Abstract
Purpose
We have previously reported on the safety and immunological response of a poxvirus-based vaccine encoding PSA used in combination with radiation therapy in patients with localized prostate cancer. We hypothesized that a “metronomic” dose of IL-2 as a biologic adjuvant would cause less toxicity while maintaining immunological response.
Experimental Design
Eighteen patients with localized prostate cancer were treated in a single-arm trial using previously established doses of vaccine and radiation therapy. The vaccine used was a recombinant vaccinia (rV) virus engineered to encode PSA admixed with an rV encoding the costimulatory molecule B7.1, followed by booster vaccinations with a recombinant fowlpox vector expressing PSA. Patients received a total of 8 planned vaccination cycles, once every 4 weeks, with GM-CSF administered on days 1 to 4 and IL-2 at a dose of 0.6 MIU/M2 administered from days 8 to 21 following each vaccination. Definitive external beam radiation therapy was initiated following the third vaccination cycle. Patients were evaluated for safety and immunological response. Toxicity and immunological activity were compared to the previously reported regimen containing a higher dose of IL-2.
Results
Seventeen of 18 patients received all 8 cycles of vaccine with IL-2. Five of 8 HLA-A2+ patients evaluated had an increase in PSA-specific T cells of ≥ 3-fold. Toxicities were generally mild, with only 7 vaccination cycles out of 140 administered resulting in grade 3 toxicities possibly attributable to IL-2.
Conclusions
Metronomic-dose IL-2 in combination with vaccine and radiation therapy is safe, can induce prostate-specific immune responses, and has immunologic activity similar to low-dose IL-2, with markedly reduced toxicities.
Keywords: cancer vaccine, interleukin-2, prostate cancer, metronomic
Introduction
Optimal treatment of localized prostate cancer remains controversial. Standard therapy with curative intent includes either surgical prostatectomy or external beam radiation therapy (EBRT) with or without androgen-deprivation therapy (ADT). Despite advances in radiation therapy approaches and surgical technique, a significant proportion of patients still relapse, often due to occult metastatic disease (1-3). The addition of ADT to EBRT has been shown to prolong survival in patients with high-risk disease (4-8). Similarly, the addition of ADT following radical prostatectomy in patients with micrometastatic lymph node involvement may improve overall survival (9). Whether other adjuvant therapies can decrease recurrence of localized prostate cancer remains a significant clinical problem and a focus of ongoing studies. We postulated that a well-tolerated systemic therapy such as vaccination could target micrometastatic disease when given with radiation therapy. Furthermore, radiation has the potential to alter the tumor phenotype, making irradiated tumor more amenable to immune-mediated killing. In addition, the combination of vaccine and radiation therapy has shown synergistic preclinical antitumor activity (10, 11). Thus, the addition of an effective vaccine could target the primary tumor as well as occult metastatic disease.
We initially evaluated whether a combination approach using a vaccine targeting prostate-specific antigen (PSA) (12), safely combined with EBRT in patients with localized or locally advanced prostate cancer, could induce immune responses specific to the vaccine. In our original study (13), patients were randomized to receive either EBRT with PSA vaccine or EBRT alone. ADT was allowed in either arm if clinically appropriate. The primary endpoint of that study was immunological response specific to the vaccine. Patients treated with EBRT and vaccine, but not those treated with EBRT alone, had a significant increase in PSA-specific T-cell responses (13). This trial demonstrated the safety and feasibility of coadministration of a PSA-specific therapeutic cancer vaccine with radiation therapy, but was not powered to demonstrate a benefit in overall survival or time to progression.
In the trial described above, however, significant toxicities were associated with the administration of adjuvant interleukin-2 (IL-2). IL-2 is a cytokine that has pleiotropic effects on T-cell function, depending on the context in which it is administered (14). IL-2 is approved for use in the United States as monotherapy for metastatic renal cell carcinoma and metastatic melanoma. The doses used for these indications—600 to 720 kU/kg (approximately 25-30 MIU/M2) administered every 8 h for up to 15 doses—are associated with significant toxicity (15). Even at the much lower dose of 4 MIU/M2 used in our study (designated standard adjuvant dose or S-IL-2), there were considerable symptomatic toxicities attributable to IL-2 (13).
At higher doses, IL-2 is not only more toxic but may be associated with negative regulation of immune response. Examination of T-cell subsets after administration of high-dose IL-2 for melanoma and renal carcinoma revealed an increase in T regulatory cells (Tregs) and a decrease in natural killer (NK) cells (16). Trials of lower-dose IL-2 (approximately 1 to 2 MIU/day) in patients with AIDS have shown a beneficial effect on expansion of the T-cell compartment, with reduced toxicity (17-19). Because of the observed toxicities and data from studies suggesting that S-IL-2 could expand the activated T-cell compartment, we sought to determine if nearly continuous, very low-dose IL-2 (designated metronomic adjuvant-dose or M-IL-2) could serve as a useful vaccine adjuvant in the same clinical setting.
Patients and Methods
Patient selection and trial design
Entry and exclusion criteria and design for this trial were the same as previously described (13); an exception in the trial reported here was that not all patients receiving M-IL-2 were required to be HLA-A2+. Briefly, patients had biopsy-proven prostatic adenocarcinoma and were considered candidates for definitive EBRT. All patients in the current study were vaccinated on a 4-week cycle with a priming dose of vaccinia PSA admixed with vaccinia B7.1, followed by boosts with fowlpox PSA for a total of 8 planned cycles. GM-CSF was administered at 100 μg/day at the vaccination site on days 1 to 4 of each cycle, and IL-2 was administered at 0.6 MIU/M2 on days 8 to 21 as a subcutaneous injection. Since approximately 1 week is needed following poxviral vector vaccination to induce a response, it was believed that starting the low-dose systemic IL-2 administration 8 days post-vaccination would preferentially boost the expansion of new antigen-specific effector cells. By dosing through day 21, the total amount of IL-2 given was similar to that given in the “standard-dose” IL-2 arm. Stopping at day 21 allowed for some resting of the T cells prior to the next vaccine (day 29). This dose was designed to provide saturation of high-affinity IL-2 receptors for 10 h/day (20, 21). All patients received EBRT, and 14 patients received ADT at the discretion of their treating physicians. All protocols were approved by the Institutional Review Board of the National Cancer Institute.
Immunological assays
Collection of mononuclear cells by apheresis, ELISPOT assays, and serologic analysis were performed as previously described (13).
Flow cytometry analysis
Three-color flow cytometry analysis was performed on peripheral blood mononuclear cells (PBMCs) for phenotypic characterization of Tregs. Cells were resuspended in staining buffer (PBS containing 3% fetal bovine serum) and stained for 30 min at 4°C with PerCP-Cy5.5-conjugated anti-CD4 and phycoerythrin (PE)-conjugated anti-CD25 (both from BD Biosciences, San Diego, CA). FoxP3 intracellular staining was performed on the cells stained with anti-CD4 and anti-CD25. Cells were fixed and permeabilized using a fix/perm kit (eBioscience, San Diego, CA) according to the manufacturer's instructions, then labeled with fluorescein isothiocyanate (FITC)-conjugated anti-FoxP3 antibody (PCH101 clone) or its isotype control antibody (eBioscience) as a negative control. Flow cytometry was performed on a Becton Dickinson LSRII (BD Biosciences); 1 × 105 cells were acquired and data were analyzed using FlowJo software (BD Biosciences). To determine the percentage of Tregs, lymphocytes were gated by plotting forward vs. side scatter, followed by gating of the CD4+ population. Then the CD25high and FoxP3+ populations were gated. The CD25high population was separated from the CD25low population on the basis of the level of CD25 expression in CD4− T cells, as previously described. A similar procedure was used for flow cytometry analysis of NK cells. PBMCs were stained with FITC-conjugated anti-CD3 and PE-conjugated anti-CD56 (BD Biosciences) and analyzed as described above (22, 23).
Statistical considerations
The toxicity profile of metronomic dosing of IL-2 was evaluated using a trial design based on a method for estimating the size of a single-stage, single-arm trial (24). Assuming that the probability of avoiding dose reduction in the S-IL-2 arm was no more than 10%, we wished to rule out 10% without reductions in favor of a 50% chance of avoiding a dose reduction. With alpha = 0.05 and beta = 0.1, it was considered unacceptable if ≤ 3 of the first 12 patients enrolled in the M-IL-2 arm did not require dose reductions, and acceptable if 4 to 12 patients avoided dose reductions. The lower bound of a 95% one-sided confidence interval about 4/12 is approximately 12%, thus demonstrating superiority to previously published results of studies using S-IL-2. Because only 3 of the first 12 patients enrolled were HLA-A2+, and 9 was the intended minimum number for evaluation, up to 7 additional HLA-A2+ patients were allowed to enroll in the M-IL-2 arm in order to obtain sufficient immunologic data.
Results
A comparison of baseline characteristics of patients receiving M-IL-2 and patients receiving S-IL-2 (Table 1) reveals that the 2 treatment arms were similar in age, ethnicity, Gleason score or disease stage, on-study PSA, and use of ADT. PSA at the time of diagnosis did not differ significantly between the 2 groups (P = NS by Student's 2-sided t test) and disease stage was also similar (not shown). Of the 19 patients in the S-IL-2 arm, 17 completed the course of 8 vaccinations. One patient withdrew from the study after a single vaccine cycle to receive immediate EBRT, and one patient was diagnosed with invasive bladder cancer after 3 cycles and was taken off study. Thus, a total of 140 vaccinations were administered to patients in the S-IL-2 arm. In the M-IL-2 arm, one patient withdrew after 4 cycles due to persistent lymphopenia attributed to EBRT; 17 completed all 8 vaccine cycles for a total of 140 cycles.
Table 1.
Patient demographics.
| Vaccine + S-IL-2a | Vaccine + M-IL-2b | |||
|---|---|---|---|---|
| n (range) | % | n (range) | % | |
| Patients enrolled | 19 | 18 | ||
| Median age | 59 (50–77) | 63 (52–75) | ||
| Race/ethnicity | ||||
| White | 15 | 78.9 | 15 | 83.3 |
| African-American | 2 | 10.5 | 3 | 16.7 |
| Hispanic | 2 | 10.5 | 0 | 0 |
| Gleason score | ||||
| 5 | 2 | 10.5 | 0 | 0 |
| 6 | 5 | 26.3 | 4 | 22.2 |
| 7 | 5 | 26.3 | 7 | 38.9 |
| 8 | 3 | 15.8 | 4 | 22.2 |
| 9 | 4 | 21.1 | 3 | 16.7 |
| Median | 7 (5–9) | 7 (6–9) | ||
| Median PSA (ng/mL) at Dx | 14.15 (3.84–206) | 9.3 (4.0–187.0) | ||
| Mean (SD) PSA (ng/mL) at Dx | 35.0 (50.7) | 27.5 (45.9) | ||
| Median PSA (ng/mL) on study | 9.86 (0.17–122.26) | 3.4 (<0.2–94.3) | ||
Adjuvant IL-2 administered at 4.0 MIU/M2 on days 8 to 12 of each cycle.
Adjuvant IL-2 administered at 0.6 MIU/M2 on days 8 to 21 of each cycle.
No grade 4 toxicities were reported in either arm. The majority of vaccinations (75.7%) were associated with ≤ grade 2 injection-site reactions (Table 2), but as in the previously reported trial, no toxicities > grade 2 were directly attributable to the vaccine. One patient experienced grade 2 dyspnea attributable to GM-CSF, the other immune adjuvant used in this trial. These toxicities did not differ appreciably from those previously described in patients receiving higher-dose IL-2. However, toxicities attributable to IL-2 differed markedly between the 2 arms (Table 2). In the S-IL-2 arm (IL-2 at 4 MIU/M2), there were 129 cycles with adverse events attributed to IL-2 (103 grade 2; 26 grade 3). In the M-IL-2 arm (IL-2 at 0.6 MIU/M2), the number of cycles leading to grade 2 and 3 toxicities was 55 (48 grade 2; 7 grade 3). In particular, there were no grade 3 constitutional symptoms in the M-IL-2 arm, and only 4.4% of cycles were associated with grade 2 fatigue possibly due to IL-2. There were no grade 3 metabolic abnormalities in the M-IL-2 arm, and the proportion of grade 2 hyperglycemia was the same in both arms. The percentage of cycles reporting grade 2 lymphopenia (S-IL-2: 16%; M-IL-2: 20.4%) and grade 3 lymphopenia (S-IL-2: 6%; M-IL-2: 3%) was roughly the same for both arms. Interestingly, a higher percentage of cycles was associated with grade 2 injection-site reactions in the M-IL-2 arm (75.7%) than in the S-IL-2 arm (45%). The clinical significance of this finding is not known.
Table 2.
On-study adverse events.
| S-IL-2a (140 cycles administered) |
M-IL-2b (140 cycles administered) |
|||
|---|---|---|---|---|
| Grade 2
# (%)* |
Grade 3
# (%)* |
Grade 2
# (%)* |
Grade 3
# (%)* |
|
| GM-CSF + vaccine-related | ||||
| Injection-site reaction | 66 (47.1) | 0 (0) | 106 (75.7) | 0 (0) |
| GM-CSF-related | ||||
| Dyspnea | 13 (9.3) | 1 (0.7) | 1 (0.7) | 0 (0) |
| Arthralgias | 12 (8.6) | 0 (0) | 0 (0) | 0 (0) |
|
| ||||
| IL-2-related | ||||
| Constitutional | ||||
| Fatigue | 31 (22.1) | 7 (5.0) | 6 (4.3) | 0 (0) |
| Fever | 4 (2.9) | 2 (1.4) | 0 (0) | 0 (0) |
| Arthralgias | 9 (6.4) | 0 (0) | 0 (0) | 0 (0) |
| Metabolic | ||||
| Hyperglycemia | 9 (6.4) | 7 (5.0) | 8 (5.7) | 0 (0) |
| Blood | ||||
| Lymphopenia | 25 (17.9) | 6 (4.3) | 28 (20.0) | 4 (2.9) |
| Gastrointestinal | ||||
| Dehydration/anorexia | 3 (2.1) | 1 (0.7) | 1 (0.7) | 0 (0) |
| Diarrhea | 7 (5.0) | 2 (1.4) | 4 (2.9) | 2 (1.4) |
| Pulmonary | ||||
| Dyspnea | 15 (10.7) | 1 (0.7) | 1 (0.7) | 1 (0.7) |
Adjuvant IL-2 administered at 4.0 MIU/M2 on days 8-12 of each cycle.
Adjuvant IL-2 administered at 0.6 MIU/M2 on days 8-21 of each cycle.
Number of cycles with adverse events and percent of total number of cycles administered.
In the S-IL-2 arm, 16 patients (84.2%) had a dose reduction of IL-2, 5 (26.3%) had IL-2 held for one or more cycles and 2 patients (11.8 %) had IL-2 discontinued for toxicities. In the M-IL-2 arm, 4 (22.2%) patients had dose reductions, 4 (22.2%) had IL-2 held for one or more cycles for IL-2-related toxicities, but no patients had IL-2 discontinued for toxicities attributable to IL-2. Thus, M-IL-2 met the criteria we had prospectively defined (see Patients and Methods) for demonstrating superiority to S-IL-2.
Patients in this study who received M-IL-2 were able to mount specific T-cell immunologic responses with a frequency similar to that of patients who received S-IL-2. Eight patients with HLA-A2 haplotype in the M-IL-2 arm were evaluated for PSA-specific immune responses by ELISPOT assay (Table 3). Of these 8 patients, 5 developed T cell-specific immune responses to PSA at some point during the course of the trial. Of the patients who developed PSA-specific responses, 3 developed at least a 3-fold increase in specific T-cell response after the third vaccine cycle, but this immune response diminished in 2 of these individuals. The third patient maintained this level of immune response beyond the eighth cycle, which was the last time titers were measured. One patient demonstrated an immune response after the fifth cycle that was maintained until the eighth cycle, and one developed an immune response after the fifth cycle but was not evaluated further. Seventeen patients with HLA-A2 haplotype who were treated with S-IL-2 were similarly evaluated for generation of PSA-specific responses (13). In this cohort, 13 of 17 patients developed specific immune responses to PSA. Interestingly, 4 of the 17 patients already had PSA-specific T-cell precursor frequencies of > 1:100,000 before starting the protocol, whereas only one patient in the M-IL-2 arm had such high titers. Eight of 17 patients evaluated who developed substantial increases in PSA-specific T cells with S-IL-2 maintained these increases until after the eighth cycle of therapy, while 3 of 8 patients in the M-IL-2 arm retained immune responses beyond the eighth cycle of therapy. Based on these limited data, M-IL-2 appears to be as effective as S-IL-2 in inducing long-lasting T-cell responses following vaccination with a PSA-containing poxviral-based vaccine.
Table 3.
Induction of PSA-specific T-cell responses in 5 patients following vaccination and administration of metronomic adjuvant dose IL-2.
| Patient | Sample* | PSA3 peptide | Flu peptide | HIV peptide |
|---|---|---|---|---|
| 31 | Pre | <1/200,000 | 1/35,294 | <1/200,000 |
| Post 3 | <1/200,000 | 1/17,143 | <1/200,000 | |
| Post 3 + 2 | 1/45,455 | 1/17,857 | <1/200,000 | |
| Post 8 | 1/60,000 | 1/46,154 | <1/200,000 | |
| 32 | Pre | 1/120,000 | 1/11,111 | <1/200,000 |
| Post 3 | 1/17,391 | 1/12,121 | <1/200,000 | |
| Post 3 + 2 | <1/200,000 | 1/14,634 | <1/200,000 | |
| Post 8 | <1/200,000 | 1/15,385 | <1/200,000 | |
| 34 | Pre | <1/200,000 | 1/13,636 | <1/200,000 |
| Post 3 | 1/46,154 | 1/14,634 | <1/200,000 | |
| Post 5 + 3 | <1/200,000 | 1/13,636 | <1/200,000 | |
| Post 8 | <1/200,000 | 1/17,143 | <1/200,000 | |
| 37 | Pre | 1/150,000 | 1/125,000 | <1/200,000 |
| Post 2 | <1/200,000 | <1/200,000 | <1/200,000 | |
| Post 5 | 1/12,000 | 1/9,234 | <1/200,000 | |
| 38 | Pre | <1/200,000 | 1/25,000 | <1/200,000 |
| Post 3 | 1/85,714 | 1/10,169 | <1/200,000 | |
| Post 8 | 1/38,462 | 1/15,385 | <1/200,000 |
Samples were obtained after indicated vaccine cycle (i.e., post 3 + 2 = 2 months after cycle 3).
The previous trial employing S-IL-2 (13) demonstrated that the combination of radiation therapy and vaccine could induce immunoreactive T cells specific to a broad range of antigens other than PSA (so-called epitope spreading or antigen cascade). To determine if M-IL-2 would induce an antigen cascade, PBMCs from 3 patients with HLA-A2 haplotype were evaluated for immune response to other antigens in the ELISPOT assay using specific peptides. As seen in Table 4, 2 patients developed immunoreactivity to XAGE-1 and a third developed immunoreactivity to PAGE-4, both members of the PAGE/GAGE gene family of antigens that are expressed on prostate carcinoma cells (25). An additional 5 patients were evaluated for response to MUC-1 only. Of these 5 patients, 2 developed T-cell responses specific to MUC-1. Of note, all patients were HIV− prior to enrollment and had no T-cell response to HIV either before or after vaccination, further demonstrating the specificity of this immune response. These data suggest that the combination of vaccine and radiotherapy can induce immunity to a range of tumor-associated antigens beyond those present in the vaccine, which may have positive implications for the efficacy of immunotherapy in this setting.
Table 4.
Antigen cascade in 3 patients after vaccination against PSA with administration of metronomic adjuvant dose IL-2.
| Patient | Sample* | MUC-1 | XAGE-1 | PAGE-4 |
|---|---|---|---|---|
| 31 | Pre | 1/85,714 | <1/200,000 | <1/200,000 |
| Post 3 | <1/200,000 | <1/200,000 | <1/200,000 | |
| Post 5+2 | <1/200,000 | <1/200,000 | <1/200,000 | |
| Post 8 | 1/37,500 | 1/27,273 | <1/200,000 | |
| 32 | Pre | <1/200,000 | 1/23,077 | 1/100,000 |
| Post 3 | <1/200,000 | 1/28,571 | 1/80,000 | |
| Post 5+2 | <1/200,000 | 1/46,154 | 1/22,222 | |
| Post 8 | <1/200,000 | 1/50,000 | 1/100,000 | |
| 33 | Pre | <1/200,000 | <1/200,000 | <1/200,000 |
| Post 3 | <1/200,000 | 1/54,545 | 1/200,000 | |
| Post 5 | <1/200,000 | <1/200,000 | <1/200,000 | |
| Post 8 | 1/46,154 | 1/24,000 | <1/200,000 |
Samples were obtained after indicated vaccine cycle.
We next evaluated the effect of M-IL-2 on levels of NK cells, which are also thought to potentially play a role in cell-mediated immunity induced by therapeutic tumor vaccines. In preclinical studies employing poxviral vaccines, NK depletion was associated with decreased survival. To determine if M-IL-2 would impair the generation of NK cells in this setting, we determined the percentage of NK cells in patients in the M-IL-2 arm before, during, and after vaccination. As seen in Table 5, S-IL-2 induced an increase in the percentage of NK cells in samples from 4 of 4 patients studied (average 80%; range 7% to 155%). Similarly, all 4 patients in the M-IL-2 arm showed increases in the percentage of NK cells (average 51%; range 19% to 97%). A representative flow cytometry result for both NK and Treg cells (see below) is shown in Figure 1. These data suggest that M-IL-2 has approximately the same effect on the generation of NK cells as the previously evaluated S-IL-2 regimen, but without the associated toxicity seen with S-IL-2.
Table 5.
NK cells in a sample of patients immunized against PSA.
| S-IL-2a | M-IL-2b | ||||
|---|---|---|---|---|---|
| Patient | Sample* | % NK cells† | Patient | Sample | % NK cells† |
| 5 | Pre | 15.26 | 35 | Pre | 7.88 |
| Post 3 | 12.64 | Post 3 | 10.38 | ||
| Post 8 | 20.87 | Post 8 | 9.71 | ||
| 10 | Pre | 5.05 | 36 | Pre | 12.52 |
| Post 3 | 8.24 | Post 3 | 13.15 | ||
| Post 8 | 11.19 | Post 8 | 14.86 | ||
| 15 | Pre | 7.52 | 39 | Pre | 19.19 |
| Post 3 | 9.03 | Post 3 | 20.01 | ||
| Post 8 | 19.22 | Post 8 | 31.42 | ||
| 9 | Pre | 15.99 | 0 | Pre | 10.99 |
| Post 3 | 9.73 | Post 3 | 16.37 | ||
| Post 8 | 17.10 | Post 8 | 21.60 | ||
| Mean (SD) | Pre | 10.95 (5.49) | Mean (SD) | Pre | 12.64 (4.77) |
| Post 3 | 9.73 (1.97) | Post 3 | 14.97 (4.15) | ||
| Post 8 | 17.10 (4.22) | Post 8 | 19.39 (9.37) | ||
Adjuvant IL-2 administered at 4.0 MIU/M2 on days 8 to 12 of each cycle.
Adjuvant IL-2 administered at 0.6 MIU/M2 on days 8 to 21 of each cycle.
Samples were obtained after indicated vaccine cycle.
Percentage of CD3−/CD56+ PBMCs.
Figure 1.
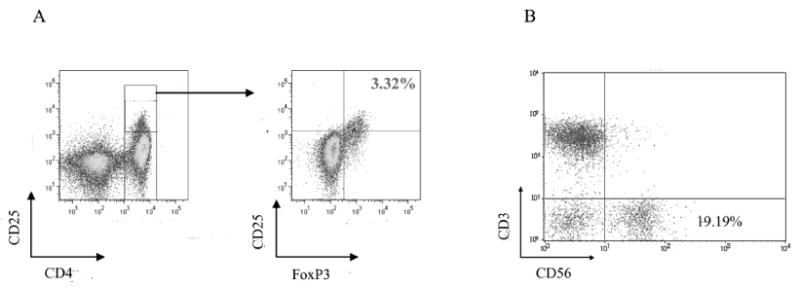
A representative flow cytometry plot of Tregs and NK cells in peripheral blood of prostate cancer patients. A. Levels of CD4+CD25highFoxP3+ Tregs in the peripheral blood of a prostate cancer patient enrolled in metronomic dose IL-2. PBMCs were analyzed by flow cytometry after cell surface labeling with PerCP-Cy5.5-conjugated anti-CD4, phycoerythrin (PE)-conjugated anti-CD25 and intracellular staining with FITC-conjugated anti-FoxP3. Levels of CD4+CD25highFoxP3+ Tregs are presented as a percentage of total CD4+ T cells. B. Levels of NK cells in the peripheral blood of a prostate cancer patient enrolled in “standard” dose IL-2 cohort. PBMCs were analyzed by flow cytometry after cell surface labeling with phycoerythrin (PE)-conjugated anti-CD3 and FITC-conjugated anti-CD56. Levels of NK cells (CD3-CD56+) are presented as a percentage of total PBMCs.
The effect of S-IL-2 on Treg generation was evaluated by flow cytometry at baseline and at multiple points following vaccination. For patients treated with both M-IL-2 and S-IL-2, the percentage of CD4+CD25high/FoxP3+ cells was determined at baseline, just after 3 and 8 cycles of vaccination, and 3 months following the last vaccination. At baseline, the percentage of Tregs in both cohorts was not significantly different from that of normal donors (data not shown). As seen in Table 6, the percentage of CD25high/FoxP3+ cells (as a percentage of total CD4+ cells) increased from baseline to maximum at cycle 8, then decreased to near baseline at 3 months after the last vaccination. In the M-IL-2 cohort, a similar increase in the percentage of Tregs was noted, with a slightly earlier rise in some patients but an average return to baseline in all patients at 3 months after the last vaccination, similar to the S-IL-2 arm. The earlier rise seen in several patients in the M-IL-2 arm may have been due to the proximity of IL-2 administration. In the S-IL-2 arm, the last dose of IL-2 was administered 12 days prior to analysis of Treg subsets, while in the M-IL-2 arm, the last dose of IL-2 was administered only 7 days prior to analysis of Treg populations. It is notable that both cohorts returned to pretreatment levels by 3 months after the last vaccination. Therefore, unlike high-dose IL-2, which has been reported to increase Tregs, neither S-IL-2 nor M-IL-2 did so in this trial.
Table 6.
Treg cells during treatment.
| S-IL-2a | ||||
|---|---|---|---|---|
| Patient | Pre* | Post 3 | Post 8 | Post 8 + 3 |
| 1 | 2.08†(1:17.92) | 2.64 (1:15.54) | 3.86 (1:11.06) | 2.92 (1:12.48) |
| 2 | 1.39 (1:12.06) | 6.21 (1:5.71) | 8.52 (1:1.35) | 4.29 (1:3.62) |
| 5 | 1.99 (1:17.17) | 3.65 (1:13.84) | 6.2 (1:6.82) | ND |
| 7 | 3.97 (1:8.61) | 5.21 (1:6.54) | 5.79 (1:4.81) | 4.21 (1:6.56) |
| 9 | 2.24 (1:16.93) | 2.84 (1:15.59) | 5.02 (1:8.21) | 2.86 (1:10.95) |
| 10 | 2.42 (1:18.91) | 2.98 (1:14.66) | 5.19 (1:7.02) | 2.33 (1:17.61) |
| 11 | 3.87 (1:12.14) | 6.26 (1:7.60) | 8.04 (1:4.27) | 4.21 (1:9.60) |
| 12 | 4.38 (1:5.58) | 5.10 (1:10.54) | 9.07 (1:4.04) | 4.10 (1:6.43) |
| 13 | 0.89 (1:53.85) | 4.51 (1:12.89) | 7.97 (1:4.51) | 4.69 (1:7.99) |
| 14 | 3.47 (1:13.04) | 5.81 (1:8.18) | 5.12 (1:8.38) | 4.28 (1:8.84) |
| 15 | 1.9 (1:27.6) | 2.32 (1:23.92) | 4.09 (1:9.82) | 2.47 (1:15.95) |
| 17 | 4.62 (1:9.83) | 4.59 (1:10.24) | 6.91 (1:5.78) | 4.15 (1:8.45) |
| Mean | 2.77 (1:17.80) | 4.34 (1:12.10) | 6.32 (1:6.34) | 3.68 (1:9.86) |
| M-IL-2b | ||||
|
|
||||
| Patient | Pre | Post 3 | Post 8 | Post 8 + 3 |
|
| ||||
| 31 | 1.51 (1:36.22) | 3.26 (1:18.21) | 3.47 (1:14.80) | 3.26 (1:14.71) |
| 32 | 4.69 (1:8.73) | 6.53 (1:6.39) | 9.2 (1:4.86) | 6.27 (1:4.66) |
| 33 | 5.81 (1:9.22) | 5.99 (1:8.76) | 7.88 (1:6.69) | 4.51 (1:10.85) |
| 34 | 3.41 (1:7.45) | 7.03 (1:3.88) | 8.56 (1:3.02) | 4.59 (1:6.36) |
| 35 | 4.15 (1:11.47) | 9.37 (1:4.54) | 3.05 (1:13.40) | 4.05 (1:8.81) |
| 36 | 2.89 (1:16.82) | 8.17 (1:5.34) | 2.96 (1:13.69) | ND |
| 37 | 4.61 (1:9.89) | 11.45 (1:2.91) | 8.89 (1:5.08) | ND |
| 38 | 3.72 (1:11.42) | 3.71 (1:8.76) | 8.18 (1:4.35) | ND |
| 39 | 4.61 (1:6.95) | 6.87 (1:4.92) | 8.32 (1:3.01) | 4.41 (1:6.60) |
| 40 | 2.09 (1:21.42) | 3.95 (1:9.26) | 7.79 (1:3.16) | 2.51 (1:9.76) |
| 41 | 3.32 (1:11.95) | 6.88 (1:6.33) | 9.33 (1:2.68) | 4.47 (1:6.60) |
| 42 | 3.43 (1:10.58) | 3.35 (1:9.62) | 2.83 (1:14.33) | 3.18 (1:10.45) |
| Mean | 3.69 (1:13.51) | 6.38 (1:7.41) | 6.71 (1:7.42) | 4.14 (1:8.76) |
Adjuvant IL-2 administered at 4.0 MIU/M2 on days 8 to 12 of each cycle.
Adjuvant IL-2 administered at 0.6 MIU/M2 on days 8 to 21 of each cycle.
Samples were obtained after indicated vaccine cycle (post 8 + 3 = 3 months after cycle 8).
Percent CD25high/FoxP3+ cells (as percentage of total CD4+ cells) and in parentheses ratio of CD4+/CD25high/FoxP3+:CD4+/FoxP3- cells. For healthy donors, 5.24% = 95th percentile.
Discussion
The uncertainty concerning the optimum adjuvant treatment for localized prostate cancer prompted us to evaluate the safety and immunological response of a viral-based vaccine in patients with high-risk localized prostate cancer. In the study reported previously (13), the number and severity of toxicities attributable to IL-2 were significant. We report now on a subsequently enrolled cohort of patients who were treated with M-IL-2 to compare the safety and immunological responses of low and metronomic dosing. In the original trial, the S-IL-2 arm received IL-2 at a dose of 4 MIU/M2 on days 8 to 12 of each cycle; in the subsequently enrolled M-IL-2 arm, 0.6 MIU/M2 of IL-2 was administered daily on days 8 to 21 of each 28-day cycle. Both treatment arms received vaccine on day 2. As our analysis demonstrates, M-IL-2 enhanced patient safety, resulted in fewer adverse events, and did not appear to significantly alter vaccine-induced T-cell responses. In this trial, IL-2 was used as an immunological adjuvant to boost T-cell function and vaccine efficacy, not as a stimulus to induce immune rejection of the tumor, as in therapy for renal cell carcinoma.
Continuous low-dose, or metronomic, therapy has received increasing interest in recent years (26). When applied to standard cytotoxic therapies, metronomic therapy has been used to potentiate theoretical antiangiogenic activity (27) through inhibition of endothelial proliferation. This method of administration limits not only endothelial regrowth through constant low-grade growth suppression, but also the toxicities associated with high doses of cytotoxic agents (26). In the context of immunotherapy, similar principles apply. Generating effective immune responses from current vaccine modalities requires administration of adjuvant therapies. IL-2 is commonly used in these circumstances for its effects on T-cell proliferation and activation. Bolus administration of S-IL-2 has several disadvantages, the primary one being the relative toxicity observed. Metronomic dosing in this context is superior, as demonstrated in this study. In the M-IL-2 arm, there were fewer ≥ grade 2 toxicities and more patients completed vaccine therapy. Based solely on safety and feasibility, M-IL-2 appears superior to standard dosing and administration.
Despite increased patient safety, however, there is still a concern that M-IL-2 therapy may not effectively induce appropriate immune responses. IL-2 causes expansion of activated T cells, but in the context of antigen presentation, IL-2 can potentiate antigen-induced cell death (14). There are, however, significant nonclinical and clinical data supporting the use of M-IL-2 as an adjuvant for vaccine therapy. High concentrations of IL-2 can cause cell death by inducing cell-cycle entry and facilitating active apoptosis through Fas ligand and tumor necrosis factor (TNF)-α (28). In HIV therapy, low-dose IL-2 has been shown to be superior to high-dose IL-2 for stimulating immune-cell function. The addition of low-dose IL-2 (1 MIU/day × 5 days every other week) to highly active anti-retroviral therapy (HAART) caused a statistically significant increase in CD4 and CD8 T cells, as well as a decrease in apoptosis compared to HAART alone in HIV+ patients (17). In the same study, IL-2 treatment decreased the expression of CD95 (Fas) on CD8+ cells. In a similar study in patients with AIDS and AIDS-associated malignancies, Khatri et al. showed that IL-2 given daily at 1.2 MIU/M2 for 3 months significantly increased IFN-γ gene expression in vivo, with normalization of a profound deficit of IFN-γ production upon in vitro stimulation (29). The source of the IFN-γ appeared to be NK cells and CD8+ T cells. In another study (30), a higher concentration of IL-2 (7.5 MIU/day s.c. × 5 days) caused an increase in spontaneous apoptosis of both CD4+ and CD8+ cells.
Both ADT and IL-2 can influence the distribution of T-cell subsets during treatment. ADT increases pre-B-cell levels in mice and thymocyte numbers in both animal models and humans treated with GnRH. This thymic expansion may increase the diversity of the T-cell repertoire, although this has not been proven (31). IL-2 therapy also has an influence on the subsets of lymphocytes that repopulate the T-cell compartment during immune reconstitution following chemotherapy. In sarcoma patients treated with cyclophosphamide and adjuvant IL-2 (at a dose of either 9 × 106 IU/M2/day CIVI 4 days/week × 3 wks starting on weeks 6, 12, 18, or 3 × 106 IU/M2/dose SQ 3 times/week × 16 weeks starting at week 6) in combination with vaccine, IL-2 caused a preferential expansion of the CD4+CD25high Treg compartment (32). In that study, the higher of 2 doses of IL-2 used seemed to induce a greater increase in Tregs, although the difference did not reach statistical significance. We have previously demonstrated that patients with prostate cancer have peripheral blood levels of Tregs similar to those of healthy donors, but with significantly greater suppressive functionality (33). In the present study, we saw a clear increase in Treg populations during the period of IL-2 administration, with a return to baseline levels 3 months after the last dose. Our data do not indicate a clear improvement in the Treg profile with metronomic dosing, but there was no prolongation of Treg expansion due to the longer period of IL-2 dosing in the M-IL-2 arm. One caveat, however, is that in the S-IL-2 arm the time from last IL-2 administration until measurement of Tregs was 14 days, whereas in the M-IL-2 arm the delay was only 7 days. It is unclear what impact this may have had on Treg levels, but the shorter time to measurement from the date of last IL-2 dose may have increased the apparent number of Tregs in the M-IL-2 arm. Any difference in Treg activity between the 2 treatment arms has not been determined.
Various doses of IL-2 have also been shown to augment NK-cell populations in humans (16, 18). Since many tumors do not express MHC molecules, which are necessary for CD8-mediated killing, NK cells (which selectively kill cells lacking MHC molecules) are a vital part of the innate immune system in this vaccine strategy. In patients with advanced malignancies, daily administration of low-dose IL-2 (1.25 MIU/M2), with pulse dosing after 4 to 6 weeks with up to 15 MIU/M2/day × 3 days (repeated every 2 weeks), expanded the T-cell population by about 50%, the NK-cell population by about 8-fold, and the subpopulation of CD56bright cells by 32-fold (34). In a study of patients with AIDS and non-Hodgkin's lymphoma, 1 MIU/M2 IL-2 administered daily for 8 weeks caused a statistically significant 1.6-fold increase in the percentage of NK cells (18). IL-2 likely causes expansion of NK-cell populations by inducing maturation of progenitors and inhibiting mature NK-cell apoptosis (35). Interestingly, in a study of renal carcinoma patients treated with IL-2, thalidomide, and radiotherapy, IL-2 increased the percentages of NK cells, but in patients concomitantly treated with radiation therapy, there was no increase (36). In contrast, all patients in our study were treated with both IL-2 and radiation therapy, and all patients tested experienced an increase in NK cells.
Our data demonstrate that administration of IL-2 in a metronomic dosing schedule (low doses administered daily for 14 of 28 days) is safe. In addition, immune responses are similar to those in patients treated with the identical vaccine strategy, but with higher doses of IL-2 given for a shorter period of time. M-IL-2 dosing is well tolerated and should allow for increased use of IL-2 in vaccine protocols. Further study is needed to determine if M-IL-2 has an effect on clinical outcomes.
Footnotes
Publisher's Disclaimer: The views expressed are those of the authors and do not represent the opinion of the NIH, FDA or HHS.
References
- 1.Dillioglugil O, Leibman BD, Kattan MW, Seale-Hawkins C, Wheeler TM, Scardino PT. Hazard rates for progression after radical prostatectomy for clinically localized prostate cancer. Urology. 1997;50:93–9. doi: 10.1016/S0090-4295(97)00106-4. [DOI] [PubMed] [Google Scholar]
- 2.Stamey TA, Yemoto CM, McNeal JE, Sigal BM, Johnstone IM. Prostate cancer is highly predictable: a prognostic equation based on all morphological variables in radical prostatectomy specimens. J Urol. 2000;163:1155–60. doi: 10.1016/s0022-5347(05)67713-0. [DOI] [PubMed] [Google Scholar]
- 3.Paulson DF, Moul JW, Walther PJ. Radical prostatectomy for clinical stage T1-2N0M0 prostatic adenocarcinoma: long-term results. J Urol. 1990;144:1180–4. doi: 10.1016/s0022-5347(17)39686-6. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 5.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 6.Granfors T, Modig H, Damber JE, Tomic R. Combined orchiectomy and external radiotherapy versus radiotherapy alone for nonmetastatic prostate cancer with or without pelvic lymph node involvement: a prospective randomized study. J Urol. 1998;159:2030–4. doi: 10.1016/S0022-5347(01)63235-X. [DOI] [PubMed] [Google Scholar]
- 7.Granfors T, Modig H, Damber JE, Tomic R. Long-term followup of a randomized study of locally advanced prostate cancer treated with combined orchiectomy and external radiotherapy versus radiotherapy alone. J Urol. 2006;176:544–7. doi: 10.1016/j.juro.2006.03.092. [DOI] [PubMed] [Google Scholar]
- 8.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–90. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 9.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781–8. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 11.Kudo-Saito C, Schlom J, Camphausen K, Coleman CN, Hodge JW. The requirement of multimodal therapy (vaccine, local tumor radiation, and reduction of suppressor cells) to eliminate established tumors. Clin Cancer Res. 2005;11:4533–44. doi: 10.1158/1078-0432.CCR-04-2237. [DOI] [PubMed] [Google Scholar]
- 12.Hodge JW, Schlom J, Donohue SJ, et al. A recombinant vaccinia virus expressing human prostate-specific antigen (PSA): safety and immunogenicity in a non-human primate. Int J Cancer. 1995;63:231–7. doi: 10.1002/ijc.2910630215. [DOI] [PubMed] [Google Scholar]
- 13.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–62. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 14.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 15.Chang AE, Rosenberg SA. Overview of interleukin-2 as an immunotherapeutic agent. Semin Surg Oncol. 1989;5:385–90. doi: 10.1002/ssu.2980050604. [DOI] [PubMed] [Google Scholar]
- 16.van der Vliet HJ, Koon HB, Yue SC, et al. Effects of the administration of high-dose interleukin-2 on immunoregulatory cell subsets in patients with advanced melanoma and renal cell cancer. Clin Cancer Res. 2007;13:2100–8. doi: 10.1158/1078-0432.CCR-06-1662. [DOI] [PubMed] [Google Scholar]
- 17.Pandolfi F, Pierdominici M, Marziali M, et al. Low-dose IL-2 reduces lymphocyte apoptosis and increases naive CD4 cells in HIV-1 patients treated with HAART. Clin Immunol. 2000;94:153–9. doi: 10.1006/clim.2000.4837. [DOI] [PubMed] [Google Scholar]
- 18.Shah MH, Freud AG, Benson DM, Jr, et al. A phase I study of ultra low dose interleukin-2 and stem cell factor in patients with HIV infection or HIV and cancer. Clin Cancer Res. 2006;12:3993–6. doi: 10.1158/1078-0432.CCR-06-0268. [DOI] [PubMed] [Google Scholar]
- 19.Lalezari JP, Beal JA, Ruane PJ, et al. Low-dose daily subcutaneous interleukin-2 in combination with highly active antiretroviral therapy in HIV+ patients: a randomized controlled trial. HIV Clin Trials. 2000;1:1–15. doi: 10.1310/T5FR-8JPX-0NEF-XDKD. [DOI] [PubMed] [Google Scholar]
- 20.Caligiuri MA, Murray C, Soiffer RJ, et al. Extended continuous infusion low-dose recombinant interleukin-2 in advanced cancer: prolonged immunomodulation without significant toxicity. J Clin Oncol. 1991;9:2110–9. doi: 10.1200/JCO.1991.9.12.2110. [DOI] [PubMed] [Google Scholar]
- 21.Caligiuri MA, Murray C, Robertson MJ, et al. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest. 1993;91:123–32. doi: 10.1172/JCI116161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cesana GC, DeRaffele G, Cohen S, et al. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24:1169–77. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 24.A'Hern RP. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20:859–66. doi: 10.1002/sim.721. [DOI] [PubMed] [Google Scholar]
- 25.Brinkmann U, Vasmatzis G, Lee B, Pastan I. Novel genes in the PAGE and GAGE family of tumor antigens found by homology walking in the dbEST database. Cancer Res. 1999;59:1445–8. [PubMed] [Google Scholar]
- 26.Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol. 2001;2:733–40. doi: 10.1016/S1470-2045(01)00587-3. [DOI] [PubMed] [Google Scholar]
- 27.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 28.Lenardo MJ. Fas and the art of lymphocyte maintenance. J Exp Med. 1996;183:721–4. doi: 10.1084/jem.183.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khatri VP, Fehniger TA, Baiocchi RA, et al. Ultra low dose interleukin-2 therapy promotes a type 1 cytokine profile in vivo in patients with AIDS and AIDS-associated malignancies. J Clin Invest. 1998;101:1373–8. doi: 10.1172/JCI2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sereti I, Herpin B, Metcalf JA, et al. CD4 T cell expansions are associated with increased apoptosis rates of T lymphocytes during IL-2 cycles in HIV infected patients. Aids. 2001;15:1765–75. doi: 10.1097/00002030-200109280-00004. [DOI] [PubMed] [Google Scholar]
- 31.Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4957–71. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Chua KS, Guimond M, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–43. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 33.Yokokawa J, Cereda V, Remondo C, et al. Enhanced Functionality of CD4+CD25highFoxP3+ Regulatory T Cells in the Peripheral Blood of Patients with Prostate Cancer. Clin Cancer Res. 2008;14:1032–40. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 34.Meropol NJ, Barresi GM, Fehniger TA, Hitt J, Franklin M, Caligiuri MA. Evaluation of natural killer cell expansion and activation in vivo with daily subcutaneous low-dose interleukin-2 plus periodic intermediate-dose pulsing. Cancer Immunol Immunother. 1998;46:318–26. doi: 10.1007/s002620050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fehniger TA, Bluman EM, Porter MM, et al. Potential mechanisms of human natural killer cell expansion in vivo during low-dose IL-2 therapy. J Clin Invest. 2000;106:117–24. doi: 10.1172/JCI6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerst JM, Bex A, Mallo H, et al. Prolonged low dose IL-2 and thalidomide in progressive metastatic renal cell carcinoma with concurrent radiotherapy to bone and/or soft tissue metastasis: a phase II study. Cancer Immunol Immunother. 2005;54:926–31. doi: 10.1007/s00262-005-0677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


