Abstract
Chronic arsenic exposure is a worldwide health problem. Although arsenic-induced cancer has been widely studied, comparatively little attention has been paid to arsenic-induced vascular disease. Epidemiological studies have shown that chronic arsenic exposure is associated with increased morbidity and mortality from cardiovascular disease. In addition, studies suggest that susceptibility to arsenic-induced vascular disease may be modified by nutritional factors in addition to genetic factors. Recently, animal models for arsenic-induced atherosclerosis and liver sinusoidal endothelial cell dysfunction have been developed. Initial studies in these models show that arsenic exposure accelerates and exacerbates atherosclerosis in apolipoprotein E–knockout mice. Microarray studies of liver mRNA and micro-RNA abundance in mice exposed in utero suggest that a permanent state of stress is induced by the arsenic exposure. Furthermore, the livers of the arsenic-exposed mice have activated pathways involved in immune responses suggesting a pro-hyperinflammatory state. Arsenic exposure of mice after weaning shows a clear dose-response in the extent of disease exacerbation. In addition, increased inflammation in arterial wall is evident. In response to arsenic-stimulated oxidative signaling, liver sinusoidal endothelium differentiates into a continuous endothelium that limits nutrient exchange and waste elimination. Data suggest that nicotinamide adenine dinucleotide phosphate oxidase–derived superoxide or its derivatives are essential second messengers in the signaling pathway for arsenic-stimulated vessel remodeling. The recent findings provide future directions for research into the cardiovascular effects of arsenic exposure.
Keywords: arsenic, inflammation, oxidative signaling, vascular disease, nutrition, microarray
Arsenic contamination of drinking water is a major worldwide public health problem. It is well established that chronic arsenic ingestion causes cancer of the skin, bladder, and lung. Less well studied is the association of chronic arsenic exposure with development of both cardiovascular disease (CVD) and peripheral vascular disease. Earlier epidemiological studies provided highly suggestive but not conclusive data supporting the hypothesis that chronic arsenic exposure causes circulatory disease. In addition, until recently animal models to study arsenic-induced circulatory diseases have not been established. A symposium entitled “Arsenic and Cardiovascular Disease” was held at the 47th Annual Meeting of the Society of Toxicology in 2008. This symposium, sponsored by the Metals Specialty Section, addressed recent epidemiological evidence and experimental results in murine models for arsenic-induced disease of the circulatory system.
EPIDEMIOLOGICAL EVIDENCE ON ARSENIC EXPOSURE AND VASCULAR DISEASE
Although inorganic arsenic has been classified as a group 1 carcinogen to humans by the International Agency for Research on Cancer, evidence for causal association of other health effects with arsenic exposure has not been well established. Arsenic is abundant in the earth's crust and can be released into groundwater under certain conditions. Epidemiological studies in parts of the world with high levels of arsenic (>300 μg/l) in groundwater have associated arsenic exposure with elevated risks for an array of CVD, including hypertension (Chen et al., 1995; Rahman et al., 1999), carotid atherosclerosis (Wang et al., 2002), ischemic heart disease (Hsueh et al., 1998; Tseng et al., 2000), and vascular disease mortality (Chen et al., 1996). The distinct association with manifestations of generalized arteriosclerosis supplies certainty on causality. However, the underlying mechanisms have not been well characterized. Large-scale prospective studies using appropriate biomarkers may help reveal pathways by which arsenic induces vascular disease.
Importantly, little is known about the associations between low-to-moderate level arsenic exposure via drinking water (<300 μg/l) and CVD (Navas-Acien et al., 2005; Wang et al., 2007a,b). Studies in areas such as the United States with low-to-moderate exposure showed inconsistent findings (Engel and Smith, 1994; Lewis et al., 1999; Meliker et al., 2007; Zierold et al., 2004). The heterogeneity of drinking water resources and the limited exposure range together pose a challenge for epidemiological studies in the United States to have valid long-term arsenic exposure measures at the individual level and a large sample size. In addition, few studies have assessed the interindividual variability to the cardiovascular effect of arsenic exposure. For weak to moderately strong associations between environmental exposures and disease outcomes, causal inference can be strengthened if the data show a stronger effect in a susceptible subgroup of the population (Khoury et al., 2005). Such data may also help risk assessment in the population and provide knowledge about the underlying mechanisms.
Reviews of epidemiological studies on arsenic and CVD have been published (Navas-Acien et al., 2005; Wang et al., 2007a). Below, we discuss recent epidemiological studies on arsenic exposure and biomarkers of CVD and studies on the interindividual susceptibility to the cardiovascular effect of arsenic exposure. The studies on interindividual susceptibility are summarized in Table 1.
TABLE 1.
Recent Epidemiological Studies of Susceptibility to the Cardiovascular Effects of Arsenic Exposure from Drinking Water
| Reference | Location | Study design | Study population | Arsenic exposure levels | Outcome | Effect modifier (susceptibility factors) |
| Chen et al. (2007a) | Bangladesh | Cross-sectional | 10,910 participants | 0.1—864 μg/l, mean 99 μg/l | Pulse pressure ≥ 55 mmHg | Low levels of dietary intake of B vitamins and folate |
| Huang et al. (2007) | Southwestern Taiwan | Cross-sectional | 871 subjects | Median in villages: 700–930 μg/l | Hypertension | High urinary MMA% and low DMA/MMA |
| Hsueh et al. (1998) | Southwestern Taiwan | Case-control | 74 ischemic heart disease patients and 193 matched healthy controls | Median in villages: 700–930 μg/l | Ischemic heart disease | Low levels of serum carotene |
| Hsueh et al. (2005) | Southwestern Taiwan | Case-control | 79 hypertensive cases and 213 controls | Median in villages: 700–930 μg/l | Hypertension | NOS3 G894T, SOD2 Ala9Val, and CYBA C242T |
| Wang et al. (2007b) | Northeastern Taiwan | Cross-sectional | 605 residents | Median in villages: undetectable to 140 μg/l | Carotid atherosclerosis | GSTP1 variant genotypes of Ile/Val and Val/Val |
EPIDEMIOLOGICAL EVIDENCE ON INTERINDIVIDUAL VARIABILITY OF ARSENIC'S CARDIOVASCULAR EFFECT
Nutritional Factors
In a case-control study in villages of southwestern Taiwan with high levels of arsenic exposure, with median arsenic concentration in villages ranging from 700 to 930 μg/l, serum samples of 74 patients with ischemic heart disease and 193 matched healthy controls were tested for serum levels of micronutrients (Hsueh et al., 1998). Multivariate analysis showed a synergistic interaction on arsenic-related ischemic heart disease between duration of consuming well water with arsenic exposure and low serum carotene level. The findings suggest that inadequate carotene intake or other factors contributing to low carotene level may increase the susceptibility to the cardiovascular effects of arsenic exposure. However, the potential mechanisms involved in the protective action remain to be studied.
More recently, in a cross-sectional study of blood pressure using data from 10,910 participants in the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh, there was a positive association between low-to-moderate levels of arsenic exposure from drinking water and high pulse pressure (pulse pressure ≥ 55 mmHg) (Chen et al., 2007a), an indicator of arterial stiffness which is associated with an increased risk of atherosclerosis (Dart and Kingwell, 2001; Safar et al., 2003). The likelihood of high pulse pressure was 1.19–1.39 times greater in subjects with higher well arsenic categories (8.1–40.8, 40.9–91.0, 91.1–176.0, and 176.1–64.0 μg/l) compared with those in the lowest category (<8 μg/l). In addition, among participants with lower than the average dietary intake level of B vitamins and folate, those with a higher well arsenic concentration were 1.83–1.89 times more likely to have a pulse pressure ≥55 mmHg compared with those in the bottom quintile of well arsenic concentration (<8 μg/l) (Chen et al., 2007a). Methylation of arsenic, a hypothesized detoxification pathway, requires the conversion of S-adenosylmethionine to S-adenosylhomocysteine, which subsequently forms homocysteine (Gamble et al., 2005; Zakharyan and Aposhian, 1999). Higher levels of plasma homocysteine have been associated with high blood pressure (Araki et al., 1989; Lim and Cassano, 2002). Because the metabolism of homocysteine requires sufficient levels of vitamin B2, B12, B6, and folic acid (Selhub, 2002), arsenic exposure may affect blood pressure through its effect on the formation of S-adenosylhomocysteine and homocysteine, especially in a background of inadequate intake of folate and B vitamins. The findings indicate that the effect of low-level arsenic exposure on blood pressure is nonlinear and may be more pronounced in persons with lower intake of nutrients related to arsenic metabolism and cardiovascular health, although the exact mechanisms are not clear.
Urinary Arsenic Metabolite Profile
Methylation of inorganic AsIII, which is primarily hepatic, first generates monomethylarsonic acid (MMAV). After the reduction of MMAV to MMAIII, a second methylation can occur to generate dimethylarsinic acid (DMAV) (Vahter and Concha, 2001). A growing number of studies in experimental model systems indicate that MMAIII is more toxic than inorganic arsenic or any of the pentavalent intermediates (Ahmad et al., 1999; Nesnow et al., 2002; Styblo et al., 2002). Epidemiological studies also suggest that an increased proportion of MMA in urine is associated with an elevated risk of cancers (Chen et al., 2003a,b; Hsueh et al., 1997; Pu et al., 2007; Yu et al., 2000) and arsenic-related skin lesions (Ahsan et al., 2007). Evidence of the effect of arsenic metabolism on CVD risk is limited. In a case-control study of hypertension conducted in southwestern Taiwan with high levels of arsenic exposure (median in villages from 700 to 930 μg/l), hypertensive cases had higher percentages of MMAV and a lower ratio of DMAV divided by MMAV than those without hypertension (Huang et al., 2007). However, study participants were exposed to arsenic 2–3 decades ago, and those with a high level of arsenic exposure in the past had a higher hypertension irrespective of differences in the composition of urinary arsenic metabolites. Studies in populations with current exposure at low-to-moderate levels are needed to assess whether CVD risk differs by levels of arsenic methylation capacity.
Genetic Susceptibility
Experimental studies have suggested that arsenic increases the production of reactive oxygen species (ROS) (Barchowsky et al., 1999; Chen et al., 1998; Wang et al., 1996). The induction of oxidative stress by arsenic may influence gene expression, inflammatory responses, and endothelial nitric oxide homeostasis (Simeonova and Luster, 2004), which play an important role in maintaining vascular tone (Willerson and Ridker, 2004). Genes involved in endogenous defenses against ROS thus may modify arsenic's effect. A study of 79 hypertensive cases and 213 controls in the high-exposed area of Taiwan investigated the roles of NOS3, the gene for endothelial nitric oxide synthase; SOD2, the gene for manganese superoxide dismutase, and CYBA, the gene for p22 phox, a critical enzyme for superoxide production and an essential component of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX). The study shows that the NOS3 G894T, SOD2 Ala9Val, and CYBA C242T are significantly associated with hypertension risk in the high exposure group (>1000 μg/l) (Hsueh et al., 2005). However, the sample size of the study was small and future large studies are needed to confirm the findings.
Glutathione S-transferases (GSTs) are a superfamily of enzymes that are key in the detoxification step of phase II metabolism. The GSTs are associated with the regulation of inflammation through modulation of prostaglandin signaling pathways and may play a major role in cellular antioxidant defense mechanisms (Hayes and McLellan, 1999; Hayes and Pulford, 1995; Ochi et al., 1994). GSTs are also involved in catalyzing the formation of arsenic-glutathione conjugates (Kitchin, 2001; Leslie et al., 2004), which are required for biliary excretion of arsenic (Leslie et al., 2004). In a study of 605 residents in northeastern Taiwan with low-to-moderate exposure (median in villages from undetectable to 140 μg/l), the prevalence of carotid atherosclerosis was significantly associated with the genetic polymorphism of glutathione S-transferase P1 (GSTP1) (Wang et al., 2007b; Wu et al., 2006). The high arsenic exposure group (>50 μg/l) with one or two variant genotypes of GSTP1 had an increased risk of carotid atherosclerosis showing an odds ratio of 2.8 compared with those with wild-type alleles.
EPIDEMIOLOGICAL EVIDENCE ON ARSENIC EXPOSURE AND ENDOTHELIAL DYSFUNCTION
Circulating markers of systemic inflammation and endothelial dysfunction such as soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular adhesion molecule-1 (sVCAM-1) are predictive for CVD (Blankenberg et al., 2001; Hwang et al., 1997; Ridker et al., 1998, 2000). In a subgroup of 115 individuals with arsenic-related skin lesions in Bangladesh, there was a positive association of urinary arsenic and well arsenic concentration with plasma levels of sICAM-1 and sVCAM-1 (Chen et al., 2007b). The positive associations of well arsenic with plasma sVCAM-1 and sICAM-1 suggest a potential pathway underlying the mechanism of action of long-term arsenic exposure in the development of CVD. However, all the participants had skin lesions and were exposed to moderate levels of arsenic exposure (median exposure 230 μg/l). Future large studies are required to study the associations of low-level arsenic exposure with markers of vascular inflammation and endothelial dysfunction in healthy persons.
Microalbuminuria, a marker of glomerular hyperfiltration, has been correlated with and may be a manifestation of impaired endothelial function (Paisley et al., 2003; Stehouwer et al., 2004). Impairment of endothelial function is recognized as one of the initial mechanisms that lead to atherosclerosis (Davignon and Ganz, 2004). Results from a study of arsenic and proteinuria detected by dipstick analysis among 11,121 persons in the HEALS in Bangladesh, showing a dose-response relationship between well arsenic concentration and prevalence of proteinuria, were discussed in the symposium. The likelihood of proteinuria in participants with higher three categories of well arsenic (40–91, 92–179, and 180–864 μg/l) was 1.36–1.64 times greater than that in the lowest category (≤7 μg/l). In addition, in cohort analysis with repeated measure of proteinuria and urinary arsenic concentration, change in urinary arsenic was positively related to incidence of proteinuria during the 4 years of follow-up. The dose-response relationship between arsenic exposure and proteinuria provides evidence of the effect of low-to-moderate level of arsenic exposure on a common causal intermediate of CVD.
ARSENIC EXPOSURE EXACERBATES ATHEROSCLEROSIS IN APOE-KNOCKOUT MICE
Atherosclerosis is an intricate disease. Primary events consist of fatty streak formation under the subendothelial space of large arteries. Accumulating evidence suggest that this process is initiated by the oxidation of low-density lipoproteins (LDLs). Deprivation of antioxidants in the subendothelial space makes the LDL susceptible to oxidation. Oxidized low-density lipoprotein (oxLDL) activates the endothelium. The activated endothelium expresses cell adhesion molecules, which recruit monocytes, which eventually transmigrate into subendothelial space and differentiate into macrophages. oxLDL is then taken up by the macrophages via scavenger receptors, resulting in foam cell formation. The evolution of fatty streaks to the more multifaceted lesions is characterized by the migration of medial smooth muscle cells into the intima. Intimal smooth muscle cells proliferate and take up oxLDL to form foam cells, synthesize extracellular matrix proteins, and form fibrous cap.
Several epidemiological studies suggest exposure to correlates with endothelial dysfunction and increased incidence of atherosclerosis. However, very little is known about the biochemical mechanisms by which arsenic exerts its proatherogenic effects. Genetically altered mice are preferred models to study atherogenesis (Table 2). Due to the high levels of high density lipoproteins, wild-type mice are resistant to atherosclerosis. However, disruption of cholesterol transport by genetic deletion of apolipoprotein E (ApoE) or low-density lipoprotein receptor (LDLr) or overexpression of apoB results in discernible increase in very low density lipoproteins (VLDL) and/or LDL cholesterol and in atherosclerotic lesion formation throughout the aortic tree that resembles human disease. Increased accumulation of arsenic in the vessel wall and increased atherosclerotic lesion formation were observed in the aorta of female ApoE-knockout mice given water containing 20 or 100 mg/l sodium arsenite for 24 weeks (Simeonova et al., 2003). Increased lesion formation was also observed in the innominate arteries in adult ApoE/LDLr double knockout mice fed sodium arsenite (133 mg/l) for 18 weeks (Bunderson et al., 2004). Data presented at the symposium showed that early life arsenic exposure (starting at 3 weeks of age) to (1–50 mg/l arsenic in drinking water) resulted in macrophage-rich lesions in the aortic sinus and the aortic arch in ApoE-knockout mice at 10 and 16 weeks of age, without increasing plasma cholesterol. Exposure of adult mice (starting at 12 weeks old) to arsenic for 24 weeks resulted in advanced lesion formation in the aortic sinus, the aortic arch, and the abdominal aorta. Characterization of these lesions illustrated increased macrophage accumulation and fibrosis in arsenic-exposed mice as compared to water-fed controls. Temporal studies showed that exposure to arsenic starting at age 3 weeks accelerated lesion formation at 10, 16, and 36 weeks of age.
TABLE 2.
Mouse Models of Arsenic-induced CVD via Drinking Water Arsenic Exposure
| Reference | Mouse strain | Arsenic exposure and level | End point assayed and outcome |
| Bunderson et al. (2004) | ApoE, LDLr double knockout | Drinking water, 5.7 mg/l, 18 weeks | Increased atherosclerotic plaque stenosis in innominate artery |
| Simeonova et al. (2003) | ApoE-knockout | Drinking water, 11.4 or 57 mg/l, 24 weeks | Increased aortic lesions |
| Srivastava et al. (2007) | ApoE-knockout | Transplacental in drinking water, 49 mg/l, gestational days 8–18 | Increased fatty streak lesions in aortic arch and valves, defective vasorelaxation response at age 10 weeks |
| Straub et al. (2007a,b) | C57Bl/6 | Drinking water, 250 μg/l, 5 weeks | Liver sinusoidal capillarization, angiogenesis in liver bile ducts, decreased lumen in hepatic arterioles, gain in sinusoidal endothelial cell caveolin and caveolae |
| Straub et al. (2008) | C57Bl/6 p47phox knockout | Drinking water, 10–250 μg/l, 2 weeks | Liver sinusoidal capillarization, loss of modified albumin scavenging, protein nitration, oxidant generation, absence of arsenic-induced effects in knockout |
ARSENIC EXPOSURE INCREASES OXIDATIVE STRESS IN VASCULAR LESIONS
Studies in experimental animals have shown that oxidized lipids are present in all stages of atherogenesis, and immunization of experimental animals with the protein adducts of the products of LDL oxidation decreases atherosclerotic lesion formation (George et al., 1998; Zhou et al., 2001). Oxidized lipids generate several bioactive molecules (e.g., ROS, peroxides, and isoprostanes), of which aldehydes are the major end products (Esterbauer et al., 1991). Malondialdehyde (MDA) and 4-hydroxy-trans-2-nonenal (HNE) are the most abundant saturated and unsaturated aldehydes generated from the oxidation of LDL (Esterbauer et al., 1991). Protein adducts of MDA and HNE have been detected in atherosclerotic lesions of experimental animals and humans (Jurgens et al., 1993; Palinski et al., 1989; Rosenfeld and Ross, 1990). Data presented at the symposium illustrated that lesions in arsenic-exposed mice showed increased accumulation of protein adducts of MDA and HNE both in early and in advanced lesions. Increased levels of free MDA and HNE were also detected in the plasma of As-exposed mice. Arsenic has also been shown to induce the expression of aldose reductase (Lau et al., 2004), an enzyme which is an efficient catalyst for the reduction of lipid-derived aldehydes (Srivastava et al., 1998a,b, 1999) and is the major aldehyde-metabolizing enzyme in the cardiovascular cells (Srivastava et al., 2001, 2004). Additionally, several studies have shown that unsaturated lipid aldehydes and arsenic are also efficiently metabolized by GSTs (Chiou et al., 1997; Yang et al., 2008). As indicated above, a recent study shows that occurrence of carotid atherosclerosis is significantly associated with genetic polymorphism of GSTP1, an enzyme involved in arsenic metabolism (Wang et al., 2007b). Since lipid aldehydes are highly reactive and can increase monocyte adhesion, cytokine production, and lipid uptake by scavenger receptors, it is conceivable that excessive generation of these aldehydes or decreased detoxification upon arsenic exposure exacerbates atherosclerotic lesion formation.
ARSENIC EXPOSURE INCREASES INFLAMMATION IN VASCULAR LESIONS
Cumulative evidence from a large number of studies indicates that inflammation plays a pivotal role in atherosclerotic plaque formation. Vascular cells including endothelial cells, macrophages/monocytes, and smooth muscle cells generate chemokines and proinflammatory cytokines including monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), and tumor necrosis factor α. MCP-1 and IL-6 are abundant in atherosclerotic lesions, and genetic deletion of these chemokines and cytokines in mice decreases atherosclerotic lesion formation (Gu et al., 1998; Schieffer et al., 2004). Recently, it has been shown that in vitro sodium arsenite induces the expression of MCP-1 and IL-6 in vascular smooth muscle cells (Lee et al., 2005). Data presented at the symposium showed increased expression of MCP-1 and IL-6 in vascular lesions in As-exposed mice. These observations are consistent with studies illustrating increased expression of circulating lymphocyte MCP-1 mRNA and plasma MCP-1 concentration in humans exposed to arsenic (Wu et al., 2003). Recent studies suggest that humans carrying two risk genotypes of ApoE and MCP1 and exposed to drinking water containing >10 μg/l have >10-fold risk of carotid atherosclerosis (Hsieh et al., 2008). Together, these observations suggest that As-induced inflammation could be an important risk factor for atherosclerosis.
ARSENIC INDUCES ENDOPLASMIC RETICULUM STRESS AND UNFOLDED PROTEIN RESPONSE
Recent studies have suggested that endoplasmic reticulum (ER) stress and subsequent unfolded protein response (UPR) could be involved in oxidized lipid–induced inflammation. Gene array analysis (Gargalovic et al., 2006) showed that in vitro oxidized lipids induce the expression of several UPR responsive genes in endothelial cells, and activation of UPR occurs in atherosclerotic plaques (Zhou et al., 2005). Data presented at the symposium showed that in vitro sodium arsenite induces the expression of several ER chaperones including 78-kDa glucose-regulated protein, homocysteine-induced endoplasmic reticulum protein, and Calnexin that stabilize protein folding in vascular endothelial cells. Accumulation of misfolded proteins in the ER activates three ER-resident transmembrane proteins, inositol-requiring kinase, activating transcription factor (ATF) 6, and protein kinase–like ER kinase (PERK), which then trigger the UPR for cell survival. Data presented at the symposium showed that sodium arsenite caused the phosphorylation of PERK-dependent UPR responsive protein, pancreatic eukaryotic initiation factor 2α (eIF2α) in vascular endothelial cells. Sodium arsenite also induced the downstream effecter of eIF2α-ATF3, a negative regulator of cytokine production, in vascular endothelial cells and bone marrow–derived macrophages. Similarly, aldehydic products of lipid oxidation (e.g., HNE) induced ER chaperones, ATF3, and phosphorylation of eIF2α. Lesions of exposed mice also showed profound increase in the expression of ATF3 as compared to control mice. Expression of ATF3 colocalized with protein-HNE adducts in the vascular lesions. In vitro induction of ATF3 by sodium arsenite or HNE was completely abolished by chemical chaperone of protein folding, phenyl butyric acid. These observations are consistent with recent studies showing that arsenic causes ER stress and UPR in human lens epithelial cells, retinal pigment epithelial cells, chronic myeloid leukemic cells, and glioma cells (Du et al., 2006; Kim et al., 2008; Roybal et al., 2005; Zhang et al., 2007). In retinal pigment epithelial cells, arsenic also increased the expression of vascular endothelial growth factor (VEGF). Arsenite-induced VEGF expression was markedly decreased in a dominant-negative ATF4 mutant and ATF4-deficient murine embryonic fibroblast cells. N-acetylcysteine, a potent antioxidant thiol, abolished arsenite-induced UPR and induction of VEGF (Roybal et al., 2004). Alternatively, As-induced ER stress could be mediated by homocysteine. As discussed above, methylation of ingested inorganic arsenic to MMA and DMA results in the formation of homocysteine (Gamble et al., 2005; Zakharyan and Aposhian, 1999), a potent inducer of ER stress, endothelial activation, inflammation, and cell proliferation (Austin et al., 2004). Increased plasma homocysteine is a risk factor for atherosclerosis. Recently, it has been shown that the risk of atherosclerosis is increased by more than fivefold in individuals with high plasma homocysteine level (>12.7mM) and high MMA (>16.5%) concentration in the urine (Wu et al., 2006). Collectively, proatherogenic affects of arsenic could be attributed at least in part to increased oxidative stress, ER stress, and vascular inflammation.
IN UTERO ARSENIC EXPOSURE
The first evidence that in utero arsenic exposure could cause arterial disease was in reports of myocardial infarction in infants whose mothers consumed water with high levels of arsenic in Antofogasta region of Chile (Rosenberg, 1973, 1974). These infants were part of a cohort of exposed people which also exhibits increased pulmonary disease (Smith et al., 2006) and CVD (Yuan et al., 2007) in more recent epidemiological studies. In these later studies, there was a marked gender bias in the increased mortality due to acute myocardial infarction associated with in utero and childhood arsenic exposure with males showing approximately 1 in 10 excess deaths caused by arsenic compared with 1 in 4 for females. Recently, direct evidence for in utero arsenic exposure induction of arterial disease was demonstrated in ApoE-knockout mice (Srivastava et al., 2007). This study showed that administration of drinking water containing arsenic as sodium arsenite caused early onset of atherosclerosis in 10-week-old male offspring and more severe disease in 16-week-old male offspring maintained on normal chow. Plasma cholesterol and phospholipids were unaltered, but significant reductions in plasma triglycerides were observed. In addition, an impaired vasorelaxation response was demonstrated in 10-week-old mice that had been exposed to arsenic in utero. These results established an experimental model in which mechanistic studies can be performed.
It is now well understood that atherosclerosis is a multifactorial disease process. More recent work also indicates that atherosclerosis may also be a multiorgan disease, with disease-modifying risks being derived from distal sites. Indeed, underlying liver disease has been shown to be a risk factor for developing atherosclerosis in humans (Brea et al., 2005; Targher et al., 2004) and is independent of other risks associated with chronic liver diseases (e.g., metabolic syndrome) (Targher et al., 2006). Whereas the mechanisms by which liver disease modulates atherosclerosis are currently poorly understood, reasonable working hypotheses exist. First, it is proposed that systemic oxidative stress and inflammation that occur during liver disease (Haukeland et al., 2006; Kugelmas et al., 2003) contribute to atherogenesis; this hypothesis is supported by the observation that the risk of atherogenesis increases with severity of hepatic inflammation (Targher et al., 2006). Second, abnormal lipid metabolism caused by underlying liver disease could contribute to atherogenesis; indeed, hepatic steatosis is associated with overproduction of VLDL in humans (Adiels et al., 2006). Furthermore, atherosclerosis shows a clear correlation with nonalcoholic fatty liver disease (NAFLD) and circulating markers of liver damage/disease associated with metabolic syndrome (Hanley et al., 2005; Schindhelm et al., 2007; Targher 2007; Targher et al., 2006; Volzke et al., 2005). Whereas most of the studies in humans have been performed with NAFLD, we propose that there are parallels between this disease and arsenic toxicity. For example, arsenic is known to cause liver damage in humans (Guha Mazumder, 2001) and animal models (Santra et al., 2000) with histological lesions in the latter analogous to those observed in advanced NAFLD. Thus, inflammation can be a cause or a consequence of liver disease. ApoE-knockout mice are constitutively hyperlipidemic, but the source of inflammation in the arsenic-exposed animals is not obvious.
Chronic arsenic exposure in adults has been associated with liver disease, notably steatosis, portal hypertension, fibrosis, and cirrhosis (Guha Mazumder, 2001). Thus, arsenic clearly is deleterious to liver. It is likely that in utero arsenic exposure affects liver development predisposing to disease. Others have demonstrated the induction of cancer in two strains of mice transplacentally exposed to arsenic (Waalkes et al., 2003, 2004, 2006). A common feature in the transplacental arsenic exposure model of As-induced carcinogenesis is the induction of hepatocellular cancer. Microarray studies of liver gene expression in these mice indicated alterations in the expression of genes related to estrogen receptor alpha (ERα) function that could contribute to the development of hepatocellular cancer in the arsenic-exposed mice (Liu et al., 2006). These gene expression changes were correlated with alterations in DNA methylation patterns in ERα (Waalkes et al., 2004), suggesting that in utero arsenic exposure caused epigenetic changes in liver. Recently, an epidemiological study of the Chilean cohort with early life arsenic exposure showed an approximately 10-fold increase in relative risk for childhood liver cancer incidence in the exposed population (Liaw et al., 2008). Thus, early life arsenic exposure clearly alters liver development and predisposes to chronic disease.
Kupffer cells are specialized macrophages that are resident in liver. These cells are a major source of cytokines that are secreted in response to infections and to hepatotoxic insult (Fainboim et al., 2007; Roberts et al., 2007). Thus, one possibility is that liver is the source of inflammatory cytokines leading to initiation of vascular disease in arsenic-exposed ApoE-knockout mice. Studies discussed in the symposium addressed the hypothesis that the in utero arsenic exposure altered liver development making the livers of exposed mice hyperresponsive to inflammatory stimuli.
Micro-RNAs (miRNAs) are small RNAs that regulate gene expression and are thought to play a critical role in modulating embryonic and fetal development (Hornstein and Shomron, 2006). Alterations in miRNAs during development are associated with alterations in histone modification and DNA methylation. Thus, miRNAs modulate epigenetic control of gene expression. If transplacental arsenic exposure is altering epigenetics in liver, then one would expect that alterations in miRNA expression would be observed in arsenic-exposed animals. Results of microarray experiments conducted to evaluate changes in liver mRNA and miRNA expression in arsenic-exposed ApoE-knockout mice on the first day of life (PND1) and at 10 weeks of age (PND70) were discussed in the symposium presentation. A 51-gene signature of mRNAs with altered expression at both PND1 and PND70 suggested that pathways associated with inflammatory responses were induced in arsenic-exposed mice. Further analyses of total mRNA and miRNA expression changes indicated that developmental trajectory was altered by arsenic exposure. Furthermore, genes common to putative targets of changed miRNAs and changed mRNAs also supported induction of pathways associated with inflammatory responses in 10-week-old arsenic-exposed ApoE-knockout mice. Thus, the data suggest that the in utero arsenic exposure altered the liver developmental program in such a way as to prime the liver to hyperrespond to an inflammatory signal.
A recent study of global gene expression changes in neonatal chord blood lymphocytes reported that stress response and inflammatory response networks were activated in human newborns exposed to arsenic in utero (Fry et al., 2007). This clear correlation between human and mouse studies support the hypothesis that in utero arsenic exposure alters developmental programming and induces a proinflammatory state. Thus, both epidemiological data and experimental data clearly show that in utero exposure to high levels of arsenic in drinking water induces vascular disease in humans and mice. The challenge for the future is to determine the impact on vascular disease of exposure to arsenic at lower levels. Elucidation of potential interacting factors such as hyperlipidemia will provide much needed information related to determining the impact of moderate-level arsenic exposure on increased mortality due to CVD in the United States and other areas of the world with low-to-moderate arsenic levels in drinking water.
ARSENIC STIMULATION OF VASCULAR REDOX SIGNALING
As discussed above, exposure to arsenic in drinking water increases risk for a number of CVDs including acute myocardial infarctions (Yuan et al., 2007), cardiac ischemic disease (Wang et al., 2007a), peripheral vascular disease (Wang et al., 2007a), systemic hypertension (Chen et al., 2007a; Kwok et al., 2007), and liver portal hypertension (Mazumder, 2005). The finding by Chen et al. of increased pulse pressure in individuals exposed to low-to-moderate levels of arsenic is consistent with a large body of literature implicating the cells in the blood vessel walls as primary targets of arsenic toxicity. In general, increased pulse pressure or enhanced peripheral resistance in hypertension results from enhanced stiffness and decreased compliance of the vessel wall. These impaired vascular functions are caused by dysregulation of vasomotor function and/or structural remodeling of blood vessels. A major drive for both disrupted signaling and remodeling is change in ROS or redox signaling in the both the vascular endothelial and the smooth muscle cells (Lee and Griendling, 2008; Lyle and Griendling, 2006).
NOX enzymes are multimeric protein complexes that produce superoxide by electron transfer from NADPH to molecular oxygen (Lee and Griendling, 2008). Increased activity of vascular NOX enzymes plays a central role in pathogenic redox signaling in vascular disease and hypertension (Lee and Griendling, 2008; Lyle and Griendling, 2006), and arsenic rapidly stimulates both endothelial (Smith et al., 2001) and smooth muscle (Lynn et al., 2000) NOX enzymes. However, the mechanism through which arsenic stimulates NOX enzyme activity remains unresolved. Previous studies demonstrated that endothelial cell NOX-generated superoxide and hydrogen peroxide levels increase within 5 min of exposure to low μM concentrations of arsenite (Barchowsky et al., 1999) and total NOX catalytic capacity remained elevated for 1 h following ex vivo arsenic exposure (Smith et al., 2001). To date, the best evidence that NOX-generated oxidants are critical mediators of the vascular actions of arsenic comes from studies investigating mechanisms for arsenic-promoted remodeling of the liver sinusoidal endothelial cells (LSECs). Exposure of mice to a moderate level of arsenic (250 μg/l) in their drinking water promoted a time-dependent closure of the LSEC fenestrations as they underwent differentiation into a normal endothelium in a process called capillarization (Straub et al., 2007a,b). This effect was dose dependent with a threshold for loss of fenestrations occurring after 2-week exposures to 10 μg/l of arsenite (Straub et al., 2008). Maximal defenestration occurred following exposure to 100 μg/l, and these exposures were also associated with increased protein nitration in the liver sinusoids and loss of scavenging of modified albumin across the liver. Capillarization and protein nitration did not occur in p47phox null mice exposed to 100 μg/l of arsenite for 2 weeks (Straub et al., 2008). Deletion of p47phox, the cytosolic regulatory subunit of multiple forms of NOXs, makes mice resistant to endogenous ligand-induced hypertension and blunts the increase in ROS production (Lee and Griendling, 2008). Further, disruption of the interaction between p47phox and gp91 of Nox2-based oxidase in primary isolates of LSEC prevented not only ex vivo arsenite-stimulated oxidant generation but also defenestration and modified albumin uptake (Straub et al., 2008). Finally, ex vivo addition of 50μM hydrogen peroxide to the LSEC was sufficient to promote defenestration. These data suggest that NOX-derived superoxide and subsequent hydrogen peroxide formation provides essential second messengers in the signaling pathway for arsenic-stimulated vessel remodeling.
The rapid increase in NOX activity and ROS second messengers suggested that the actions of arsenic were not random and that they might involve a signal amplification cascade. In support of this hypothesis, arsenite mobilizes another essential subunit of the NOX complex, Rac1-GTPase, to the endothelial cell membrane within 5 min of exposure (Smith et al., 2001). Exposing mice to arsenite also causes sustained localization of Rac1 to the LSEC membranes in vivo (Straub et al., 2007a), and inhibiting or eliminating endothelial cell Rac1-GTPase inhibits arsenic-stimulated NOX activity (Qian et al., 2005; Smith et al., 2001). In addition to activating NOX complexes, Rac1 is essential for endothelial and smooth muscle cell migration that is required for both angiogenesis and vessel remodeling (Brown et al., 2006). Thus, activation of Rac1 may represent another key regulatory step in the signal amplification scheme for arsenic-stimulated vascular cell dysfunction.
Endogenous hypertensive peptides, such as angiotensin II or endothelin-1, interact with cognate G-protein coupled receptors (GPCR) to initiate Rac1-GTPase–dependent superoxide generation by NOX enzymes (Lee and Griendling, 2008). More specifically, it is the Gαi-coupled receptor subtypes for these ligands that amplify signals to increase Rac1-GTPase activity and stimulate NOX (Brown et al., 2006). To determine whether arsenite also targeted a GPCR as an initial step in NOX activation, human microvascular endothelial cells were preincubated with Gαi-selective inhibitor, Pertussis toxin (Ptx). Relative to the effects of arsenite on untreated cells, Ptx prevented arsenite from activating Rac1-GTPase activity and increasing downstream changes in angiogenic and inflammatory gene expression. However, neither Ptx nor a direct inhibitor of Rac1 prevented arsenite from stimulating an adaptive or stress-responsive increase in heme oxygenase-1 mRNA levels.
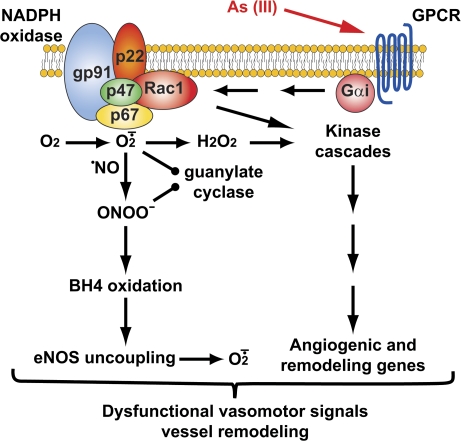
The majority of reports in the literature and the data presented support the vascular NOX system as being critical in the molecular pathology of arsenic-related vascular disease. Data from the p47phox null mice and recent epidemiological studies (Hsueh et al., 2005) provide support that NOX activity contributes to both vascular dysfunction and remodeling. The novel data presented here further define the signaling pathways that contribute to these conditions by demonstrating that arsenic interactions with vascular cells are amplified through a signaling cascade that requires both GTPase and oxidase-generated second messengers. Figure 1 presents a hypothetical scheme linking action of arsenite on a Gαi-coupled receptor to downstream NOX activation. Generation of excess reactive oxygen second messengers is a primary mechanism of hypertension and vascular disease that diminishes relaxation and promotes inappropriate signaling for proliferation and remodeling that stiffens or occludes blood vessels (Lee and Griendling, 2008; Lyle and Griendling, 2006). Arsenite may similarly increase risk of vascular disease by adding additional receptor-mediated signaling for vascular dysfunction. A major goal of current research is identification of the GPCR on endothelial cells that respond to arsenic. Further, it is important to note that the majority of cell culture–based studies of arsenite signaling have ignored the effects of methylated metabolites since the vascular cells do not significantly contribute to arsenite metabolism. However, one study that systematically analyzed dose dependence of the organic metabolite effects on cell signaling and cytotoxicity demonstrated that the trivalent MMA was approximately an order of magnitude more potent than arsenite (Hirano et al., 2004). It will be intriguing to determine whether the methylated metabolites also require GPCR receptors to produce cellular effects and whether differences in sensitivity to inorganic and organic species relate to differences in receptor affinities or actions on different receptors.
FIG. 1.
Hypothetical scheme of arsenic-stimulated redox signaling for vascular cell dysfunction. Arsenic may interact with GPCR to initiate signal amplification schemes regulating NOX-dependent redox signaling. Note that the membrane-bound NOX subunits may more likely reside in intracellular membranes. Downstream signaling for dysfunction was adapted from Lee and Griendling (2008).
SUMMARY AND FUTURE DIRECTIONS
In summary, the presentations in this symposium discussed current evidence for the association between arsenic and vascular disease from epidemiological studies and the underlying mechanisms from animal/experimental studies.
The epidemiological literature to date suggests that the cardiovascular effects of arsenic exposure are modified by nutritional factors, genetics, and arsenic metabolism capacity. In particular, the data suggest an important role played by factors related to oxidative stress and vascular inflammation. However, most of the existing epidemiological studies were conducted in populations with high levels of arsenic exposure and with a relatively small sample size. The challenges for future epidemiological studies of arsenic and vascular disease include (1) longitudinal studies to capture long-term arsenic exposure at the individual level, (2) use of reliable biomarkers, (3) inclusion of persons with low-to-moderate exposure, and (4) use of a comprehensive genomic approach to evaluate genetic susceptibility.
Findings from mechanistic studies indicate that arsenic causes inflammation in vascular tissues and activates oxidative signaling. Observed endothelial cell dysfunction is likely a consequence of direct effects on the vascular cells and indirect effects on the liver vasculature caused by arsenic exposure–promoted inflammation, disrupted lipid metabolism, and increased lipid oxidation. Thus, the liver appears to be a major site of arsenic action from the standpoint of both generation of potentially more toxic metabolites and sensitivity to toxic actions on its unique vasculature. In addition to the metabolic changes, epigenetic changes in liver also are a consequence of chronic arsenic exposure. The epigenetic changes appear to result in a chronic state of cellular stress with a predisposition to heightened inflammatory responses. These changes can result in overt liver disease and also likely play a role in development of systemic vascular disease. The role of nutrition in modulating the vascular disease manifestations of chronic arsenic exposure is likely related to modification of arsenic's impact on liver function. Clearly, more studies are needed to elucidate the interactions of arsenic exposure, liver function, nutrition, and vascular disease.
Since CVD constitutes about one-third of total mortality in the world and arsenic exposure at low-to-moderate levels is more prevalent, a small increased risk associated with arsenic exposure would translate into large numbers of excess deaths and would be of wide public health significance. Additional large epidemiological and mechanistic studies are needed to identify susceptibility factors and underlying mechanisms especially for low-level exposure.
FUNDING
The National Institutes of Health (R01ES011314, R21ES015812, F30ES013372, T32ES011564, P30ES014443, R01ES013781, R01HL65618, R01ES011594, P01ES11860, P30ES00989, P30ES000260, P42ES010349).
References
- Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, Vehkavaara S, Hakkinen A, Olofsson SO, Yki-Jarvinen H, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49(4):755–765. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Anderson WL, Kitchin KT. Dimethylarsinic acid effects on DNA damage and oxidative stress related biochemical parameters in B6C3F1 mice. Cancer Lett. 1999;139(2):129–135. doi: 10.1016/s0304-3835(99)00022-1. [DOI] [PubMed] [Google Scholar]
- Ahsan H, Chen Y, Kibriya MG, Slavkovich V, Parvez F, Jasmine F, Gamble MV, Graziano JH. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol. Biomarkers Prev. 2007;16(6):1270–1278. doi: 10.1158/1055-9965.EPI-06-0676. [DOI] [PubMed] [Google Scholar]
- Araki A, Sako Y, Fukushima Y, Matsumoto M, Asada T, Kita T. Plasma sulfhydryl-containing amino acids in patients with cerebral infarction and in hypertensive subjects. Atherosclerosis. 1989;79(2–3):139–146. doi: 10.1016/0021-9150(89)90118-4. [DOI] [PubMed] [Google Scholar]
- Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004;11(Suppl. 1):S56–S64. doi: 10.1038/sj.cdd.4401451. [DOI] [PubMed] [Google Scholar]
- Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radic. Biol. Med. 1999;27(11–12):1405–1412. doi: 10.1016/s0891-5849(99)00186-0. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, Meyer J. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104(12):1336–1342. doi: 10.1161/hc3701.095949. [DOI] [PubMed] [Google Scholar]
- Brea A, Mosquera D, Martin E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: A case-control study. Arterioscler. Thromb. Vasc. Biol. 2005;25(5):1045–1050. doi: 10.1161/01.ATV.0000160613.57985.18. [DOI] [PubMed] [Google Scholar]
- Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: A decade of hypertrophic signaling hits. Circ. Res. 2006;98(6):730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- Bunderson M, Brooks DM, Walker DL, Rosenfeld ME, Coffin JD, Beall HD. Arsenic exposure exacerbates atherosclerotic plaque formation and increases nitrotyrosine and leukotriene biosynthesis. Toxicol. Appl. Pharmacol. 2004;201(1):32–39. doi: 10.1016/j.taap.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler. Thromb. Vasc. Biol. 1996;16(4):504–510. doi: 10.1161/01.atv.16.4.504. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Hsueh YM, Lai MS, Shyu MP, Chen SY, Wu MM, Kuo TL, Tai TY. Increased prevalence of hypertension and long-term arsenic exposure. Hypertension. 1995;25(1):53–60. [PubMed] [Google Scholar]
- Chen Y, Factor-Litvak P, Howe GR, Graziano JH, Brandt-Rauf P, Parvez F, van GA, Ahsan H. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: A population-based, cross-sectional study. Am. J. Epidemiol. 2007a;165(5):541–552. doi: 10.1093/aje/kwk037. [DOI] [PubMed] [Google Scholar]
- Chen Y, Santella RM, Kibriya MG, Wang Q, Kappil M, Verret WJ, Graziano JH, Ahsan H. Association between arsenic exposure from drinking water and plasma levels of soluble cell adhesion molecules. Environ. Health Perspect. 2007b;115(10):1415–1420. doi: 10.1289/ehp.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Guo YL, Su HJ, Hsueh YM, Smith TJ, Ryan LM, Lee MS, Chao SC, Lee JY, Christiani DC. Arsenic methylation and skin cancer risk in southwestern Taiwan. J. Occup. Environ. Med. 2003a;45(3):241–248. doi: 10.1097/01.jom.0000058336.05741.e8. [DOI] [PubMed] [Google Scholar]
- Chen YC, Lin-Shiau SY, Lin JK. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J. Cell. Physiol. 1998;177(2):324–333. doi: 10.1002/(SICI)1097-4652(199811)177:2<324::AID-JCP14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Chen YC, Su HJ, Guo YL, Hsueh YM, Smith TJ, Ryan LM, Lee MS, Christiani DC. Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Control. 2003b;14(4):303–310. doi: 10.1023/a:1023905900171. [DOI] [PubMed] [Google Scholar]
- Chiou HY, Hsueh YM, Hsieh LL, Hsu LI, Hsu YH, Hsieh FI, Wei ML, Chen HC, Yang HT, Leu LC, et al. Arsenic methylation capacity, body retention, and null genotypes of glutathione S-transferase M1 and T1 among current arsenic-exposed residents in Taiwan. Mutat. Res. 1997;386(3):197–207. doi: 10.1016/s1383-5742(97)00005-7. [DOI] [PubMed] [Google Scholar]
- Dart AM, Kingwell BA. Pulse pressure—a review of mechanisms and clinical relevance. J. Am. Coll. Cardiol. 2001;37(4):975–984. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23) Suppl. 1:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- Du Y, Wang K, Fang H, Li J, Xiao D, Zheng P, Chen Y, Fan H, Pan X, Zhao C, et al. Coordination of intrinsic, extrinsic, and endoplasmic reticulum-mediated apoptosis by imatinib mesylate combined with arsenic trioxide in chronic myeloid leukemia. Blood. 2006;107(4):1582–1590. doi: 10.1182/blood-2005-06-2318. [DOI] [PubMed] [Google Scholar]
- Engel RR, Smith AH. Arsenic in drinking water and mortality from vascular disease: An ecologic analysis in 30 counties in the United States. Arch. Environ. Health. 1994;49(5):418–427. doi: 10.1080/00039896.1994.9954996. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Fainboim L, Chernavsky A, Paladino N, Flores AC, Arruvito L. Cytokines and chronic liver disease. Cytokine Growth Factor Rev. 2007;18(1–2):143–157. doi: 10.1016/j.cytogfr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, Luo M, Bhattacharya S, Kandjanapa K, Soontararuks S, Nookabkaew S, et al. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. PLoS Genet. 2007;3(11):e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Ahsan H, Pilsner R, Ilievski V, Slavkovich V, Parvez F, Levy D, Factor-Litvak P, Graziano JH. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ. Health Perspect. 2005;113(12):1683–1688. doi: 10.1289/ehp.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargalovic PS, Imura M, Zhang B, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Patel S, et al. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc. Natl. Acad. Sci. USA. 2006;103(34):12741–12746. doi: 10.1073/pnas.0605457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J, Afek A, Gilburd B, Levkovitz H, Shaish A, Goldberg I, Kopolovic Y, Wick G, Shoenfeld Y, Harats D. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis. 1998;138(1):147–152. doi: 10.1016/s0021-9150(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell. 1998;2(2):275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- Guha Mazumder DN. Arsenic and liver disease. J. Indian Med. Assoc. 2001;99(6) 311, 314–315, 320. [PubMed] [Google Scholar]
- Hanley AJ, Williams K, Festa A, Wagenknecht LE, D'Agostino RB, Jr, Haffner SM. Liver markers and development of the metabolic syndrome: The insulin resistance atherosclerosis study. Diabetes. 2005;54(11):3140–3147. doi: 10.2337/diabetes.54.11.3140. [DOI] [PubMed] [Google Scholar]
- Haukeland JW, Damas JK, Konopski Z, Loberg EM, Haaland T, Goverud I, Torjesen PA, Birkeland K, Bjoro K, Aukrust P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J. Hepatol. 2006;44(6):1167–1174. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 1999;31(4):273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995;30(6):445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Hirano S, Kobayashi Y, Cui X, Kanno S, Hayakawa T, Shraim A. The accumulation and toxicity of methylated arsenicals in endothelial cells: Important roles of thiol compounds. Toxicol. Appl. Pharmacol. 2004;198(3):458–467. doi: 10.1016/j.taap.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Hornstein E, Shomron N. Canalization of development by microRNAs. Nat. Genet. 2006;38(Suppl.):S20–S24. doi: 10.1038/ng1803. [DOI] [PubMed] [Google Scholar]
- Hsieh YC, Hsieh FI, Lien LM, Chou YL, Chiou HY, Chen CJ. Risk of carotid atherosclerosis associated with genetic polymorphisms of apolipoprotein E and inflammatory genes among arsenic exposed residents in Taiwan. Toxicol. Appl. Pharmacol. 2008;227(1):1–7. doi: 10.1016/j.taap.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Hsueh YM, Chiou HY, Huang YL, Wu WL, Huang CC, Yang MH, Lue LC, Chen GS, Chen CJ. Serum beta-carotene level, arsenic methylation capability, and incidence of skin cancer. Cancer Epidemiol. Biomarkers Prev. 1997;6(8):589–596. [PubMed] [Google Scholar]
- Hsueh YM, Lin P, Chen HW, Shiue HS, Chung CJ, Tsai CT, Huang YK, Chiou HY, Chen CJ. Genetic polymorphisms of oxidative and antioxidant enzymes and arsenic-related hypertension. J. Toxicol. Environ. Health A. 2005;68(17–18):1471–1484. doi: 10.1080/15287390590967414. [DOI] [PubMed] [Google Scholar]
- Hsueh YM, Wu WL, Huang YL, Chiou HY, Tseng CH, Chen CJ. Low serum carotene level and increased risk of ischemic heart disease related to long-term arsenic exposure. Atherosclerosis. 1998;141(2):249–257. doi: 10.1016/s0021-9150(98)00178-6. [DOI] [PubMed] [Google Scholar]
- Huang YK, Tseng CH, Huang YL, Yang MH, Chen CJ, Hsueh YM. Arsenic methylation capability and hypertension risk in subjects living in arseniasis-hyperendemic areas in southwestern Taiwan. Toxicol. Appl. Pharmacol. 2007;218(2):135–142. doi: 10.1016/j.taap.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr, Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: The Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96(12):4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- Jurgens G, Chen Q, Esterbauer H, Mair S, Ledinski G, Dinges HP. Immunostaining of human autopsy aortas with antibodies to modified apolipoprotein B and apoprotein(a) Arterioscler. Thromb. 1993;13(11):1689–1699. doi: 10.1161/01.atv.13.11.1689. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Davis R, Gwinn M, Lindegren ML, Yoon P. Do we need genomic research for the prevention of common diseases with environmental causes? Am. J. Epidemiol. 2005;161(9):799–805. doi: 10.1093/aje/kwi113. [DOI] [PubMed] [Google Scholar]
- Kim EH, Yoon MJ, Kim SU, Kwon TK, Sohn S, Choi KS. Arsenic trioxide sensitizes human glioma cells, but not normal astrocytes, to TRAIL-induced apoptosis via CCAAT/enhancer-binding protein homologous protein-dependent DR5 up-regulation. Cancer Res. 2008;68(1):266–275. doi: 10.1158/0008-5472.CAN-07-2444. [DOI] [PubMed] [Google Scholar]
- Kitchin KT. Recent advances in arsenic carcinogenesis: Modes of action, animal model systems, and methylated arsenic metabolites. Toxicol. Appl. Pharmacol. 2001;172(3):249–261. doi: 10.1006/taap.2001.9157. [DOI] [PubMed] [Google Scholar]
- Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: A pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38(2):413–419. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- Kwok RK, Mendola P, Liu ZY, Savitz DA, Heiss G, Ling HL, Xia Y, Lobdell D, Zeng D, Thorp JM, Jr, et al. Drinking water arsenic exposure and blood pressure in healthy women of reproductive age in Inner Mongolia, China. Toxicol. Appl. Pharmacol. 2007;222(3):337–343. doi: 10.1016/j.taap.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Lau AT, He QY, Chiu JF. A proteome analysis of the arsenite response in cultured lung cells: Evidence for in vitro oxidative stress-induced apoptosis. Biochem. J. 2004;382(Pt 2):641–650. doi: 10.1042/BJ20040224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid. Redox. Signal. 2008;10(6):1045–1059. doi: 10.1089/ars.2007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PC, Ho IC, Lee TC. Oxidative stress mediates sodium arsenite-induced expression of heme oxygenase-1, monocyte chemoattractant protein-1, and interleukin-6 in vascular smooth muscle cells. Toxicol. Sci. 2005;85(1):541–550. doi: 10.1093/toxsci/kfi101. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Haimeur A, Waalkes MP. Arsenic transport by the human multidrug resistance protein 1 (MRP1/ABCC1). Evidence that a tri-glutathione conjugate is required. J. Biol. Chem. 2004;279(31):32700–32708. doi: 10.1074/jbc.M404912200. [DOI] [PubMed] [Google Scholar]
- Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, Calderon RL. Drinking water arsenic in Utah: A cohort mortality study. Environ. Health Perspect. 1999;107(5):359–365. doi: 10.1289/ehp.99107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw J, Marshall G, Yuan Y, Ferreccio C, Steinmaus C, Smith AH. Increased childhood liver cancer mortality and arsenic in drinking water in northern Chile. Cancer Epidemiol. Biomarkers Prev. 2008;17(8):1982–1987. doi: 10.1158/1055-9965.EPI-07-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim U, Cassano PA. Homocysteine and blood pressure in the Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Epidemiol. 2002;156(12):1105–1113. doi: 10.1093/aje/kwf157. [DOI] [PubMed] [Google Scholar]
- Liu J, Xie Y, Ducharme DM, Shen J, Diwan BA, Merrick BA, Grissom SF, Tucker CJ, Paules RS, Tennant R, et al. Global gene expression associated with hepatocarcinogenesis in adult male mice induced by in utero arsenic exposure. Environ. Health Perspect. 2006;114(3):404–411. doi: 10.1289/ehp.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda) 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- Lynn S, Gurr JR, Lai HT, Jan KY. NADH oxidase activation is involved in arsenite-induced oxidative DNA damage in human vascular smooth muscle cells. Circ. Res. 2000;86(5):514–519. doi: 10.1161/01.res.86.5.514. [DOI] [PubMed] [Google Scholar]
- Mazumder DN. Effect of chronic intake of arsenic-contaminated water on liver. Toxicol. Appl. Pharmacol. 2005;206(2):169–175. doi: 10.1016/j.taap.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Meliker JR, Wahl RL, Cameron LL, Nriagu JO. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: A standardized mortality ratio analysis. Environ. Health. 2007;6:4. doi: 10.1186/1476-069X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Sharrett AR, Silbergeld EK, Schwartz BS, Nachman KE, Burke TA, Guallar E. Arsenic exposure and cardiovascular disease: A systematic review of the epidemiologic evidence. Am. J. Epidemiol. 2005;162(11):1037–1049. doi: 10.1093/aje/kwi330. [DOI] [PubMed] [Google Scholar]
- Nesnow S, Roop BC, Lambert G, Kadiiska M, Mason RP, Cullen WR, Mass MJ. DNA damage induced by methylated trivalent arsenicals is mediated by reactive oxygen species. Chem. Res. Toxicol. 2002;15(12):1627–1634. doi: 10.1021/tx025598y. [DOI] [PubMed] [Google Scholar]
- Ochi T, Kaise T, Oya-Ohta Y. Glutathione plays different roles in the induction of the cytotoxic effects of inorganic and organic arsenic compounds in cultured BALB/c 3T3 cells. Experientia. 1994;50(2):115–120. doi: 10.1007/BF01984946. [DOI] [PubMed] [Google Scholar]
- Paisley KE, Beaman M, Tooke JE, Mohamed-Ali V, Lowe GD, Shore AC. Endothelial dysfunction and inflammation in asymptomatic proteinuria. Kidney Int. 2003;63(2):624–633. doi: 10.1046/j.1523-1755.2003.00768.x. [DOI] [PubMed] [Google Scholar]
- Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew TE, Steinberg D, Witztum JL. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl. Acad. Sci. USA. 1989;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu YS, Yang SM, Huang YK, Chung CJ, Huang SK, Chiu AW, Yang MH, Chen CJ, Hsueh YM. Urinary arsenic profile affects the risk of urothelial carcinoma even at low arsenic exposure. Toxicol. Appl. Pharmacol. 2007;218(2):99–106. doi: 10.1016/j.taap.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Qian Y, Liu KJ, Chen Y, Flynn DC, Castranova V, Shi X. Cdc42 regulates arsenic-induced NADPH oxidase activation and cell migration through actin filament reorganization. J. Biol. Chem. 2005;280(5):3875–3884. doi: 10.1074/jbc.M403788200. [DOI] [PubMed] [Google Scholar]
- Rahman M, Tondel M, Ahmad SA, Chowdhury IA, Faruquee MH, Axelson O. Hypertension and arsenic exposure in Bangladesh. Hypertension. 1999;33(1):74–78. doi: 10.1161/01.hyp.33.1.74. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351(9096):88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- Roberts RA, Ganey PE, Ju C, Kamendulis LM, Rusyn I, Klaunig JE. Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol. Sci. 2007;96(1):2–15. doi: 10.1093/toxsci/kfl173. [DOI] [PubMed] [Google Scholar]
- Rosenberg HG. Systemic arterial disease with myocardial infarction. Report on two infants. Circulation. 1973;47(2):270–275. doi: 10.1161/01.cir.47.2.270. [DOI] [PubMed] [Google Scholar]
- Rosenberg HG. Systemic arterial disease and chronic arsenicism in infants. Arch. Pathol. 1974;97(6):360–365. [PubMed] [Google Scholar]
- Rosenfeld ME, Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of WHHL and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1990;10(5):680–687. doi: 10.1161/01.atv.10.5.680. [DOI] [PubMed] [Google Scholar]
- Roybal CN, Hunsaker LA, Barbash O, Vander Jagt DL, Abcouwer SF. The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J. Biol. Chem. 2005;280(21):20331–20339. doi: 10.1074/jbc.M411275200. [DOI] [PubMed] [Google Scholar]
- Roybal CN, Yang S, Sun CW, Hurtado D, Vander Jagt DL, Townes TM, Abcouwer SF. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J. Biol. Chem. 2004;279(15):14844–14852. doi: 10.1074/jbc.M312948200. [DOI] [PubMed] [Google Scholar]
- Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107(22):2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- Santra A, Maiti A, Das S, Lahiri S, Charkaborty SK, Mazumder DN. Hepatic damage caused by chronic arsenic toxicity in experimental animals. J. Toxicol. Clin. Toxicol. 2000;38(4):395–405. doi: 10.1081/clt-100100949. [DOI] [PubMed] [Google Scholar]
- Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, Trautwein C, Luchtefeld M, Schmittkamp C, Heeneman S, et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110(22):3493–3500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CD, Heine RJ, Diamant M. Alanine aminotransferase predicts coronary heart disease events: A 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007;191(2):391–396. doi: 10.1016/j.atherosclerosis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J. Nutr. Health Aging. 2002;6(1):39–42. [PubMed] [Google Scholar]
- Simeonova PP, Hulderman T, Harki D, Luster MI. Arsenic exposure accelerates atherogenesis in apolipoprotein E(-/-) mice. Environ. Health Perspect. 2003;111(14):1744–1748. doi: 10.1289/ehp.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeonova PP, Luster MI. Arsenic and atherosclerosis. Toxicol. Appl. Pharmacol. 2004;198(3):444–449. doi: 10.1016/j.taap.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von EO, Steinmaus C, Bates MN, Selvin S. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ. Health Perspect. 2006;114(8):1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Klei LR, Barchowsky A. Arsenite stimulates plasma membrane NADPH oxidase in vascular endothelial cells. Am. J. Physiol Lung Cell. Mol. Physiol. 2001;280(3):L442–L449. doi: 10.1152/ajplung.2001.280.3.L442. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Chandra A, Ansari NH, Srivastava SK, Bhatnagar A. Identification of cardiac oxidoreductase(s) involved in the metabolism of the lipid peroxidation-derived aldehyde-4-hydroxynonenal. Biochem. J. 1998a;329:469–475. doi: 10.1042/bj3290469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Chandra A, Wang LF, Seifert WE, Jr, Dague BB, Ansari NH, Srivastava SK, Bhatnagar A. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. J. Biol. Chem. 1998b;273(18):10893–10900. doi: 10.1074/jbc.273.18.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Conklin DJ, Liu SQ, Prakash N, Boor PJ, Srivastava SK, Bhatnagar A. Identification of biochemical pathways for the metabolism of oxidized low-density lipoprotein derived aldehyde-4-hydroxy trans-2-nonenal in vascular smooth muscle cells. Atherosclerosis. 2001;158(2):339–350. doi: 10.1016/s0021-9150(01)00454-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, D'Souza SE, Sen U, States JC. In utero arsenic exposure induces early onset of atherosclerosis in ApoE-/- mice. Reprod. Toxicol. 2007;23(3):449–456. doi: 10.1016/j.reprotox.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Spite M, Trent JO, West MB, Ahmed Y, Bhatnagar A. Aldose reductase-catalyzed reduction of aldehyde phospholipids. J. Biol. Chem. 2004;279(51):53395–53406. doi: 10.1074/jbc.M403416200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Watowich SJ, Petrash JM, Srivastava SK, Bhatnagar A. Structural and kinetic determinants of aldehyde reduction by aldose reductase. Biochemistry. 1999;38(1):42–54. doi: 10.1021/bi981794l. [DOI] [PubMed] [Google Scholar]
- Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: Further evidence for a link between microalbuminuria and endothelial dysfunction–the Hoorn Study. Kidney Int. Suppl. 2004;92:S42–S44. doi: 10.1111/j.1523-1755.2004.09211.x. [DOI] [PubMed] [Google Scholar]
- Straub AC, Clark KA, Ross MA, Chandra AG, Li S, Gao X, Pagano PJ, Stolz DB, Barchowsky A. Arsenic-stimulated liver sinusoidal capillarization in mice requires NADPH oxidase-generated superoxide. J. Clin. Invest. 2008 doi: 10.1172/JCI35092. doi:10.1172/JCI35092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub AC, Stolz DB, Ross MA, Hernandez-Zavala A, Soucy NV, Klei LR, Barchowsky A. Arsenic stimulates sinusoidal endothelial cell capillarization and vessel remodeling in mouse liver. Hepatology. 2007a;45(1):205–212. doi: 10.1002/hep.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub AC, Stolz DB, Vin H, Ross MA, Soucy NV, Klei LR, Barchowsky A. Low level arsenic promotes progressive inflammatory angiogenesis and liver blood vessel remodeling in mice. Toxicol. Appl. Pharmacol. 2007b;222(3):327–336. doi: 10.1016/j.taap.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styblo M, Drobna Z, Jaspers I, Lin S, Thomas DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic: A research update. Environ. Health Perspect. 2002;110(Suppl. 5):767–771. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: The plot thickens. Diabet. Med. 2007;24(1):1–6. doi: 10.1111/j.1464-5491.2007.02025.x. [DOI] [PubMed] [Google Scholar]
- Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Zenari L, Cigolini M, Falezza G, Arcaro G. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006;29(6):1325–1330. doi: 10.2337/dc06-0135. [DOI] [PubMed] [Google Scholar]
- Targher G, Bertolini L, Padovani R, Zenari L, Zoppini G, Falezza G. Relation of nonalcoholic hepatic steatosis to early carotid atherosclerosis in healthy men: Role of visceral fat accumulation. Diabetes Care. 2004;27(10):2498–2500. doi: 10.2337/diacare.27.10.2498. [DOI] [PubMed] [Google Scholar]
- Tseng CH, Tai TY, Chong CK, Tseng CP, Lai MS, Lin BJ, Chiou HY, Hsueh YM, Hsu KH, Chen CJ. Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: A cohort study in arseniasis-hyperendemic villages in Taiwan. Environ. Health Perspect. 2000;108(9):847–851. doi: 10.1289/ehp.00108847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M, Concha G. Role of metabolism in arsenic toxicity. Pharmacol. Toxicol. 2001;89(1):1–5. doi: 10.1034/j.1600-0773.2001.d01-128.x. [DOI] [PubMed] [Google Scholar]
- Volzke H, Robinson DM, Kleine V, Deutscher R, Hoffmann W, Ludemann J, Schminke U, Kessler C, John U. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J. Gastroenterol. 2005;11(12):1848–1853. doi: 10.3748/wjg.v11.i12.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Chen H, Xie Y, Achanzar WE, Zhou YS, Cheng ML, Diwan BA. Estrogen signaling in livers of male mice with hepatocellular carcinoma induced by exposure to arsenic in utero. J Natl. Cancer Inst. 2004;96(6):466–474. doi: 10.1093/jnci/djh070. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Ward JM, Diwan BA. Enhanced urinary bladder and liver carcinogenesis in male CD1 mice exposed to transplacental inorganic arsenic and postnatal diethylstilbestrol or tamoxifen. Toxicol. Appl. Pharmacol. 2006;215(3):295–305. doi: 10.1016/j.taap.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Ward JM, Liu J, Diwan BA. Transplacental carcinogenicity of inorganic arsenic in the drinking water: Induction of hepatic, ovarian, pulmonary, and adrenal tumors in mice. Toxicol. Appl. Pharmacol. 2003;186(1):7–17. doi: 10.1016/s0041-008x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- Wang CH, Hsiao CK, Chen CL, Hsu LI, Chiou HY, Chen SY, Hsueh YM, Wu MM, Chen CJ. A review of the epidemiologic literature on the role of environmental arsenic exposure and cardiovascular diseases. Toxicol. Appl. Pharmacol. 2007a;222(3):315–326. doi: 10.1016/j.taap.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Wang CH, Jeng JS, Yip PK, Chen CL, Hsu LI, Hsueh YM, Chiou HY, Wu MM, Chen CJ. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation. 2002;105(15):1804–1809. doi: 10.1161/01.cir.0000015862.64816.b2. [DOI] [PubMed] [Google Scholar]
- Wang TS, Kuo CF, Jan KY, Huang H. Arsenite induces apoptosis in Chinese hamster ovary cells by generation of reactive oxygen species. J. Cell. Physiol. 1996;169(2):256–268. doi: 10.1002/(SICI)1097-4652(199611)169:2<256::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Wang YH, Wu MM, Hong CT, Lien LM, Hsieh YC, Tseng HP, Chang SF, Su CL, Chiou HY, Chen CJ. Effects of arsenic exposure and genetic polymorphisms of p53, glutathione S-transferase M1, T1, and P1 on the risk of carotid atherosclerosis in Taiwan. Atherosclerosis. 2007b;192(2):305–312. doi: 10.1016/j.atherosclerosis.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 Suppl. 1):I, I2–I10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- Wu MM, Chiou HY, Ho IC, Chen CJ, Lee TC. Gene expression of inflammatory molecules in circulating lymphocytes from arsenic-exposed human subjects. Environ. Health Perspect. 2003;111(11):1429–1438. doi: 10.1289/ehp.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Chiou HY, Hsueh YM, Hong CT, Su CL, Chang SF, Huang WL, Wang HT, Wang YH, Hsieh YC, et al. Effect of plasma homocysteine level and urinary monomethylarsonic acid on the risk of arsenic-associated carotid atherosclerosis. Toxicol. Appl. Pharmacol. 2006;216(1):168–175. doi: 10.1016/j.taap.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yang Y, Xu Y, Lick SD, Awasthi YC, Boor PJ. Endothelial glutathione-S-transferase A4-4 protects against oxidative stress and modulates iNOS expression through NF-kappaB translocation. Toxicol. Appl. Pharmacol. 2008;230(2):187–196. doi: 10.1016/j.taap.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RC, Hsu KH, Chen CJ, Froines JR. Arsenic methylation capacity and skin cancer. Cancer Epidemiol. Biomarkers Prev. 2000;9(11):1259–1262. [PubMed] [Google Scholar]
- Yuan Y, Marshall G, Ferreccio C, Steinmaus C, Selvin S, Liaw J, Bates MN, Smith AH. Acute myocardial infarction mortality in comparison with lung and bladder cancer mortality in arsenic-exposed region II of Chile from 1950 to 2000. Am. J. Epidemiol. 2007;166(12):1381–1391. doi: 10.1093/aje/kwm238. [DOI] [PubMed] [Google Scholar]
- Zakharyan RA, Aposhian HV. Arsenite methylation by methylvitamin B12 and glutathione does not require an enzyme. Toxicol. Appl. Pharmacol. 1999;154(3):287–291. doi: 10.1006/taap.1998.8587. [DOI] [PubMed] [Google Scholar]
- Zhang H, Duncan G, Wang L, Liu P, Cui H, Reddan JR, Yang BF, Wormstone IM. Arsenic trioxide initiates ER stress responses, perturbs calcium signalling and promotes apoptosis in human lens epithelial cells. Exp. Eye Res. 2007;85(6):825–835. doi: 10.1016/j.exer.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111(14):1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001;21(1):108–114. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]
- Zierold KM, Knobeloch L, Anderson H. Prevalence of chronic diseases in adults exposed to arsenic-contaminated drinking water. Am. J. Public Health. 2004;94(11):1936–1937. doi: 10.2105/ajph.94.11.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]