (C and D) gelsolin (−1.98); (E and F) NSF (−1.91 times); and (G and H) PDI (−2.48 times).
Neurofilament triplet L protein (NFL), gelsolin (Gsn), protein disulfide isomerase (PDI), glutathione S-transferase (GST), NADH dehydrogenase 1 α, pyruvate kinase (Pkm), and fatty acid synthase (Fasn) were previously reported by our group as being targeted by 1,2-DAB, the γ-diketone–like aromatic cousin of 2,5-HD. A qualitative difference in the 2,5-HD- versus 1,2-DAB expression of the aforementioned set of proteins was that 1,2-DAB induced changes in the Yb1 subunit of GST, whereas 2,5-HD affected the expression of GST μ5. Western blot analyses were conducted to confirm the change in the expression level PDI, a selected representative of the differentially expressed proteins (data not shown).
Proteins with expression levels that were modified by 2,5-HD but not reported as such in the 1,2-DAB-study included: Spna2, the intermediate filaments GFAPδ, internexin α, NFM, and NFH; and certain enzymes mainly involved in supporting the energy metabolism. Most of these proteins were however suspected as being modified by 1,2-DAB but were not reported because they had a lower confidence score on mass spectrometry identification. In this study, the expression of structural proteins, that is, GFAPδ, internexin α, NF-M, and NF-H were reduced in abundance following treatment with 2,5-HD whereas that of proteins associated with the energy metabolism was increased. In addition, this study has shown an increase in the expression of the ubiquitin-like modifier activating enzyme E1 (UbmE1).
DISCUSSION
We and others have pointed to the axon as the site of genesis of γ-diketone axonopathy (Tshala-Katumbay et al., 2005; Zagoren et al., 1983). Although most studies have focused on changes in individual axonal proteins, this is the first study that provides a global proteomic picture revealing targets common to 2,5-HD and 1,2-DAB, the γ-diketone–like metabolites of the aliphatic solvent n-hexane and the aromatic solvent 1,2-DEB, respectively. The mechanisms by which changes in axonal proteins lead to axonopathy and/or nerve fiber degeneration have yet to be understood. Our global proteomic approach has allowed the identification of several proteins of interest in the pathogenesis of γ-diketone axonopathy. Both 1,2-DAB and 2,5-HD reduce the expression level of proteins involved in maintaining the physical integrity of axons (e.g., Gsn) or assisting redox/folding mechanisms (e.g., PDI). In contrast, most of the proteins involved in supporting the energy metabolism (e.g., Pkm) showed higher levels of proteomic expression, a proteomic pattern seen in animals treated with 1,2-DAB (Tshala-Katumbay et al., 2008).
Changes in the expression of structural/membrane proteins by γ-diketones may reflect changes in protein synthesis and/or degradation after posttranslational modifications possibly by γ-diketo-adduction and crosslinking (polymerization) (Spencer et al., 2002). Protein polymerization and/or breakdown of proteins likely occur as evidenced by the presence of protein spots with similar IP but higher MW and identified as a single protein (e.g., GFAP δ) on the SDS gels or by the migration of certain proteins at gel spots of which IP and or MW values differ from the theoretical ones (e.g., NADH). Reduction of protein synthesis is less probable as we and others have previously suggested (Opanashuk et al., 2001; Tshala-Katumbay et al., 2008). Increased protein degradation likely occurs as indicated by the present findings that show an increased expression of the ubiquitin-activating enzyme UbmE1, an activator of the ubiquitin proteasome system, in samples from animals treated with 2,5-HD (Groettrup et al., 2008).
The increase in the expression levels of enzymes involved in energy metabolism, except for glycogen phosphorylase (GLPH), possibly represents a stress response involving unbalanced protein turnover associated with protein degradation and/or chemical attack by γ-diketones. Whether the change in the expression levels of these enzymes is accompanied by a production of deleterious species such as free radicals is not known and needs to be investigated (Genova et al., 2004). Reduction in the expression levels of GLPH suggests that impaired glucose metabolism may contribute to the pathogenesis of γ-diketone neurotoxicity.
We have sought to understand whether the magnitude of γ-diketone–induced proteomic changes was related to the physicochemical properties of individual proteins. Our findings indicate no correlation between the protein MW, IP, total LC, or CC and the observed modifications in the abundance of individual proteins in samples from animals treated with 2,5-HD. Because ε-amino-groups of lysine and/or thiol (SH) residues of cysteine are preferentially targeted by γ-diketones, we propose that chemical adduction of (neuro)proteins that are critical to the functions of neurons may be dictated by the position of the aforementioned amino residues within (neuro)protein sequences and their active/catalytic sites. This proposal is consistent with findings from studies that show that chemical attack on ϵ-amino- and/or SH-groups located on active/catalytic sites of (neuro)proteins such as glutamate dehydrogenase (GDH) is accompanied by loss of enzymatic function (Ahn et al., 1999). In this respect, analytical mass spectrometry is needed to understand how the putative γ-diketone-induced (neuro)protein-adduction affects ϵ-amino and/or SH-containing active sites of the (neuro)proteins identified in this study and their relationship to nerve fiber (axon) pathology.
The interpretation of the present findings requires caution for two reasons. First, our studies were conducted on a limited number of animals. Second, the observed proteomic modifications represent changes observed in samples that likely contain multiple cellular components (e.g., cell bodies of neurons, glial cells, or endothelial cells). Nevertheless, we have shown that 2,5-HD induces a 1,2-DAB-like pattern of neuroproteomic changes in the lumbar spinal cord of Sprague-Dawley rats. The similarity of the neuroproteomic patterns of 2,5-HD and 1,2-DAB axonopathy suggests common biomarkers and/or mechanisms of neurotoxicity associated with exposure to their respective parent compounds n-hexane and 1,2-DEB. In addition, this study has revealed two other proteins, that is, spna2 and/or NSF that appear to be of interest in the pathogenesis of γ-diketone axonopathy. Neuronal Spna2 participates in the formation of axo-glial junctions that are themselves critically involved in maintaining the regular shape and organization of the cytoskeleton (Garcia-Fresco et al., 2006). Change in the level of NSF is intriguing because this n-ethylmaleimide-sensitive protein has been reported as a target for axonopathic acrylamide, a neurotoxic type-2 alkene (LoPachin et al., 2008).
Analytical studies on the γ-diketone susceptibility of selected proteins that are commonly targeted by 1,2-DAB and 2,5-HD (e.g., Gsn, PDI, Spna2, or certain enzymes involved in energy metabolism such as NADH) and investigations on the time course of proteomic changes as shown in recent studies (Song et al., 2008; Wang et al., 2008) may provide with new insights into mechanisms by which nerve fibers (axons) undergo degeneration.
FUNDING
National Institute of Health (P42ES10338, U19ES11384, K01NS052183); and Oregon Worker Benefit Fund.
Acknowledgments
The technical expertise of Dan Austin (OHSU, Portland, OR) and guidance of Paul Desjardins and Roger Butterworth (Université de Montréal, Canada) are appreciated.
References
- Ahn JY, Choi S, Cho SW. Identification of lysine residue involved in inactivation of brain glutamate dehydrogenase isoproteins by o-phthalaldehyde. Biochimie. 1999;81:1123–1129. doi: 10.1016/s0300-9084(99)00349-1. [DOI] [PubMed] [Google Scholar]
- DeCaprio AP. n-hexane, metabolites and derivatives. In: Spencer PS, Schaumburg HS, editors. Experimental and Clinical Neurotoxicology. 2nd edn. New York: Oxford University Press; 2000. pp. 633–648. [Google Scholar]
- Gagnaire F, Ensminger A, Marignac B, De Ceaurriz J. Possible involvement of 1,2-diacetylbenzene in diethylbenzene-induced neuropathy in rats. J. Appl. Toxicol. 1991;11:261–268. doi: 10.1002/jat.2550110406. [DOI] [PubMed] [Google Scholar]
- Gagnaire F, Marignac B, De Ceaurriz J. Diethylbenzene-induced sensorimotor neuropathy in rats. J. Appl. Toxicol. 1990;10:105–112. doi: 10.1002/jat.2550100208. [DOI] [PubMed] [Google Scholar]
- Garcia-Fresco GP, Sousa AD, Pillai AM, Moy SS, Crawley JN, Tessarollo L, Dupree JL, Bhat MA. Disruption of axo-glial junctions causes cytoskeletal disorganization and degeneration of Purkinje neuron axons. Proc. Natl. Acad. Sci. U. S. A. 2006;103:5137–5142. doi: 10.1073/pnas.0601082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova ML, Pich MM, Bernacchia A, Bianchi C, Biondi A, Bovina C, Falasca AI, Formiggini G, Castelli GP, Lenaz G. The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann. N. Y. Acad. Sci. 2004;1011:86–100. doi: 10.1007/978-3-662-41088-2_10. [DOI] [PubMed] [Google Scholar]
- Groettrup M, Pelzer C, Schmidtke G, Hofmann K. Activating the ubiquitin family: UBA6 challenges the field. Trends Biochem. Sci. 2008;33:230–237. doi: 10.1016/j.tibs.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Kim MS, Sabri MI, Miller VH, Kayton RJ, Dixon DA, Spencer PS. 1,2-diacetylbenzene, the neurotoxic metabolite of a chromogenic aromatic solvent, induces proximal axonopathy. Toxicol. Appl. Pharmacol. 2001;177:121–131. doi: 10.1006/taap.2001.9301. [DOI] [PubMed] [Google Scholar]
- LoPachin RM, Barber DS, Gavin T. Molecular mechanisms of the conjugated α,β-unsaturated carbonyl derivatives: Relevance to neurotoxicity and neurodegenerative diseases. Toxicol. Sci. 2008;104:235–249. doi: 10.1093/toxsci/kfm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opanashuk LA, He DK, Lehning EJ, LoPachin RM. γ-Diketone peripheral neuropathy III. Neurofilament gene expression. Neurotoxicology. 2001;22:215–220. doi: 10.1016/s0161-813x(00)00011-5. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. 2007 Available at: http://www.R-project.org. Accessed July 15, 2008. [Google Scholar]
- Song F, Yu S, Zhang C, Zhou G, Wang Q, Xie K. The reversibility of neurofilament decline induced by 2,5-hexanedione in rate nerve tissues. Biochem. Pharmacol. 2008;75:737–744. doi: 10.1016/j.bcp.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Kim MS, Sabri MI. Aromatic as well as aliphatic hydrocarbon solvent axonopathy. Int. J. Hyg. Environ. Health. 2002;205:131–136. doi: 10.1078/1438-4639-00138. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Schaumburg HH. Ultrastructural studies of the dying-back process. III. The evolution of experimental peripheral giant axonal degeneration. J. Neuropathol. Exp. Neurol. 1977;36:276–299. doi: 10.1097/00005072-197703000-00005. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Schaumburg HH, Sabri MI, Veronesi B. The enlarging view of hexacarbon neurotoxicity. Crit. Rev. Toxicol. 1980;7:279–356. doi: 10.3109/10408448009037489. [DOI] [PubMed] [Google Scholar]
- Tshala-Katumbay D, Monterroso V, Kayton R, Lasarev M, Sabri M, Spencer P. Probing mechanisms of axonopathy. Part I: Protein targets of 1,2-diacetylbenzene, the neurotoxic metabolite of aromatic solvent 1,2-diethylbenzene. Toxicol. Sci. 2008;105:134–141. doi: 10.1093/toxsci/kfn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tshala-Katumbay DD, Palmer VS, Kayton RJ, Sabri MI, Spencer PS. A new murine model of giant proximal axonopathy. Acta Neuropathol. 2005;109:405–410. doi: 10.1007/s00401-005-0982-z. [DOI] [PubMed] [Google Scholar]
- Wang QS, Hou LY, Zhang CL, Song FY, Xie KQ. Changes of cytoskeletal proteins in nerve tissues and serum rats treated with 2,5-hexanedione. Toxicology. 2008;244:166–178. doi: 10.1016/j.tox.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Zagoren JC, Politis MJ, Spencer PS. Rapid reorganization of the axonal cytoskeleton induced by a γ-diketone. Brain Res. 1983;270:162–164. doi: 10.1016/0006-8993(83)90807-7. [DOI] [PubMed] [Google Scholar]