Abstract
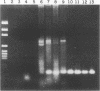
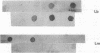
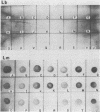
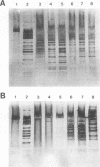
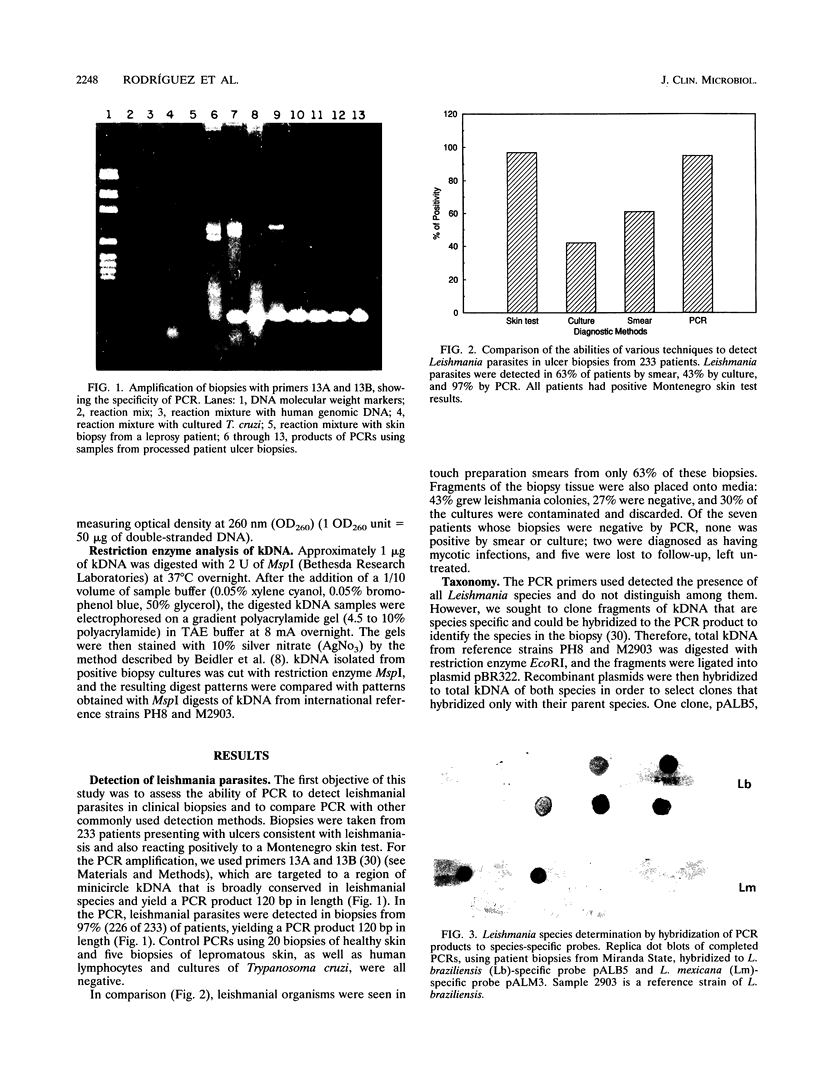
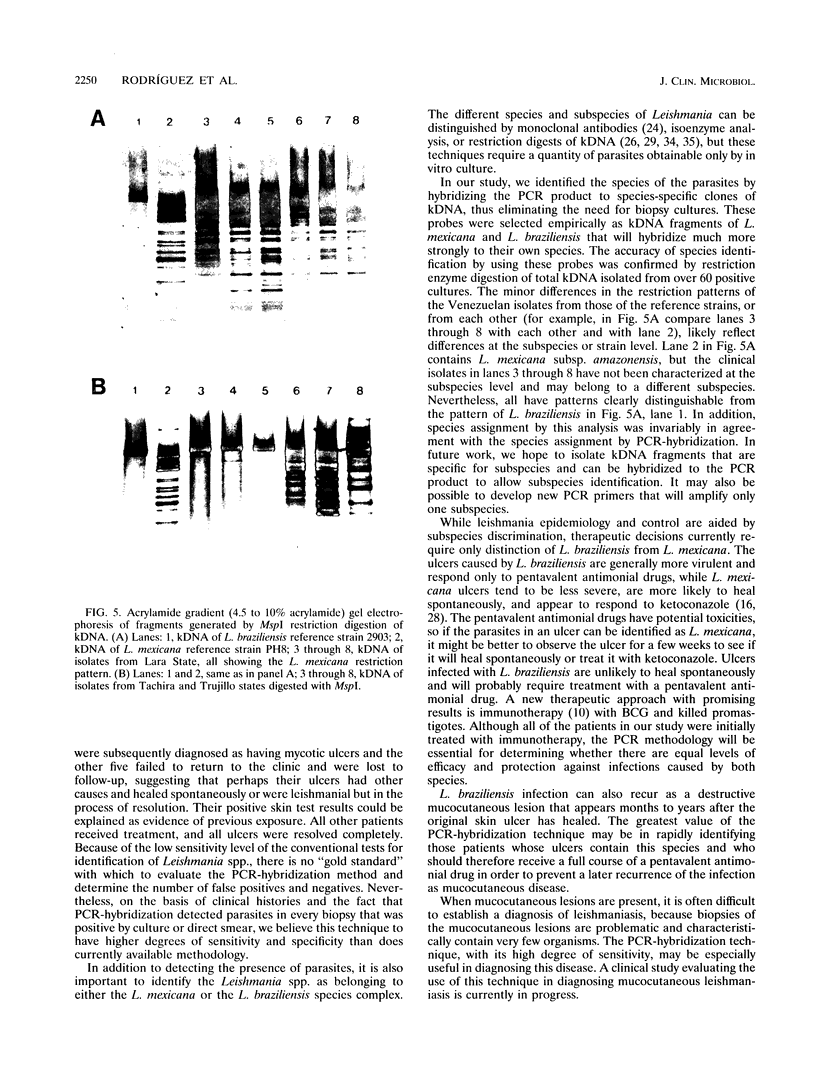
The aim of this study was to assess the efficacy of PCR methodology in establishing the diagnosis of cutaneous leishmaniasis in patients from areas of endemicity in Venezuela. Biopsies from 233 patients with cutaneous ulcers suggestive of leishmaniasis were analyzed by PCR, employing oligonucleotides directed against conserved regions of kinetoplast DNA (kDNA), and the PCR products were then hybridized to nonradioactively labeled, species-specific, cloned kDNA fragments. The ability of PCR to detect Leishmania cells was compared with those of the conventional methodologies: skin testing with killed promastigotes (Montenegro test), examination of Giemsa-stained biopsy smears, and in vitro culture of biopsy tissue. The PCR-hybridization technique detected the presence of Leishmania cells in 98% of patients clinically diagnosed as having leishmaniasis and also positive by the Montenegro skin test. In comparison, leishmania positivity was found in only 42% of cultures and 64% of biopsy smears. By hybridizing the PCR product to new kDNA probes specific for either Leishmania mexicana or Leishmania braziliensis, we found that both species are major causes of cutaneous leishmaniasis in Venezuela, and the species identification was confirmed by restriction enzyme analysis of kDNA from biopsy cultures. This work demonstrates that PCR coupled with hybridization is useful not only for the diagnosis of cutaneous leishmaniasis but also for the taxonomic discrimination essential for both epidemiology and therapy. This technique can be used to diagnose leishmaniasis in a country in which the disease is endemic and can perhaps be adapted for use in a rural clinic.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias J. R., Naiff R. D. The principal reservoir host of cutaneous leishmaniasis in the urban areas of Manaus, Central Amazon of Brazil. Mem Inst Oswaldo Cruz. 1981 Jul-Sep;76(3):279–286. doi: 10.1590/s0074-02761981000300005. [DOI] [PubMed] [Google Scholar]
- Arnot D. E., Barker D. C. Biochemical identification of cutaneous leishmanias by analysis of kinetoplast DNA. II. Sequence homologies in Leishmania kDNA. Mol Biochem Parasitol. 1981 May;3(1):47–56. doi: 10.1016/0166-6851(81)90076-1. [DOI] [PubMed] [Google Scholar]
- Ashall F., Miles M. A. Diagnosis of parasitic diseases using DNA-to-DNA hybridization. Trans R Soc Trop Med Hyg. 1988;82(2):235–236. doi: 10.1016/0035-9203(88)90428-2. [DOI] [PubMed] [Google Scholar]
- Añez N., Nieves E., Scorza J. V. El "status" taxonómico de Leishmania garnhami, indicado por su patrón de desarrollo en el vector. Mem Inst Oswaldo Cruz. 1985 Jan-Mar;80(1):113–119. doi: 10.1590/s0074-02761985000100017. [DOI] [PubMed] [Google Scholar]
- Barker D. C., Butcher J. The use of DNA probes in the identification of leishmanias: discrimination between isolates of the Leishmania mexicana and L. braziliensis complexes. Trans R Soc Trop Med Hyg. 1983;77(3):285–297. doi: 10.1016/0035-9203(83)90146-3. [DOI] [PubMed] [Google Scholar]
- Barker D. C. Molecular approaches to DNA diagnosis. Parasitology. 1989;99 (Suppl):S125–S146. doi: 10.1017/s0031182000083463. [DOI] [PubMed] [Google Scholar]
- Beidler J. L., Hilliard P. R., Rill R. L. Ultrasensitive staining of nucleic acids with silver. Anal Biochem. 1982 Nov 1;126(2):374–380. doi: 10.1016/0003-2697(82)90530-9. [DOI] [PubMed] [Google Scholar]
- Convit J., Ulrich M., Aranzazu N., Castellanos P. L., Pinardi M. E., Reyes O. The development of a vaccination model using two microorganisms and its application in leprosy and leishmaniasis. Lepr Rev. 1986 Dec;57 (Suppl 2):263–273. doi: 10.5935/0305-7518.19860080. [DOI] [PubMed] [Google Scholar]
- Corredor A., Kreutzer R. D., Tesh R. B., Boshell J., Palau M. T., Caceres E., Duque S., Pelaez D., Rodriguez G., Nichols S. Distribution and etiology of leishmaniasis in Colombia. Am J Trop Med Hyg. 1990 Mar;42(3):206–214. doi: 10.4269/ajtmh.1990.42.206. [DOI] [PubMed] [Google Scholar]
- Cuba Cuba C. A., Netto E. M., Marsden P. D., Rosa A. de C., Llanos Cuentas E. A., Costa J. L. Cultivation of Leishmania braziliensis braziliensis from skin ulcers in man under field conditions. Trans R Soc Trop Med Hyg. 1986;80(3):456–457. doi: 10.1016/0035-9203(86)90342-1. [DOI] [PubMed] [Google Scholar]
- Herwaldt B. L., Arana B. A., Navin T. R. The natural history of cutaneous leishmaniasis in Guatemala. J Infect Dis. 1992 Mar;165(3):518–527. doi: 10.1093/infdis/165.3.518. [DOI] [PubMed] [Google Scholar]
- Kennedy W. P. Novel identification of differences in the kinetoplast DNA of Leishmania isolates by recombinant DNA techniques and in situ hybridisation. Mol Biochem Parasitol. 1984 Jul;12(3):313–325. doi: 10.1016/0166-6851(84)90088-4. [DOI] [PubMed] [Google Scholar]
- Lainson R., Shaw J. J. Leishmaniasis of the New World: taxonomic problems. Br Med Bull. 1972 Jan;28(1):44–48. doi: 10.1093/oxfordjournals.bmb.a070892. [DOI] [PubMed] [Google Scholar]
- Lopes U. G., Wirth D. F. Identification of visceral Leishmania species with cloned sequences of kinetoplast DNA. Mol Biochem Parasitol. 1986 Jul;20(1):77–84. doi: 10.1016/0166-6851(86)90144-1. [DOI] [PubMed] [Google Scholar]
- Massamba N. N., Mutinga M. J. Recombinant kinetoplast DNA (kDNA) probe for identifying Leishmania tropica. Acta Trop. 1992 Sep;52(1):1–15. doi: 10.1016/0001-706x(92)90002-f. [DOI] [PubMed] [Google Scholar]
- Mayrink W., Williams P., Coelho M. V., Dias M., Martins A. V., Magalhães P. A., Da Costa C. A., Falcão A. R., Melo M. N., Falcão A. L. Epidemiology of dermal leishmaniasis in the Rio Doce Valley, State of Minas Gerais, Brazil. Ann Trop Med Parasitol. 1979 Apr;73(2):123–137. doi: 10.1080/00034983.1979.11687239. [DOI] [PubMed] [Google Scholar]
- McMahon-Pratt D., Bennett E., David J. R. Monoclonal antibodies that distinguish subspecies of Leishmania braziliensis. J Immunol. 1982 Sep;129(3):926–927. [PubMed] [Google Scholar]
- Morel C., Simpson L. Characterization of pathogenic trypanosomatidae by restriction endonuclease fingerprinting of kinetoplast DNA minicircles. Am J Trop Med Hyg. 1980 Sep;29(5 Suppl):1070–1074. doi: 10.4269/ajtmh.1980.29.1070. [DOI] [PubMed] [Google Scholar]
- Navin T. R., Arana B. A., Arana F. E., Berman J. D., Chajón J. F. Placebo-controlled clinical trial of sodium stibogluconate (Pentostam) versus ketoconazole for treating cutaneous leishmaniasis in Guatemala. J Infect Dis. 1992 Mar;165(3):528–534. doi: 10.1093/infdis/165.3.528. [DOI] [PubMed] [Google Scholar]
- Navin T. R., Arana F. E., de Mérida A. M., Arana B. A., Castillo A. L., Silvers D. N. Cutaneous leishmaniasis in Guatemala: comparison of diagnostic methods. Am J Trop Med Hyg. 1990 Jan;42(1):36–42. doi: 10.4269/ajtmh.1990.42.36. [DOI] [PubMed] [Google Scholar]
- Pacheco R. S., Grimaldi Júnior G., Morel C. M. Inhibition of growth of Leishmania mexicana mexicana by Leishmania mexicana amazonensis during "in vitro" co-cultivation. Mem Inst Oswaldo Cruz. 1987 Oct-Dec;82(4):537–542. doi: 10.1590/s0074-02761987000400011. [DOI] [PubMed] [Google Scholar]
- Rodgers M. R., Popper S. J., Wirth D. F. Amplification of kinetoplast DNA as a tool in the detection and diagnosis of Leishmania. Exp Parasitol. 1990 Oct;71(3):267–275. doi: 10.1016/0014-4894(90)90031-7. [DOI] [PubMed] [Google Scholar]
- Rogers W. O., Wirth D. F. Kinetoplast DNA minicircles: regions of extensive sequence divergence. Proc Natl Acad Sci U S A. 1987 Jan;84(2):565–569. doi: 10.1073/pnas.84.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spithill T. W., Grumont R. J. Identification of species, strains and clones of Leishmania by characterization of kinetoplast DNA minicircles. Mol Biochem Parasitol. 1984 Jun;12(2):217–236. doi: 10.1016/0166-6851(84)90137-3. [DOI] [PubMed] [Google Scholar]
- Ulrich M., Centeno M., Mattout Z., Convit J. Serological patterns and specificity in American cutaneous leishmaniasis. Am J Trop Med Hyg. 1988 Aug;39(2):179–184. doi: 10.4269/ajtmh.1988.39.179. [DOI] [PubMed] [Google Scholar]
- Weigle K. A., de Dávalos M., Heredia P., Molineros R., Saravia N. G., D'Alessandro A. Diagnosis of cutaneous and mucocutaneous leishmaniasis in Colombia: a comparison of seven methods. Am J Trop Med Hyg. 1987 May;36(3):489–496. doi: 10.4269/ajtmh.1987.36.489. [DOI] [PubMed] [Google Scholar]
- Wirth D. F., Pratt D. M. Rapid identification of Leishmania species by specific hybridization of kinetoplast DNA in cutaneous lesions. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6999–7003. doi: 10.1073/pnas.79.22.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn M. H., Barker D. C. Diagnosis of New World leishmaniasis: specific detection of species of the Leishmania braziliensis complex by amplification of kinetoplast DNA. Acta Trop. 1992 Sep;52(1):45–58. doi: 10.1016/0001-706x(92)90006-j. [DOI] [PubMed] [Google Scholar]
- de Brujin M. H., Labrada L. A., Smyth A. J., Santrich C., Barker D. C. A comparative study of diagnosis by the polymerase chain reaction and by current clinical methods using biopsies from Colombian patients with suspected leishmaniasis. Trop Med Parasitol. 1993 Sep;44(3):201–207. [PubMed] [Google Scholar]