Abstract
The lymphatic vascular system functions to maintain fluid homeostasis by removing fluid from the interstitial space and returning it to venous circulation. This process is dependent upon the maintenance and modulation of a semi-permeable barrier between lymphatic endothelial cells of the lymphatic capillaries. However, our understanding of the lymphatic endothelial barrier and the molecular mechanisms that govern its function remains limited. Adrenomedullin (AM) is a 52 amino acid secreted peptide which has a wide range of effects on cardiovascular physiology and is required for the normal development of the lymphatic vascular system. Here, we report that AM can also modulate lymphatic permeability in cultured dermal microlymphatic endothelial cells (HMVEC-dLy). AM stimulation caused a reorganization of the tight junction protein ZO-1 and the adherens protein VE-cadherin at the plasma membrane, effectively tightening the endothelial barrier. Stabilization of the lymphatic endothelial barrier by AM occurred independently of changes in junctional protein gene expression and AM−/− endothelial cells showed no differences in the gene expression of junctional proteins compared to wildtype endothelial cells. Nevertheless, local administration of AM in the mouse tail decreased the rate of lymph uptake from the interstitial space into the lymphatic capillaries. Together, these data reveal a previously unrecognized role for AM in controlling lymphatic endothelial permeability and lymphatic flow through reorganization of junctional proteins.
Keywords: Adrenomedullin, VEGFA, Permeability, Lymphatic, Endothelial, Lymphography
1. Introduction
1.1
The lymphatic vascular system is a blind-ended network of endothelial cell lined vessels that functions to maintain fluid homeostasis by unidirectionally transporting tissue fluid, extravasated plasma proteins, lipids, and cells from the interstitium to the circulatory system by way of the thoracic duct. When the lymphatic vascular system fails to function properly patients are at risk of developing serious and debilitating lymphedema. Pressure sensing, fibrillin-rich anchoring filaments that tether lymphatic endothelial cells (LECs) to the extracellular matrix contract in response to increases in interstitial pressure, thereby stretching LECs apart and facilitating lymph uptake into lymphatic capillaries. However, recent evidence suggests that LECs are also active participants in lymph transport through formation of an endothelial barrier that can regulate both ion and protein transport [3, 7, 19]. While these studies have implicated vascular endothelial growth factors A and C (VEGFA, VEGFC) and the intracellular signaling molecule cAMP as potential players in LEC permeability regulation, there remains a need to identify pharmacologically tractable targets that can efficiently modulate lymphatic permeability.
Adrenomedullin (AM) is a highly conserved 52 amino acid peptide vasodilator that is upregulated in a variety of cardiovascular conditions [12, 13, 23]. First recognized for its ability to maintain vascular smooth muscle tone, AM is also an important regulator of endothelial cell biology. For example, numerous in vitro studies have shown that AM is a potent angiogenic factor [10, 20, 32]. Our own recent studies using knockout mice have revealed that AM and its receptors are required for normal lymphatic vascular development [11].
With regard to endothelial permeability, AM treatment of human umbilical vein endothelial cell (HUVEC) monolayers dose dependently reduced hyperpermeability caused by inflammatory mediators including H2O2, thrombin, E. coli hemolysin and S. aureus α-toxin [2, 14, 16] The protective effect of AM on the endothelial barrier has also been shown in vivo in rat ileum exposed to S. aureus α-toxin and ex vivo in rabbit lungs exposed to H2O2 [2, 14]. Further, in the blood-brain barrier, several studies have shown that AM treatment increased transendothelial electrical resistance thereby reducing endothelial permeability [18, 21, 22]. Taken together, these data suggest AM functions as a potent factor in maintaining the blood endothelial barrier; however, whether this function is conserved in LECs and to what extent remains unknown.
The purpose of this study was to explore the role of AM in the regulation of lymphatic permeability. We evaluated the effects of AM on the expression and localization of LEC junction components. Specifically, the response to AM treatment of the tight junction molecules Zonulus Occludin (ZO-1), Claudin-5, Claudin-12, and Junction Adhesion Molecular C (JAMC) as well as the endothelial-specific adherens protein VE-cadherin were assessed. Further, the ability of AM to modulate LEC permeability was measured utilizing both in vitro and in vivo approaches. The in vivo studies exploited the technique of fluorescent tail microlymphography and provided functional validation of our in vitro observations. Together, these data establish a previously unrecognized role of AM in stabilizing of the endothelial barrier and modulating lymphatic flow in vivo.
2. Materials and Methods
2.1. Cell Culture
Cryopreserved adult human dermal lymphatic microvascular endothelial cells (HMVEC-dLys) were obtained from Cambrex (Walkersville, MD) and maintained in Clonetics (East Rutherford, NJ) microvascular endothelial growth medium (EGM-2MV) and endothelial basal medium (EBM), respectively. Human AM and human AM 22–52 were purchased from American Peptide (Sunnyvale, CA), Forskolin from Sigma-Aldrich, and recombinant human VEGFA from Pierce Biotechnology. Goat anti-VE-cadherin (C-19) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti- ZO-1 antibody (33–9100) was obtained from Zymed (San Francisco, CA). Cy2 and Cy3-labeled secondary antibodies were from Jackson Immunoresearch (West Grove, PA).
2.2 Immunofluorescence labeling and microscopy
For ZO-1 and VE-cadherin localization, cells were grown on 0.2% gelatin coated coverslips until they achieved confluence. The medium was changed to serum-free conditions and the cells were stimulated with AM (100 nM), VEGFA (10 ng/mL) or vehicle (0.1% BSA) for 30 min. The cells were then washed twice with PBS with calcium and then fixed/permeabilized in ice-cold ethanol for 30 min. Cells were blocked with 3% BSA in PBS for 1 h at RT and incubated with primary antibody in 3% BSA overnight at 4°C and with appropriate secondary antibody for 1 h at RT. Nuclei were labeled with Hoechst 33258 (Sigma). The coverslips were mounted on glass slides and images were obtained with a Nikon E800 microscope with a Hammamatsu ORCA-ER CCD camera using Metamorph Software (Molecular Devices) and processed in Adobe Photoshop 8.0 (Adobe).
2.3. Endothelial cell permeability
HMVEC-dLys permeability was studied as previously described [6, 24, 27]. Briefly, cells were plated at a density of 1 × 105 cells/cm2 on gelatin-coated membranes (Corning Costar Transwells, 0.4 µm pore size, 6.5 mm diameter) and medium was changed every 24 h. Permeability of monolayers was measured in terms of Trypan Blue-BSA (TB-BSA) transfer [27]. At 96 h post seeding, membranes were incubated with test ligands in HBSS containing 0.03 M HEPES (HBSS/HEPES) in both the apical and basal-lateral chambers. In the apical chamber, 4% TB-BSA was added with or without test ligands followed by gentle shaking at 37°C/5% CO2 under sterile conditions for 90 min. At the end of the incubation, samples were taken from the lower chamber and the absorbance at 590nm was measured. The relative permeability was calculated by dividing the OD of treated samples by vehicle control.
2.4. Gene Expression Analysis
Total RNA was isolated from cells using the Qiagen RNAEasy Mini Kit and DNase Treated with Promega DNase per manufactures instructions. RNA was reverse transcribed using MMV reverse transcriptase (Invitrogen). cDNA was used for semi-quantitative RT-PCR or quantitative RT-PCR using gene-specific primers, SYBR Green (Stratagene), and the Stratagene MXP3000 and MxPro Software (Stratagene). The following murine primers sequences were used: VE-Cadherin: Forward: 5’-GGTGGCCAAAGACCCTGAC-3’ Reverse: 5’-ACTGGTCTTGCGGATGGAGT-3’, JAMC: Forward: 5’-GCTGGGAGAGCACATGCAA-3’ Reverse: 5’-CAGGAGCTCTGGGCTCACA-3’. Primers sequences for ZO-1, Claudin-5 and Claudin-12 were designed by the report by Holmes et al. [17]. The following human primers sequences were used: VE-Cadherin: Forward: 5’-GCCAGGTATGAGATCGTGGT-3’ Reverse: 5’-GTGTCTTCAGGCACGACAAA-3. Primer sets for human ZO-1, Claudin-5, Claudin-12, and JAMC were purchased from Qiagen.
2.5. Isolation of AM−/− Endothelial Cells
The generation and characterization of mice with targeted deletion of the Adm gene has been previously described [4]. To isolate AM−/− endothelial cells, timed matings were established between AM+/− mice and embryos were harvested at embryonic day 13.5. Genotyping was performed as previously described [4] on genomic DNA isolated from yolk sacs. Whole embryos were treated with enzyme solution containing 2.4U/mL Neutral Protease and 150U/mL Collagenase Type II (Worthington Biochemical) at 37°C for 30 minutes to generate a single cell suspension. Cells were incubated with 3 µg Rat anti-Mouse CD31 (BD Pharmigen) at 4°C for 30 minutes and then washed 3 times. The cells were then incubated with Sheep anti-Rat IgG Dynabeads (Invitrogen) at 4°C for 20 minutes and then washed 4 times. Bead-bound cells were collected for RNA purification and levels of gene expression for junctional proteins were determined as described above.
2.6. Quantitative Tail Microlymphography
The flow velocity of the dermal lymphatic vessels in the mouse tail was measured as previously described [25]. Briefly, adult male 129S6/SvEv mice were anesthetized by subcutaneous injection of avertin at a concentration of 0.5mg/gm body weight. Mice placed on a heating pad in the working space underneath a Leica MZ 16 FA dissecting microscope. The tail was immobilized on the pad with two-sided tape. Next, 1 µL of a 25% FITC-Dextran (2,000,000 kDa) with vehicle or AM peptide [10 ng/μL] was loaded into a microsyringe (Hamilton, Reno, NV) fitted with a 30-gauge needle and was injected intradermally at the tip of the mouse tail. The flow of FITC-Dextran through the dermal lymphatics was acquired with a camera fitted onto the Leica microscope every minute for 15 minutes. Flow rates were determined using offline image analysis (Adobe Photoshop 8.0 and Image J) and application of a stepwise polynomial equation [25]. The data for each subregion were fit to this equation with a least squares nonlinear regression algorithm. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
2.7. Statistics
Results are represented as +/− S.E.M. Statistical analyses were performed with a Student t test with unequal variance. A P value of<.05 was considered statistically significant.
3. Results
3.1. AM prevents VEGFA-mediated increase in lymphatic endothelial cell permeability
HMVEC-dLys were grown to a monolayer in transwells and paracellular permeability was measured by the ability of Trypan Blue-labeled bovine serum albumin (TB-BSA) to pass through a monolayer from the apical chamber to the basal lateral chamber in the presence or absence of AM or VEGFA [6]. Treatment with AM did not change the basal permeability of the HMVEC-dLy monolayer compared to vehicle control (Figure 1). As expected, treatment with VEGFA resulted in a significant increase in permeability across the HMVEC-dLys monolayer. AM stimulation of LECs in the presence of VEGFA dose-dependently prevented the VEGFA-induced increase in permeability of the HMVEC-dLys monolayer to albumin. Therefore, although AM did not affect LEC permeability under basal conditions, it was highly effective at counteracting the permeabilizing effects of VEGFA on cultured LECs.
Figure 1.
Effects of AM on the permeability of HMVEC-dLy monolayers. Cells were treated with 0.1% BSA alone, AM (100 nM), VEGFA (10 ng/mL), or VEGFA with increasing concentrations of AM (0.1 nM–100 nM) for 90 min. Relative changes in permeability were determined by measuring TB-BSA transfer for each treatment normalized to vehicle. control. #P<0.01 compared to vehicle control. *P<0.01 compared to VEGFA treated. Data represented as mean +/− SEM from 3 independent experiments, each performed in triplicate.
3.2. AM stabilizes lymphatic endothelial cell-cell junctions
HMVEC-dLys were grown to confluence and then treated with vehicle control, AM, VEGFA, or the combination of AM with VEGFA. Vehicle treated HMVEC-dLys displayed a mosaic pattern of ZO-1 and VE-cadherin staining that was characterized by both continuous and discontinuous patches along the cell-cell membranes (Figure 2A and 2D). Treatment of HMVEC-dLys with AM resulted in the formation of continuous tight junction strands as indicated by the tight junction protein ZO-1 (Figure 2B). Further, AM caused a reorganization of VE-cadherin at the plasma membrane which appeared as a linearized adherence junction band compared to vehicle treated cells (Figure 2F). As expected, treatment with VEGFA dramatically disrupted of both ZO-1 and VE-cadherin localization, indicated by gaps and an intermittent, zipper-like staining pattern (Figures 2C and 2G). Surprisingly, co-treatment of AM with VEGFA completely abrogated the VEGFA induced disruption ZO-1 and VE-cadherin localization at the plasma membrane (Figure 2D and 2H). We also noticed that AM treatment appeared to only affect the localization of ZO-1 and VE-cadherin, but not the total amount of protein present at the cellular junctions. These data demonstrate that AM functions to reorganize LEC paracellular junctions by forming a tighter paracellular seal between cells and preventing their disruption by permeabilizing agents such as VEGFA.
Figure 2.
AM stabilized the endothelial barrier in HMVEC-dLys in the presence of VEGFA. (A–B) Treatment with 0.1% BSA (Veh.) showed intact monolayer as visualized by ZO-1 (A) and VE-cadherin (B). (C–D) Stimulation with 100 nM AM resulted in linearization of both ZO-1 (C) and VE-cadherin (D) at the cell-cell junction. (E–F) VEGFA (10 ng/mL) treated cells displayed discontinued ZO-1 (E) and VE-cadherin staining (F). (G–H) Co-treatment of AM with VEGFA stabilized ZO-1 (G) and VE-cadherin (H); completely preventing VEGFA-mediated barrier disruption. Original magnification 400X. Each image is representative of each experimental condition imaged from 4 separate fields repeated 3 times in triplicate.
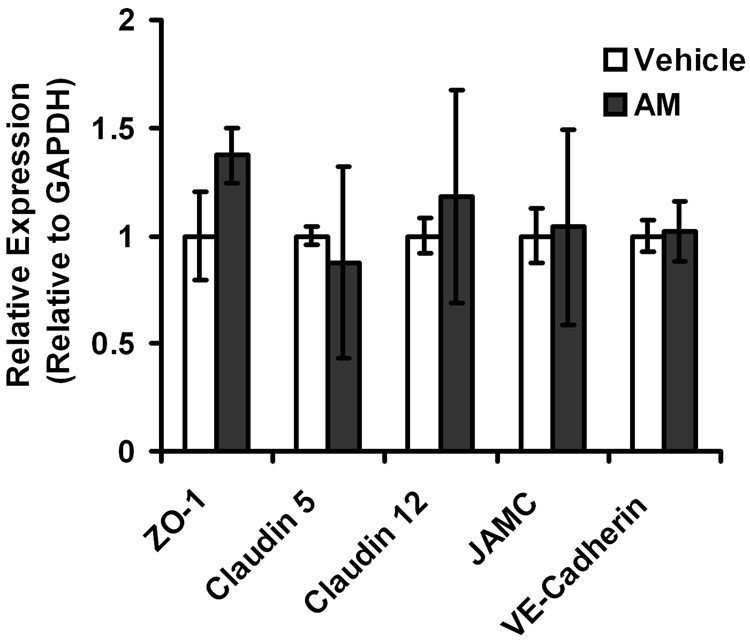
3.3. AM stimulation does not change the expression of junction genes at the RNA level
RNA was isolated from LECs treated with AM or with vehicle control and gene expression was measured by quantitative RT-PCR. There was no significant difference in the expression of ZO-1, Claudin-5, Claudin-12, JAMC, or VE-cadherin between AM treated and vehicle control (Figure 3). Therefore, the effects of AM on LEC junction composition are independent of changes in gene expression, suggesting that modulation of lymphatic function by AM is likely to occur rapidly in vivo.
Figure 3.
Stimulation of HMVEC-dLys with AM does not affect gene expression of junction components. Total RNA was isolated from HMVEC-dLys treated with 0.1% BSA or 100 nM AM and the gene expression of ZO-1, Claudin-5, Claudin-12, JAMC, and VE-cadherin were assessed relative to GAPDH. No statistical significant differences were found between the two groups suggesting the effect of AM on barrier function is independent to changes in gene expression of junction components. n>3 with each assay performed in duplicate.
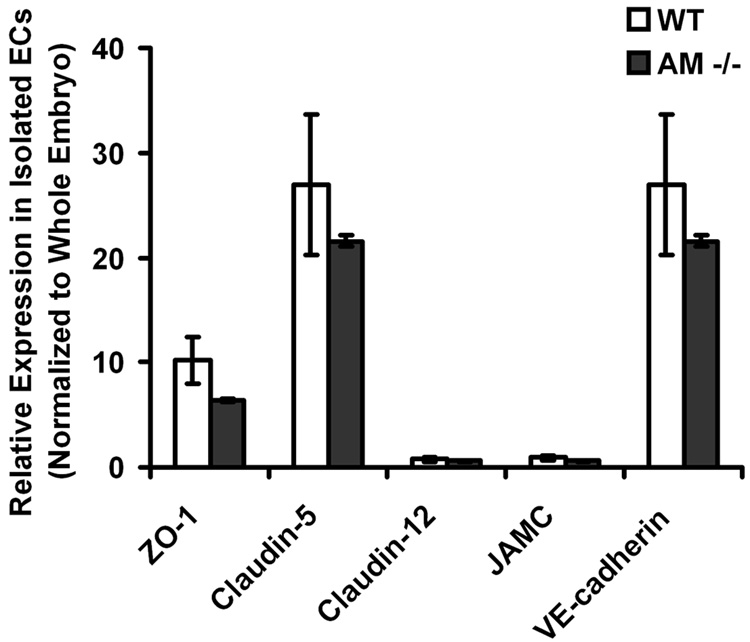
3.4. Genetic depletion of AM does not change the expression of junction genes at the RNA level
To determine whether a genetic reduction in AM could influence the expression of junction genes in vivo, we isolated endothelial cells from AM−/− mice at embryonic day 13.5. Although somewhat reduced, the gene expression levels of ZO-1, Claudin-5, Claudin-12, JAMC and VE-cadherin were not statistically different in AM−/− endothelial cells compared to endothelial cells isolated from wildtype littermate controls (Figure 4). Therefore, even though AM−/− mice die at mid-gestation with vascular defects, the loss of AM in endothelial cells does not affect the expression of genes important for endothelial barrier function.
Figure 4.
Genetic loss of AM does not affect gene expression of junction components in vivo. Using magnetic bead purification, endothelial cells were isolated from AM−/− and wildtype littermate embryos at embryonic day 13.5. The gene expression of ZO-1, Claudin-5, Claudin-12, JAMC, and VE-cadherin were determined and normalized to their expression in whole embryo. Although AM−/− endothelial cells had somewhat diminished gene expression levels, there were no statistically significant differences compared to wildtype endothelial cells. n>3 with each assay performed in duplicate.
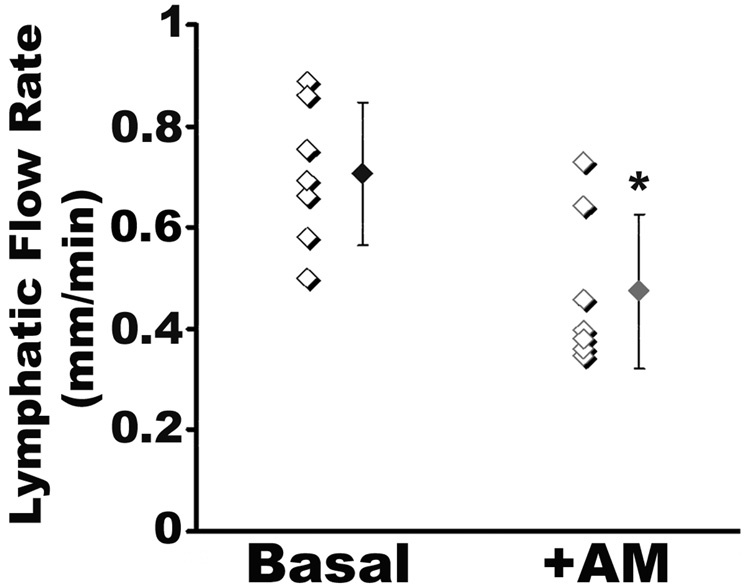
3.5. AM reduces lymphatic flow in vivo
To address whether the permeability effect of AM on LECs could result in altered lymphatic flow in vivo we used fluorescent tail microlymphography in adult male mice. Like in humans, lymphography of the mouse tail provides a sensitive and quantitative measure of dermal lymphatic capillary flow that is largely independent of the extrinsic effects of blood flow on lymphatic function. Briefly, FITC-dextran in the presence or absence of AM was injected into the interstitial space of the mouse tail, and the ability of the dextran to enter the lymphatic capillaries was monitored over time. Consistent with previously published results from other groups [1, 25, 31], the basal lymphatic flow rates in the wild-type SvEV129/6 mouse tail was 0.78 mm/min (Figure 5). When AM was co-administered with the FITC-dextran, we consistently found a significant decrease in lymphatic flow rates, with an average uptake rate of 0.52 mm/min. Therefore, these data demonstrate that AM can potently reduce lymphatic flow rates in vivo.
Figure 5.
AM decreases lymph uptake into the lymphatic capillaries in the mouse tail. Tails of SvEv129/6 wildtype mice were injected with 200 kDa FITC-Dextran and movement of the labeled-dye into the lymphatic capillaries was measured over time. The rate of uptake in the absence of AM was 0.78mm/min while the rate of uptake in the presence of AM was significantly decreased to 0.52mm/min. P*<0.01. n>7 for each treatment group.
4. Discussion
4.1
In the present study, we demonstrate that AM stabilizes the barrier function of LECs both in vitro and in vivo. AM dose-dependently prevented VEGFA-mediated increases in permeability of cultured HMVEC-dLys to albumin. Molecularly, AM treatment reorganized ZO-1 and VE-cadherin at the plasma membrane to form continuous cell-cell contacts, thereby limiting paracellular transport. In addition, we showed that exogenous AM peptide effectively slowed lymphatic flow rates in the mouse tail. Importantly, the ability of AM to modulate lymphatic barrier functions was independent of changes in gene expression of tight junction and adherens junction components both in vitro and in a genetic knockout mouse model.
Previous studies have shown that AM can stabilize the endothelial barrier of various blood vascular beds during inflammatory conditions (H2O2, thrombin, S. aureus α-toxin, E. coli. Hemolysin) and thus presumably reduce edema formation [2, 14, 16, 32]. However in another study, AM increased inflammatory edema accumulation caused by substance P and bradykinin [5]. Although these studies were not able to separate the direct anti-inflammatory functions of AM from its effects on vascular permeability, our study sheds new light of the in vivo functions of AM in vascular beds that contain both blood and lymphatic capillaries. We found that the ability of AM to maintain barrier function is not limited to blood endothelial cells but also affects LECs. Because lymphatic uptake was significantly reduced after co-administration of AM in vivo, our results suggest an essential role for AM in perpetuating inflammatory edema.
The VEGF family members VEGFA and VEGFC have both been shown to modulate lymphatic permeability. Targeted overexpression of VEGFA in the mouse epidermis exacerbated lymphatic leakage after acute UVB irradiation [19], while polymorphisms in the VEGFA promoter region leading to enhanced VEGFA expression have been attributed to hydrocele development in lymphatic filariasis [7]. In addition, VEGFC has been demonstrated to reduce the transendothelial electrical resistance (TEER) of a LEC monolayer [3]. While both VEGFA and VEGFC increase lymphatic permeability, AM represents one of the first angiogenic factors that can stabilize the lymphatic endothelial barrier. Therefore, the local interactions between VEGF family members and AM in regulating blood and lymphatic microcirculation warrant additional investigation.
We have previously shown that AM and its receptors are essential for the development and function of the lymphatic vasculature [11]. AM transduces its signal through a G-protein coupled receptor, calcitonin receptor-like receptor (CLR) when the receptor is associated with receptor activity modifying protein 2 (RAMP2) [26]. Mice that have targeted deletions of the genes that encode for either AM, CLR, or RAMP2 die at mid-gestation with generalized edema that is characterized by diminished proliferation of LECs [11]. A conditional knockout of CLR in endothelial cells using the Cre-LoxP homologous recombination system also resulted in a similar phenotype, supporting an essential function for AM signaling in endothelial cells [11]. Our studies, and those of others [28, 29], have shown that AM and its receptors are intrinsically enriched in LECs compared to blood vascular endothelial cells. Therefore, the enhanced sensitivity of LECs to local AM provides an explanation, in part, for the remarkable phenotype of the null mice. Our current study extends our understanding of AM function in LECs and suggests that an additional factor leading to the embryonic edema of the null mice may be leaky, hyperpermeable lymphatic capillaries that are unable to support lymphatic flow in vivo. Consistent with findings from other groups [22, 32], the fact that isolated endothelial cells lacking AM had normal expression of genes encoding junctional proteins further demonstrates that the effects of AM on the endothelial cell barrier are largely mediated by structural reorganization of the plasma membrane rather than changes in gene expression.
The unique molecular interface formed between the association of CLR with RAMPs provides a distinctive target for pharmacological drug design. In fact, two compounds that target the CLR-RAMP1 interface are currently in clinical trials for the treatment of migraine pain associated with calcitonin gene related peptide biology [8, 9, 15, 30]. Our current study suggests that modulation of AM activity through small molecule targeting of the CLR-RAMP2 complex could provide an effective means of altering lymphatic permeability for either the treatment of lymphedema or the inhibition of tumor metastasis through the lymphatic vasculature.
Acknowledgements
The authors gratefully acknowledge Drs. James M. Anderson, Allen Fanning and Christina Van Itallie for providing us with the ZO-1 antibodies and helpful advice and discussions. This work was supported in part by the UNC Developmental Biology Training Grant and AHA Pre-doctoral Fellowship (0815050E) to W.P.D. as well as The Burroughs Wellcome Fund, NIH/NHLBI (HL091973), and an American Heart Association grant (0555424U) to K.M.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berk DA, Swartz MA, Leu AJ, Jain RK. Transport in lymphatic capillaries. II. Microscopic velocity measurement with fluorescence photobleaching. Am J Physiol. 1996;270:H330–H337. doi: 10.1152/ajpheart.1996.270.1.H330. [DOI] [PubMed] [Google Scholar]
- 2.Brell B, Temmesfeld-Wollbruck B, Altzschner I, Frisch E, Schmeck B, Hocke AC, et al. Adrenomedullin reduces Staphylococcus aureus alpha-toxin-induced rat ileum microcirculatory damage. Crit Care Med. 2005;33:819–826. doi: 10.1097/01.ccm.0000159194.53695.7a. [DOI] [PubMed] [Google Scholar]
- 3.Breslin JW, Yuan SY, Wu MH. VEGF-C alters barrier function of cultured lymphatic endothelial cells through a VEGFR-3-dependent mechanism. Lymphat Res Biol. 2007;5:105–113. doi: 10.1089/lrb.2007.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caron KM, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc Natl Acad Sci U S A. 2001;98:615–619. doi: 10.1073/pnas.021548898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu DQ, Choy M, Foster P, Cao T, Brain SD. A comparative study of the ability of calcitonin gene-related peptide and adrenomedullin(13 – 52) to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589–1596. doi: 10.1038/sj.bjp.0703502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullen VC, Mackarel AJ, Hislip SJ, O'Connor CM, Keenan AK. Investigation of vascular endothelial growth factor effects on pulmonary endothelial monolayer permeability and neutrophil transmigration. Gen Pharmacol. 2000;35:149–157. doi: 10.1016/s0306-3623(01)00102-1. [DOI] [PubMed] [Google Scholar]
- 7.Debrah AY, Mand S, Toliat MR, Marfo-Debrekyei Y, Batsa L, Nurnberg P, et al. Plasma vascular endothelial growth Factor-A (VEGF-A) and VEGF-A gene polymorphism are associated with hydrocele development in lymphatic filariasis. Am J Trop Med Hyg. 2007;77:601–608. [PubMed] [Google Scholar]
- 8.Doods H. Development of CGRP antagonists for the treatment of migraine. Curr Opin Investig Drugs. 2001;2:1261–1268. [PubMed] [Google Scholar]
- 9.Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, et al. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Sauze S, Delfino C, Mabrouk K, Dussert C, Chinot O, Martin PM, et al. Effects of adrenomedullin on endothelial cells in the multistep process of angiogenesis: involvement of CRLR/RAMP2 and CRLR/RAMP3 receptors. Int J Cancer. 2004;108:797–804. doi: 10.1002/ijc.11663. [DOI] [PubMed] [Google Scholar]
- 11.Fritz-Six KL, Dunworth WP, Li M, Caron KM. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest. 2008;118:40–50. doi: 10.1172/JCI33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbons C, Dackor R, Dunworth W, Fritz-Six K, Caron KM. Receptor activitymodifying proteins: RAMPing up adrenomedullin signaling. Mol Endocrinol. 2007;21:783–796. doi: 10.1210/me.2006-0156. [DOI] [PubMed] [Google Scholar]
- 13.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 14.Hippenstiel S, Witzenrath M, Schmeck B, Hocke A, Krisp M, Krull M, et al. Adrenomedullin reduces endothelial hyperpermeability. Circ Res. 2002;91:618–625. doi: 10.1161/01.res.0000036603.61868.f9. [DOI] [PubMed] [Google Scholar]
- 15.Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, et al. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70:1304–1312. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- 16.Hocke AC, Temmesfeld-Wollbrueck B, Schmeck B, Berger K, Frisch EM, Witzenrath M, et al. Perturbation of endothelial junction proteins by Staphylococcus aureus alpha-toxin: inhibition of endothelial gap formation by adrenomedullin. Histochem Cell Biol. 2006;126:305–316. doi: 10.1007/s00418-006-0174-5. [DOI] [PubMed] [Google Scholar]
- 17.Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns. 2006;6:581–588. doi: 10.1016/j.modgep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Honda M, Nakagawa S, Hayashi K, Kitagawa N, Tsutsumi K, Nagata I, et al. Adrenomedullin improves the blood-brain barrier function through the expression of claudin-5. Cell Mol Neurobiol. 2006;26:109–118. doi: 10.1007/s10571-006-9028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kajiya K, Hirakawa S, Detmar M. Vascular endothelial growth factor-A mediates ultraviolet B-induced impairment of lymphatic vessel function. Am J Pathol. 2006;169:1496–1503. doi: 10.2353/ajpath.2006.060197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim W, Moon SO, Sung MJ, Kim SH, Lee S, So JN, et al. Angiogenic role of adrenomedullin through activation of Akt, mitogen-activated protein kinase, and focal adhesion kinase in endothelial cells. Faseb J. 2003;17:1937–1939. doi: 10.1096/fj.02-1209fje. [DOI] [PubMed] [Google Scholar]
- 21.Kis B, Deli MA, Kobayashi H, Abraham CS, Yanagita T, Kaiya H, et al. Adrenomedullin regulates blood-brain barrier functions in vitro. Neuroreport. 2001;12:4139–4142. doi: 10.1097/00001756-200112210-00055. [DOI] [PubMed] [Google Scholar]
- 22.Kis B, Snipes JA, Deli MA, Abraham CS, Yamashita H, Ueta Y, et al. Chronic adrenomedullin treatment improves blood-brain barrier function but has no effects on expression of tight junction proteins. Acta Neurochir Suppl. 2003;86:565–568. doi: 10.1007/978-3-7091-0651-8_115. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 24.Klausner JM, Abu-Abid S, Alexander JS, Hanshke-Mineau R, Goldman G, Morel N, et al. Thromboxane modulates endothelial permeability. Mediators Inflamm. 1994;3:149–153. doi: 10.1155/S0962935194000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leu AJ, Berk DA, Yuan F, Jain RK. Flow velocity in the superficial lymphatic network of the mouse tail. Am J Physiol. 1994;267:H1507–H1513. doi: 10.1152/ajpheart.1994.267.4.H1507. [DOI] [PubMed] [Google Scholar]
- 26.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 27.McQuaid KE, Smyth EM, Keenan AK. Evidence for modulation of hydrogen peroxide-induced endothelial barrier dysfunction by nitric oxide in vitro. Eur J Pharmacol. 1996;307:233–241. doi: 10.1016/0014-2999(96)00271-3. [DOI] [PubMed] [Google Scholar]
- 28.Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. Embo J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roesli C, Mumprecht V, Neri D, Detmar M. Identification of the surfaceaccessible, lineage-specific vascular proteome by two-dimensional peptide mapping. Faseb J. 2008;22:1933–1944. doi: 10.1096/fj.07-100529. [DOI] [PubMed] [Google Scholar]
- 30.Salvatore CA, Hershey JC, Corcoran HA, Fay JF, Johnston VK, Moore EL, et al. Pharmacological characterization of MK-0974 [N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3- yl]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carbox amide], a potent and orally active calcitonin gene-related peptide receptor antagonist for the treatment of migraine. J Pharmacol Exp Ther. 2008;324:416–421. doi: 10.1124/jpet.107.130344. [DOI] [PubMed] [Google Scholar]
- 31.Swartz MA, Berk DA, Jain RK. Transport in lymphatic capillaries. I. Macroscopic measurements using residence time distribution theory. Am J Physiol. 1996;270:H324–H329. doi: 10.1152/ajpheart.1996.270.1.H324. [DOI] [PubMed] [Google Scholar]
- 32.Temmesfeld-Wollbruck B, Hocke AC, Suttorp N, Hippenstiel S. Adrenomedullin and endothelial barrier function. Thromb Haemost. 2007;98:944–951. doi: 10.1160/th07-02-0128. [DOI] [PubMed] [Google Scholar]