Abstract
Our skin is constantly challenged by microbes but is rarely infected. Cutaneous production of antimicrobial peptides (AMPs) is a primary system for protection, and expression of some AMPs further increases in response to microbial invasion. Cathelicidins are unique AMPs that protect the skin through 2 distinct pathways: (1) direct antimicrobial activity and (2) initiation of a host response resulting in cytokine release, inflammation, angiogenesis, and reepithelialization. Cathelicidin dysfunction emerges as a central factor in the pathogenesis of several cutaneous diseases, including atopic dermatitis, in which cathelicidin is suppressed; rosacea, in which cathelicidin peptides are abnormally processed to forms that induce inflammation; and psoriasis, in which cathelicidin peptide converts self-DNA to a potent stimulus in an autoinflammatory cascade. Recent work identified vitamin D3 as a major factor involved in the regulation of cathelicidin. Therapies targeting control of cathelicidin and other AMPs might provide new approaches in the management of infectious and inflammatory skin diseases.
Keywords: Antimicrobial peptides; alarmins; skin; cathelicidin; rosacea; atopic dermatitis; psoriasis; 1,25 dihydroxy vitamin D3
Antimicrobial peptides (AMPs) were first thought to act as endogenous antibiotics whose function was only to kill microbes. Today, although it is clear that AMPs act to form a chemical shield on the surface of the skin, they are also thought to trigger and coordinate multiple components of the innate and adaptive immune system.1,2 Many cell types that permanently reside in the skin produce AMPs, including keratinocytes, sebocytes, eccrine glands, and mast cells.3–6 Circulating cells recruited to the skin, such as neutrophils and natural killer cells, are also significant contributors to the total amount of AMPs present.7 Cathelicidins and β-defensins are the most well characterized of the AMPs found in the skin, but a list of the known cutaneous AMPs can identify more than 20 individual proteins that have shown antimicrobial activity (Table I).6,8–39 This extensive list of skin-derived AMPs is complicated by the nature of the experimental assays and the concentrations used to identify antimicrobial activity. Thus many molecules better known for other biologic activity, such as α-melanocyte–stimulating hormone or serine leukocyte protease inhibitor, can also be considered as functional AMPs in the skin.8 Unfortunately, because adequate animal model systems do not always permit direct testing of the AMP activity of many of these peptides, it remains difficult to determine the primary function of a peptide that shows antimicrobial and additional biologic functions. In general, the AMPs are structurally extremely diverse but considered together only because of their antimicrobial activity.
TABLE I.
Mammalian peptides with antimicrobial activity in skin (AMPs)*
| AMP | Reference |
|---|---|
| AMPs identified in resident cells | |
| Cathelicidins | Frohm et al (1997)9 |
| Marchini et al (2002)10 | |
| β-Defensins | Harder et al (1997)11 |
| Liu et al (1998)12 | |
| Bactericidal/permeability-increasing protein (BPI) | Takahashi et al (2004)13 |
| Lactoferrin | Cumberbatch et al (2000)14 |
| Lysozyme | Marchini et al (2002)10 |
| Dermcidin | Schittek et al (2001)15 |
| Murakami et al (2002)6 | |
| Histones | Rose et al (1998)16 |
| S100A15 | Büchau et al (2007)17 |
| RNase 7 | Harder et al (2002)18 |
| AMPs identified in infiltrating cells | |
| Cathelicidins | Gallo et al (1994)19 |
| Marchini et al (2002)10 | |
| α-Defensins | Harwig et al (1993)20 |
| Lactoferrin | Caccavo et al (2002)21 |
| Granulysin | Stenger et al (1998)22 |
| Perforin | Stenger et al (1998)22 |
| Eosinophil cationic protein (ECP)/RNase 3 | Domachowske et al (1998)23 |
| Eosinophil-derived neurotoxin (EDN)/RNase 2 | Domachowske et al (1998)24 |
| RANTES | Tang et al (2002)25 |
| AMPs identified as proteinase inhibitors | |
| hCAP18/LL-37 prosequence (cathelin-like domain) | Zaiou et al (2003)26 |
| Secretory leukocyte proteinase inhibitor (SLPI)/antileukoprotease | Wingens et al (1998)27 |
| Elafin/skin-derived antileukoprotease (SKALP) | Simpson et al (1999)28 |
| Meyer-Hoffert et al (2003)29 | |
| P-cystatin A | Takahashi et al (2004)13 |
| Cystatin C | Blankenvoorde et al (1998)30 |
| AMPs identified as chemokines | |
| Psoriasin | Glaser et al (2005)31 |
| Monokine induced by IFN-γ (MIG/CXCL9) | Cole et al (2001)32 |
| IFN-γ–inducible protein of 10 kd (IP-10/CXCL10) | Cole et al (2001)32 |
| IFN-γ–inducible T cell α chemoattractant (ITAC/CXCL11) | Cole et al (2001)32 |
| AMPs identified as neuropeptides | |
| α-Melanocyte–stimulating hormone (α-MSH) | Cutuli et al (2000)33 |
| Substance P | Kowalska et al (2002)34 |
| Bradykinin | Kowalska et al (2002)34 |
| Neurotensin | Kowalska et al (2002)34 |
| Vasostatin-1 and chromofungin (chromogranin A) | Tasiemski et al (2002)35 |
| Secretolytin (chromogranin B) | Tasiemski et al (2002)35 |
| Enkelytin and peptide B (proenkephalin A) | Tasiemski et al (2002)35 |
| Ubiquitin | Kieffer et al (2003)36 |
| Neuropeptide Y | Lambert et al (2002)37 |
| Polypeptide YY/skin-polypeptide Y | Lambert et al (2002)37 |
| Catestatin | Radek et al (2008)38 |
| Adrenomedullin | Allaker et al (1999)39 |
References are limited because of space restrictions.
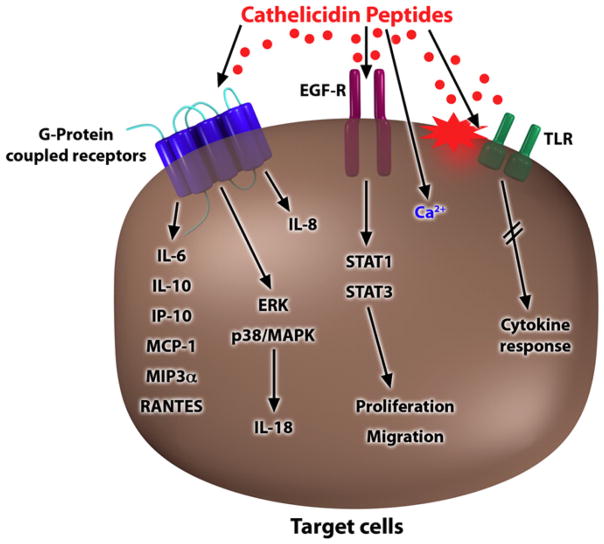
Cathelicidins are an important AMP family in the skin because they were the first AMP5 found in mammalian skin, and since then, the most compelling animal models that support their antimicrobial function have been compiled.40–42 Human cathelicidin is often referred to by one of its peptide forms (LL-37) or by the nomenclature assigned to its precursor protein (hCAP18).43,44 Peptide processing has emerged as a critical element in the control of cathelicidin activity. In its nascent form hCAP18 is thought to be inactive. On cleavage by serine proteases, the generation of the mature peptide results in multiple potential activities.45,46 The 37-amino-acid peptide LL-37 forms an α-helix in solution and can disrupt both bacterial membranes and viral envelopes.8 In addition, cathelicidin LL-37 shows antifungal activity.47 Furthermore, LL-37 can interact with mammalian cells to trigger a host response. These functions have been called the alarmin activity of AMPs,48 and cathelicidin peptides can act through multiple potential mechanisms. Alarmin functions include direct interactions of LL-37 with cell-surface receptors, such as the formyl-peptide receptor–like 1 or G protein–coupled receptors, resulting in direct effects on intracellular signaling pathways (Fig 1).8,49,50 Furthermore, LL-37 was shown to influence Toll-like receptor (TLR) signaling in immune cells through interaction with the cellular membrane and epidermal growth factor receptor transactivation and to increase intracellular Ca2+mobilization.51–55 Cathelicidin also synergizes with endogenous inflammatory mediators to enhance the induction of specific inflammatory effectors through a complex mechanism involving multiple pathways.56 As a result, cathelicidin peptides increase cell migration and secretion of chemokines and other signaling molecules from activated cells (Fig 1).2,50 All these activities complement the role of the cathelicidins as direct antimicrobial agents, and they have established their role as essential defense molecules in innate immune responses.
FIG 1.
Models for cell activation by cathelicidins. Multiple mechanisms have been proposed for cathelicidins to stimulate a cellular response. Responses are dependent on activation of G protein–coupled receptors and transactivation of the epidermal growth factor receptor or secondary to intracellular Ca2+ mobilization or a change in cell membrane function, leading to alterations in receptor responses. Finally, cathelicidins can influence the function of TLRs through both direct and indirect pathways. EGF-R, Epidermal growth factor receptor; IP-10, IFN-γ–inducible protein 10; MCP-1, monocyte chemoattractant protein 1; MIP3α, macrophage inflammatory protein 3α; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; STAT, signal transducer and activator of transcription.
ROLE OF CATHELICIDIN IN INFLAMMATORY SKIN DISEASES
The presence of cathelicidin in the skin has been shown to offer increased protection against bacterial and viral infections.40,57 In healthy skin keratinocytes express low amounts of cathelicidin. On infection or barrier disruption, cathelicidin is strongly induced.9,58,59 However, in several common skin diseases the normal barrier against infection is diminished or the control of inflammation is abnormal. One example is atopic dermatitis. Here viral and bacterial infections perpetuate cutaneous inflammation and complicate successful therapy. Observations of the expression of AMPs of atopic patients demonstrated that the process of AMP induction was greatly reduced in lesional skin.60 The resulting diminished antimicrobial barrier correlated with an increased susceptibility of these patients to microbial superinfections.57,61 Diminished inducibility of cathelicidin and defensins in atopic dermatitis appears to be partially a consequence of the altered cytokine micromilieu.57 In particular, TH2 cytokines, such as IL-4 and IL-13, suppress the induction of AMPs and contribute to a disturbed cutaneous antimicrobial response. Thus in this disorder a decrease in the amount of AMPs released by the skin barrier contributes to disease.
Other associations of AMPs with skin diseases appear to be a consequence of host stimulatory effects rather than action as an antimicrobial agent. As discussed earlier, the cathelicidin peptide LL-37 induces the expression of proinflammatory cytokines in keratinocytes, chemotaxis of adaptive immune cells, and angiogenesis.2,62 On the skin surface, LL-37 is normally processed to smaller peptides with enhanced antimicrobial functions but lesser inflammatory effects.63 Individuals with rosacea were studied because this disease is defined by abnormal inflammation and vascular reactivity in facial skin, and these responses resembled activities associated with cathelicidin. It was found that patients with rosacea express abnormally high levels of cathelicidin in the LL-37 peptide form.64 In addition, proteolytically processed forms of cathelicidin found in patients with rosacea were found to be dramatically different from those in healthy individuals, where LL-37 is rare and shorter forms predominate. The cathelicidin peptides in patients with rosacea were a result of a posttranslational processing abnormality associated with an increase in protease activity in the epidermis.64 In mice increasing cutaneous protease activity or injection of the identified cathelicidin peptides resulted in inflammation, erythema, and telangiectasia that mimicked the disease in human subjects.64 The central role of cathelicidin was further supported in mice with a targeted deletion of the cathelicidin gene Camp: in these mice increased serine protease activity did not induce inflammation. Thus in patients with rosacea, too much AMP and abnormal processing lead to disease.
A third example of a human inflammatory skin disease associated with abnormal AMP expression and activity is psoriasis.60,65 As mentioned earlier, cathelicidin is increased in lesional skin in patients with psoriasis.60,66 Psoriasis is a chronic inflammatory skin disease, and an autoimmune reaction is suspected to play a major role in the course of the disease. The auto-antigens triggering inflammation in psoriasis remain unknown. In a recent study LL-37 isolated from lesional skin was shown to form complexes with human self-DNA to activate plasmacytoid dendritic cells (pDCs).66 pDCs do not normally respond to self-DNA, but binding to LL-37 converted DNA in a potent stimulus for pDC activation. LL-37/self-DNA complexes signaled through TLR9 and elicited IFN-α release from pDCs. IFN-α subsequently activated a T-cell response that can lead to cutaneous inflammation.66 Because cathelicidin LL-37 expression is low in healthy skin but strongly induced after skin injury, binding of self-DNA released from damaged or apoptotic cells to LL-37 might result in the creation of a potent immune stimulus. Therefore in this third example of a human skin disease associated with cathelicidin, the response of an AMP might be normal but critical to the amplification loop that results in disease.
CONTROL OF AMP EXPRESSION IN THE SKIN
The disorders associated with AMP expression all highlight the importance of understanding mechanisms that control their expression. Cathelicidins, like most AMPs, are produced in keratinocytes, neutrophils, and many other cell types.7,67 In initial observations cathelicidin expression in skin followed a pattern that was expected for a molecule involved in defense function. Cathelicidin expression is high in bacterial skin infection and induced by cutaneous barrier disruption, such as in invasive bacterial infection or physical injury of the skin.9,58 Still, the molecular regulation of cathelicidin transcription was long unclear because classic mediators of inflammation or infection did not influence expression.68
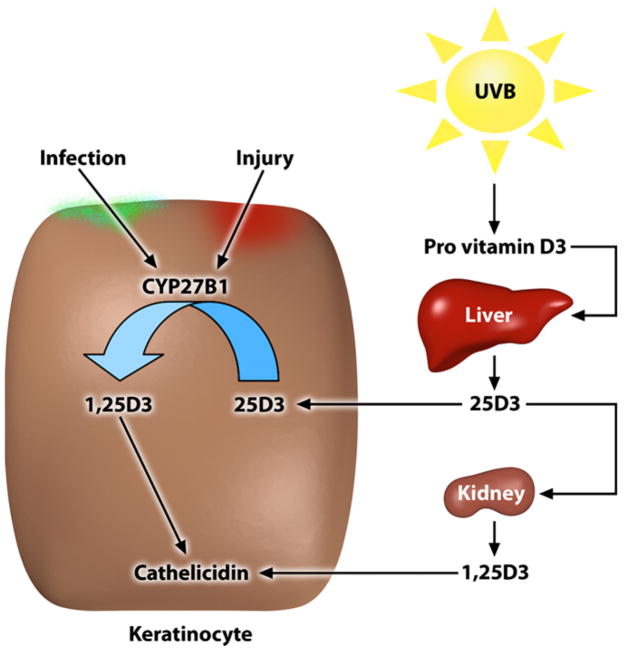
A breakthrough in the understanding of cathelicidin expression in the skin came with the identification of a vitamin D response element in the cathelicidin promoter.69 In the meantime, several research groups confirmed that cathelicidin is a direct target of vitamin D3 in keratinocytes.69–71 Additional elements of the vitamin D3 signaling cascade have been identified that lead to increased cathelicidin expression, such as recruitment of coactivators or epigenetic changes.72 Still, it was unclear how cathelicidin is induced in bacterial infections or in wounds, situations in which a sudden change in vitamin D3 levels seemed unlikely. The solution to this dilemma came with recognition that 1α-hydroxylase (CYP27B1) executes a hydroxylation step in the skin that generates the most biologically active form of vitamin D3 (1,25D3).73 This activation step for vitamin D3 occurs in monocytes and keratinocytes by CYP27B1 and is under the control of inflammatory stimuli combined with TLR2 (Fig 2).59,73,74 On skin injury or bacterial infection, there is a local increase in expression of CYP27B1, and as a direct consequence, more vitamin D3 is activated to induce cathelicidin expression and function.59,73
FIG 2.
Mechanisms of vitamin D3 activation and cathelicidin response. Extrarenal metabolism of vitamin D3 by keratinocytes provides a system for rapid control of cathelicidin expression. Activation of 25D3 to 1,25D3 requires 2 hydroxylation steps that occur sequentially in the liver and kidney. However, keratinocytes also express CYP27B1, a 1α-hydroxylase that activates 1,25D3. CYP27B1 expression in keratinocytes is controlled by danger signals during skin infection and tissue damage.
VITAMIN D3 AND SKIN IMMUNE DEFENSE
Vitamin D3 has been well studied as an essential factor for calcium homeostasis and bone metabolism but is less known as a regulator of immunity.75 In particular, vitamin D3 has been suggested to enable efficient antimicrobial defense at epithelial surfaces, such as airways or skin.68,76 These data are epidemiologically relevant because vitamin D3 deficiency is common, especially in the elderly, and might contribute to increased morbidity and mortality.77,78 Low vitamin D3 levels are suggested to arise mainly from insufficient dietary intake and a predominant indoor lifestyle. Recommendations to limit sun exposure to prevent skin cancer further complicate the ongoing debate about the health benefits of vitamin D3.78 Vitamin D3 or cholecalciferol is added in food fortification, and it is suggested that people in industrialized countries maintain their vitamin D3 needs through the intake of such fortified foods. However, the human body is able to produce sufficient vitamin D3 provided that there is adequate vitamin D3 precursor and minimal UVB exposure.75 Several human cell types are involved in synthesizing and activating vitamin D3. Synthesis of previtamin D3 from 7-dehydrocholesterol occurs in the skin and involves UVB radiation that penetrates the epidermis. 7-Dehydrocholesterol absorbs UV light most effectively at wavelengths between 270 and 290 nm, and thus the production of vitamin D3 will occur at those wavelengths. Calciol, which is the product of the transformation of 7-dehydrocholesterol, is an inactive, unhydroxylated form of vitamin D3. Caciol must be hydroxylated twice to form calcidiol (25 hydrox vitamin D3 [25D3]) and finally active calcitriol (1,25D3; Fig 2) to form active prohormone vitamin D3. The 2 enzymes responsible for activating vitamin D3, vitamin D 25-hydroxylase (CYP2R1) and 25-hydroxyvitamin D3 1α-hydroxylase (CYP27B1), were initially identified in the liver and kidney.79 Also, keratinocytes express both enzymes and are capable of producing active 1,25D3 independent of renal and hepatic hydroxylation steps (Fig 2).74 In skin this is important because the presence of vitamin D3 is essential for normal keratinocyte development and function.80 In an autocrine fashion 1,25D3 regulates keratinocyte proliferation, differentiation, and the formation of an intact epidermal barrier. Alterations in local vitamin D3 concentrations, activation, or both will likely affect normal keratinocyte function and formation of the antimicrobial barrier.80–82
THERAPEUTIC TARGETING OF CATHELICIDIN THROUGH THE VITAMIN D3 PATHWAY
Understanding the molecular elements of cathelicidin expression might lead to new treatments for inflammatory skin diseases (and help explain mechanisms of current therapies). As mentioned above, cathelicidin expression is regulated through the vitamin D3 pathway and involves epigenetic changes, such as histone acetylation.72 Targeting vitamin D3 metabolism and signaling might be beneficial in atopic dermatitis, rosacea, and psoriasis. Several possible clinical applications are conceivable.
In the treatment of atopic dermatitis, UVB therapy is frequently used. Currently, the effect of UVB irradiation is attributed to its effects on T cells and T cell–mediated immune responses.83 As outlined above, the underlying beneficial effect of UVB therapy could also be a result of the activation of cutaneous vitamin D3 synthesis.84 Oral supplementation of 1,25D3 or vitamin D3 precursors might be beneficial in atopic dermatitis as well. 1,25D3 increases cathelicidin expression and antimicrobial activity in keratinocytes in vitro.68,69 Increasing vitamin D3 metabolism or increasing vitamin D3 serum levels could contribute to the restoration of an effective barrier in atopic dermatitis. However, because topical 1,25D3 has been reported to induce skin irritation and an atopic dermatitis–mimicking phenotype in mice, further clinical and experimental studies have to be performed to prove its benefits.85
Patients with rosacea might benefit from therapies blocking cathelicidin expression and processing. Polymorphisms in the vitamin D receptor gene have been described in patients with severe rosacea, indicating that vitamin D3 signaling is involved in pathogenesis.86 Blocking cathelicidin expression by targeting the vitamin D3 pathway might represent a novel therapeutic approach in rosacea. As an example, vitamin D3 analogs without intrinsic activity at the vitamin D receptor have been shown to inhibit 1,25D3-induced cathelicidin in keratinocytes in vitro.59 Blocking protease activity in the skin of patients with rosacea might serve as an alternative approach. Interestingly, tetracyclines, which are commonly used in the treatment of rosacea, can inhibit metallo-proteases.64 It is conceivable that tetracyclines exhibit beneficial effects in patients with rosacea by inhibiting cathelicidin processing and thereby blocking the alarmin functions of this AMP.
Finally, in psoriasis blocking cathelicidin peptide could break the vicious cycle of increased LL-37 expression, pDC activation, and cutaneous inflammation. Again, strategies to decrease cathelicidin expression in keratinocytes could target vitamin D3 signaling. Paradoxically, for a long time, vitamin D3 analogs have been used in the therapy of psoriasis. Vitamin D3 analogs bind to and activate the vitamin D receptor and should therefore increase cathelicidin expression in keratinocytes, presumably worsening inflammation in patients with psoriasis. However, the opposite is true: vitamin D analogs resemble one of the pillars of topical psoriasis treatment. They ameliorate cutaneous inflammation and reverse morphologic changes within lesional skin.87 Understanding the molecular effects of vitamin D3 analogs on cutaneous innate immune function will eventually also lead to better treatment.
In summary, influencing cathelicidin expression through vitamin D3 signaling might offer a new approach in the therapy of very common skin diseases. However, until the “sunshine vitamin” can be targeted, additional experimental work and clinical studies have to be performed to prove its safety and benefits. Overall, current data overwhelmingly support the importance of AMPs to healthy human skin, but the key steps to put this information to therapeutic use remain to be examined.
Abbreviations
- AMP
Antimicrobial peptide
- 1,25D3
1,25 dihydroxy vitamin D3
- 25D3
25 hydroxy vitamin D3
- pDC
Plasmacytoid dendritic cell
- TLR
Toll-like receptor
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
References
- 1.Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005;125:9–13. doi: 10.1111/j.0022-202X.2004.23587.x. [DOI] [PubMed] [Google Scholar]
- 2.Schauber J, Gallo RL. Expanding the roles of antimicrobial peptides in skin: alarming and arming keratinocytes. J Invest Dermatol. 2007;127:510–2. doi: 10.1038/sj.jid.5700761. [DOI] [PubMed] [Google Scholar]
- 3.Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274–8. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 4.Lee DY, Yamasaki K, Rudsil J, Zouboulis CC, Park GT, Yang JM, et al. Sebocytes express functional cathelicidin antimicrobial peptides and can act to kill Propionibacterium acnes. J Invest Dermatol. 2008;17:17. doi: 10.1038/sj.jid.5701235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005;73:6771–81. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murakami M, Ohtake T, Dorschner RA, Schittek B, Garbe C, Gallo RL. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J Invest Dermatol. 2002;119:1090–5. doi: 10.1046/j.1523-1747.2002.19507.x. [DOI] [PubMed] [Google Scholar]
- 7.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–93. [PubMed] [Google Scholar]
- 8.Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006;306:91–110. doi: 10.1007/3-540-29916-5_4. [DOI] [PubMed] [Google Scholar]
- 9.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 10.Marchini G, Lindow S, Brismar H, Stabi B, Berggren V, Ulfgren AK, et al. The newborn infant is protected by an innate antimicrobial barrier: peptide antibiotics are present in the skin and vernix caseosa. Br J Dermatol. 2002;147:1127–34. doi: 10.1046/j.1365-2133.2002.05014.x. [DOI] [PubMed] [Google Scholar]
- 11.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Wang L, Jia HP, Zhao C, Heng HH, Schutte BC, et al. Structure and mapping of the human beta-defensin HBD-2 gene and its expression at sites of inflammation. Gene. 1998;222:237–44. doi: 10.1016/s0378-1119(98)00480-6. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi M, Horiuchi Y, Tezuka T. Presence of bactericidal/permeability-increasing protein in human and rat skin. Exp Dermatol. 2004;13:55–60. doi: 10.1111/j.0906-6705.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 14.Cumberbatch M, Dearman RJ, Uribe-Luna S, Headon DR, Ward PP, Conneely OM, et al. Regulation of epidermal Langerhans cell migration by lactoferrin. Immunology. 2000;100:21–8. doi: 10.1046/j.1365-2567.2000.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schittek B, Hipfel R, Sauer B, Bauer J, Kalbacher H, Stevanovic S, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2:1133–7. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- 16.Rose FR, Bailey K, Keyte JW, Chan WC, Greenwood D, Mahida YR. Potential role of epithelial cell-derived histone H1 proteins in innate antimicrobial defense in the human gastrointestinal tract. Infect Immun. 1998;66:3255–63. doi: 10.1128/iai.66.7.3255-3263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchau AS, Hassan M, Kukova G, Lewerenz V, Kellermann S, Wurthner JU, et al. S100A15, an antimicrobial protein of the skin: regulation by E. coli through Toll-like receptor 4. J Invest Dermatol. 2007;127:2596–604. doi: 10.1038/sj.jid.5700946. [DOI] [PubMed] [Google Scholar]
- 18.Harder J, Schroder JM. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem. 2002;277:46779–84. doi: 10.1074/jbc.M207587200. [DOI] [PubMed] [Google Scholar]
- 19.Gallo RL, Ono M, Povsic T, Page C, Eriksson E, Klagsbrun M, et al. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci U S A. 1994;91:11035–9. doi: 10.1073/pnas.91.23.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harwig SS, Chen NP, Park AS, Lehrer RI. Purification of cysteine-rich bioactive peptides from leukocytes by continuous acid-urea-polyacrylamide gel electrophoresis. Anal Biochem. 1993;208:382–6. doi: 10.1006/abio.1993.1065. [DOI] [PubMed] [Google Scholar]
- 21.Caccavo D, Pellegrino NM, Altamura M, Rigon A, Amati L, Amoroso A, et al. Antimicrobial and immunoregulatory functions of lactoferrin and its potential therapeutic application. J Endotoxin Res. 2002;8:403–17. doi: 10.1179/096805102125001000. [DOI] [PubMed] [Google Scholar]
- 22.Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–5. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 23.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177:1458–64. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 24.Domachowske JB, Dyer KD, Adams AG, Leto TL, Rosenberg HF. Eosinophil cationic protein/RNase 3 is another RNase A-family ribonuclease with direct antiviral activity. Nucleic Acids Res. 1998;26:3358–63. doi: 10.1093/nar/26.14.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang YQ, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun. 2002;70:6524–33. doi: 10.1128/IAI.70.12.6524-6533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaiou M, Nizet V, Gallo RL. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J Invest Dermatol. 2003;120:810–6. doi: 10.1046/j.1523-1747.2003.12132.x. [DOI] [PubMed] [Google Scholar]
- 27.Wingens M, van Bergen BH, Hiemstra PS, Meis JF, van Vlijmen-Willems IM, Zeeuwen PL, et al. Induction of SLPI (ALP/HUSI-I) in epidermal keratinocytes. J Invest Dermatol. 1998;111:996–1002. doi: 10.1046/j.1523-1747.1998.00425.x. [DOI] [PubMed] [Google Scholar]
- 28.Simpson AJ, Maxwell AI, Govan JR, Haslett C, Sallenave JM. Elafin (elastase-specific inhibitor) has anti-microbial activity against gram-positive and gram-negative respiratory pathogens. FEBS Lett. 1999;452:309–13. doi: 10.1016/s0014-5793(99)00670-5. [DOI] [PubMed] [Google Scholar]
- 29.Meyer-Hoffert U, Wichmann N, Schwichtenberg L, White PC, Wiedow O. Supernatants of Pseudomonas aeruginosa induce the Pseudomonas-specific antibiotic elafin in human keratinocytes. Exp Dermatol. 2003;12:418–25. doi: 10.1034/j.1600-0625.2002.120409.x. [DOI] [PubMed] [Google Scholar]
- 30.Blankenvoorde MF, van’t Hof W, Walgreen-Weterings E, van Steenbergen TJ, Brand HS, Veerman EC, et al. Cystatin and cystatin-derived peptides have antibacterial activity against the pathogen Porphyromonas gingivalis. Biol Chem. 1998;379:1371–5. [PubMed] [Google Scholar]
- 31.Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 32.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR-CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–7. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 33.Cutuli M, Cristiani S, Lipton JM, Catania A. Antimicrobial effects of alpha-MSH peptides. J Leukoc Biol. 2000;67:233–9. doi: 10.1002/jlb.67.2.233. [DOI] [PubMed] [Google Scholar]
- 34.Kowalska K, Carr DB, Lipkowski AW. Direct antimicrobial properties of substance P. Life Sci. 2002;71:747–50. doi: 10.1016/s0024-3205(02)01740-x. [DOI] [PubMed] [Google Scholar]
- 35.Tasiemski A, Hammad H, Vandenbulcke F, Breton C, Bilfinger TJ, Pestel J, et al. Presence of chromogranin-derived antimicrobial peptides in plasma during coronary artery bypass surgery and evidence of an immune origin of these peptides. Blood. 2002;100:553–9. doi: 10.1182/blood.v100.2.553. [DOI] [PubMed] [Google Scholar]
- 36.Kieffer AE, Goumon Y, Ruh O, Chasserot-Golaz S, Nullans G, Gasnier C, et al. The N- and C-terminal fragments of ubiquitin are important for the antimicrobial activities. FASEB J. 2003;17:776–8. doi: 10.1096/fj.02-0699fje. [DOI] [PubMed] [Google Scholar]
- 37.Lambert RW, Campton K, Ding W, Ozawa H, Granstein RD. Langerhans cell expression of neuropeptide Y and peptide YY. Neuropeptides. 2002;36:246–51. doi: 10.1016/s0143-4179(02)00020-3. [DOI] [PubMed] [Google Scholar]
- 38.Radek KA, Lopez-Garcia B, Hupe M, Niesman IR, Elias PM, Taupenot L, et al. The neuroendocrine peptide catestatin is a cutaneous antimicrobial and induced in the skin after injury. J Invest Dermatol. 2008 Jan 10; doi: 10.1038/sj.jid.5701225. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allaker RP, Zihni C, Kapas S. An investigation into the antimicrobial effects of adrenomedullin on members of the skin, oral, respiratory tract and gut microflora. FEMS Immunol Med Microbiol. 1999;23:289–93. doi: 10.1111/j.1574-695X.1999.tb01250.x. [DOI] [PubMed] [Google Scholar]
- 40.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 41.Iimura M, Gallo RL, Hase K, Miyamoto Y, Eckmann L, Kagnoff MF. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J Immunol. 2005;174:4901–7. doi: 10.4049/jimmunol.174.8.4901. [DOI] [PubMed] [Google Scholar]
- 42.Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–41. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 43.Cowland JB, Johnsen AH, Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–6. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 44.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, Gudmundsson GH. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci U S A. 1995;92:195–9. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–80. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 46.Murakami M, Lopez-Garcia B, Braff M, Dorschner RA, Gallo RL. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J Immunol. 2004;172:3070–7. doi: 10.4049/jimmunol.172.5.3070. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005;125:108–15. doi: 10.1111/j.0022-202X.2005.23713.x. [DOI] [PubMed] [Google Scholar]
- 48.Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007;601:185–94. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- 49.Yang D, Chertov O, Oppenheim JJ. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37) J Leukoc Biol. 2001;69:691–7. [PubMed] [Google Scholar]
- 50.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 51.Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–8. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 52.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol. 2001;31:1066–75. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 53.Niyonsaba F, Ushio H, Nagaoka I, Okumura K, Ogawa H. The human beta-defensins (−1, −2, −3, −4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J Immunol. 2005;175:1776–84. doi: 10.4049/jimmunol.175.3.1776. [DOI] [PubMed] [Google Scholar]
- 54.Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol. 2007;157:1124–31. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]
- 55.Di Nardo A, Braff MH, Taylor KR, Na C, Granstein RD, McInturff JE, et al. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J Immunol. 2007;178:1829–34. doi: 10.4049/jimmunol.178.3.1829. [DOI] [PubMed] [Google Scholar]
- 56.Yu J, Mookherjee N, Wee K, Bowdish DM, Pistolic J, Li Y, et al. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J Immunol. 2007;179:7684–91. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]
- 57.Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–8. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–7. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 59.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 61.Howell MD, Wollenberg A, Gallo RL, Flaig M, Streib JE, Wong C, et al. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006;117:836–41. doi: 10.1016/j.jaci.2005.12.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murakami M, Dorschner RA, Stern LJ, Lin KH, Gallo RL. Expression and secretion of cathelicidin antimicrobial peptides in murine mammary glands and human milk. Pediatr Res. 2005;57:10–5. doi: 10.1203/01.PDR.0000148068.32201.50. [DOI] [PubMed] [Google Scholar]
- 64.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–80. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 65.Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008;40:23–5. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 67.Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005;124:394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
- 68.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–19. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang T-T, Nestel F, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 Is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 70.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 71.Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124:1080–2. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 72.Schauber J, Oda Y, Buchau AS, Yun QC, Steinmeyer A, Zugel U, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D(3) J Invest Dermatol. 2008;128:816–24. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- 73.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 74.Bikle DD, Pillai S, Gee E, Hincenbergs M. Regulation of 1,25-dihydroxyvitamin D production in human keratinocytes by interferon-gamma. Endocrinology. 1989;124:655–60. doi: 10.1210/endo-124-2-655. [DOI] [PubMed] [Google Scholar]
- 75.Bikle DD. What is new in vitamin D: 2006–2007. Curr Opin Rheumatol. 2007;19:383–8. doi: 10.1097/BOR.0b013e32818e9d58. [DOI] [PubMed] [Google Scholar]
- 76.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3) J Cyst Fibros. 2007;6:403–10. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Allain TJ, Dhesi J. Hypovitaminosis D in older adults. Gerontology. 2003;49:273–8. doi: 10.1159/000071707. [DOI] [PubMed] [Google Scholar]
- 78.Moan J, Porojnicu AC, Dahlback A, Setlow RB. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proc Natl Acad Sci U S A. 2008;105:668–73. doi: 10.1073/pnas.0710615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29:664–73. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 80.Bikle DD. Vitamin D regulated keratinocyte differentiation. J Cell Biochem. 2004;92:436–44. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- 81.Oda Y, Sihlbom C, Chalkley RJ, Huang L, Rachez C, Chang CP, et al. Two distinct coactivators, DRIP/mediator and SRC/p160, are differentially involved in VDR transactivation during keratinocyte differentiation. J Steroid Biochem Mol Biol. 2004;89–90:273–6. doi: 10.1016/j.jsbmb.2004.03.106. [DOI] [PubMed] [Google Scholar]
- 82.Bikle D, Chang S, Crumrine D, Elalieh H, Man M, Dardenne O, et al. Mice lacking 25OHD 1alpha-hydroxylase demonstrate decreased epidermal differentiation and barrier function. J Steroid Biochem Mol Biol. 2004;1–5:347–53. doi: 10.1016/j.jsbmb.2004.03.113. [DOI] [PubMed] [Google Scholar]
- 83.Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem Photobiol. 2008;84:10–8. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 84.Lehmann B, Genehr T, Knuschke P, Pietzsch J, Meurer M. UVB-induced conversion of 7-dehydrocholesterol to 1alpha, 25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J Invest Dermatol. 2001;117:1179–85. doi: 10.1046/j.0022-202x.2001.01538.x. [DOI] [PubMed] [Google Scholar]
- 85.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. 2006;103:11736–41. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jansen T, Krug S, Kind P, Plewig G, Messer G. BsmI polymorphism of the vitamin D receptor gene in patients with the fulminant course of rosacea conglobata (rosacea fulminans) J Dermatol. 2004;31:244–6. doi: 10.1111/j.1346-8138.2004.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 87.Lebwohl M, Menter A, Koo J, Feldman SR. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004;50:416–30. doi: 10.1016/j.jaad.2002.12.002. [DOI] [PubMed] [Google Scholar]