SUMMARY
In both developed and developing countries, trans fatty acids (TFA) are largely consumed from partially hydrogenated vegetable oils. This article focuses on TFA as a modifiable dietary risk factor for cardiovascular disease, reviewing the evidence for lipid and non lipid effects; the relations of trans fat intake with clinical endpoints; and current policy and legislative issues. In both observational cohort studies and randomized clinical trials, TFA adversely affect lipid profiles (including raising LDL and triglyceride levels, and reducing HDL levels), systemic inflammation, and endothelial function. More limited but growing evidence suggests that TFA also exacerbate visceral adiposity and insulin resistance. These pluripotent effects of TFA on a plethora of cardiovascular risk factors are consistent with the strong associations seen in prospective cohort studies between TFA consumption and risk of myocardial infarction and coronary heart disease (CHD) death. The documented harmful effects of TFA along with the feasibility of substituting partially hydrogenated vegetable oils with healthy alternatives indicate little reason for continued presence of industrially-produced TFA in food preparation and manufacturing or in home cooking fats/oils. A comprehensive strategy to eliminate use of industrial TFA in both developed and developing countries, including education, food labeling, and policy and legislative initiatives, would likely prevent tens of thousands of CHD events worldwide each year.
Keywords: trans-fatty acids, cardiovascular, coronary heart disease, nutrition, diet, policy, review
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death in nearly all nations. In the US alone, 1.2 million myocardial infarctions and deaths from coronary heart disease (CHD) occur each year [1]. CVD risk factors include both nonmodifiable risk factors (e.g., age, gender, family history) and modifiable risk factors, particularly poor dietary habits, physical inactivity and smoking. Poor control of these lifestyle risk factors results in endothelial dysfunction, metabolic dysfunction, and adiposity, that in turn together lead to dyslipidemia, hypertension, diabetes, systemic inflammation, pro-thrombosis, and risk of arrhythmia. The ultimate result is subclinical and then clinically apparent CVD, including CHD, cardiac arrhythmias, heart failure, stroke, and cognitive decline [2]. Thus, lifestyle habits – diet, physical activity, smoking – and their sequelae are the major causes of most CVD.
In the 1960s and 1970s, ecologic studies (i.e., comparisons across countries or populations) and relatively simple biochemical and metabolic experiments led to the generation of the traditional diet-heart paradigm: that dietary total fat and saturated fat, by means of their effects on serum total cholesterol and low density lipoprotein cholesterol (LDL-C) concentrations, were the major dietary causes of CHD. Since that time, dramatic advances in experimental and epidemiologic nutritional science have occurred, including development of sophisticated metabolic and experimental studies to examine effects of diet on a wide range of both lipid and nonlipid pathways, and of large prospective cohort studies and randomized controlled trials (RCTs) to examine how dietary factors relate to clinical events among individuals (rather than across populations). These advances have led to a more complete diet-heart paradigm, in which numerous dietary factors (e.g., trans fatty acids [TFA], n-3 fatty acids, whole grains and carbohydrate quality, fruits and vegetables, unsaturated fats, legumes and nuts, food processing, and preparation methods) affect not only serum lipid profiles but also arrhythmia, hemodynamics, inflammation, endothelial function, satiety and weight gain, insulin sensitivity, and thrombosis; and in which these numerous intermediate risk factors cause a variety of clinical endpoints, including atherosclerosis, acute plaque rupture, sudden death, congestive heart failure, atrial fibrillation, cerebrovascular disease, obesity, and diabetes mellitus [3].
This article focuses on TFA as a modifiable dietary risk factor for CVD, reviewing the evidence for lipid and non lipid effects; the relations of trans fat intake with clinical endpoints; and current policy and legislative issues.
TRANS FATTY ACIDS IN THE DIET
TFA are unsaturated fatty acids with at least one carbon-carbon double bond in the trans configuration. In mammals, endogenously synthesized unsaturated fatty acids have double bonds in the cis configuration. The main exception are ruminants such as cows, sheep, and goats, in which small amounts of TFA are produced enzymatically by the action of bacteria in the ruminant stomach. Because TFA comprise a minority (typically <5%) of ruminant fatty acids, consumption of naturally-occurring ruminant TFA typically contributes to less than 0.5% of total energy intake. Thus, in both developed and developing countries, the major source of dietary TFA is most commonly industrially-produced TFA, contributing 2 to 3% to total energy intake in the US [4] and 4% or more in developing countries [5]. These TFA are created during the partial hydrogenation of vegetable oils, which alters 30–50% of the unsaturated fats from having double bonds in the natural cis to the trans configuration. Due to the presence of these TFA, partially hydrogenated oils have some commercial advantages over many unhydrogenated oils, such as longer shelf-life, solidity at room temperature, and greater stability during high temperature commercial deep-frying. In developed countries, major dietary sources of industrial TFA include bakery products (e.g., cakes, cookies, pies), deep fried and frozen foods (e.g., french fries, breaded chicken and fish), packaged snacks (e.g., popcorn), margarines, and crackers. In contrast, in developing countries, major dietary sources of industrial TFA are partially hydrogenated fats directly used for cooking (typically blends of partially hydrogenated soybean, sunflower, cottonseed and palm oils), that are often subsidized by the government for distribution as dietary oils and thus most prevalent in poor and rural areas [5]. Importantly, whereas mean population intakes of TFA typically average between 2–4% of energy, a substantial minority of the population can have much higher intakes. For example, because TFA are present in a number of commercially available products, it is not difficult to consume up to 20 grams (9% of energy) in one day [6], far exceeding the recommended maximum of 2 grams (<1% of energy) per day [7]. TFA are also transported across the placenta and are present in breast milk in proportion to the mother’s dietary intake [8].
“Natural” (ruminant) trans fatty acids
The most predominant trans-isomer in ruminant TFA is vaccenic acid. Smaller amounts of conjugated linolenic acid (CLA, another TFA) can also be formed from vaccenic acid. By modifying the feed of ruminant animals, the TFA content of meat and dairy products can be altered [9], but the content of TFA in ruminant products is generally quite low (~1–8% of total fatty acids). Vaccenic acid and CLA have been hypothesized to have different physiologic effects compared with industrialized TFA, due for example to some structural (isomeric) differences [9]. The current evidence for such claims is inconclusive. In feeding trials, CLA consumption has increased insulin resistance, increased lipid peroxidation, and had mixed effects on markers of inflammation and immune function [10–13]. Four prospective studies have investigated the associations between consumption of ruminant TFA and CHD risk. None demonstrated a positive association between ruminant TFA and CHD risk, and three studies observed trends toward non significant inverse (protective) associations [14–17]. Thus, whether due to low levels of consumption, structural differences, or potential health benefits of other nutrients in dairy and meat products that may counterbalance any adverse effects, the public health implications of ruminant TFA consumption appear to be limited. The remainder of this review focuses on effects of industrial (or total, largely industrial) TFA.
TRANS FATTY ACIDS – LIPID EFFECTS
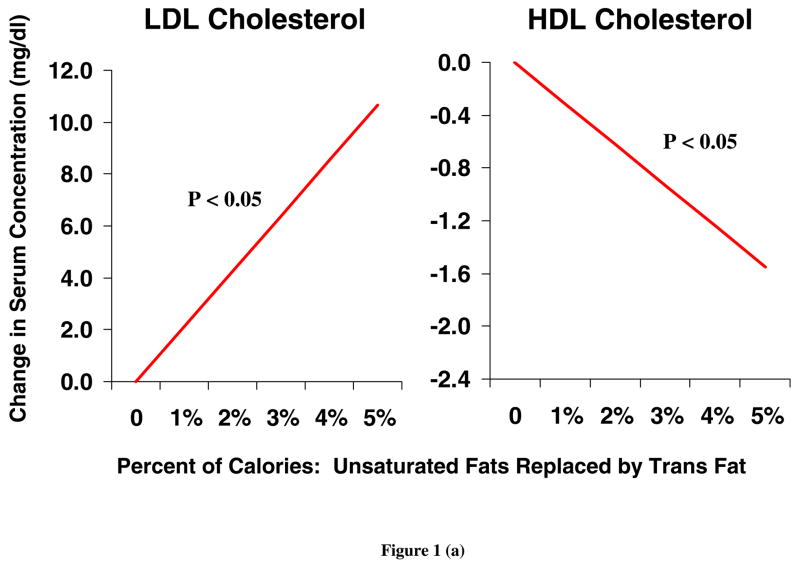
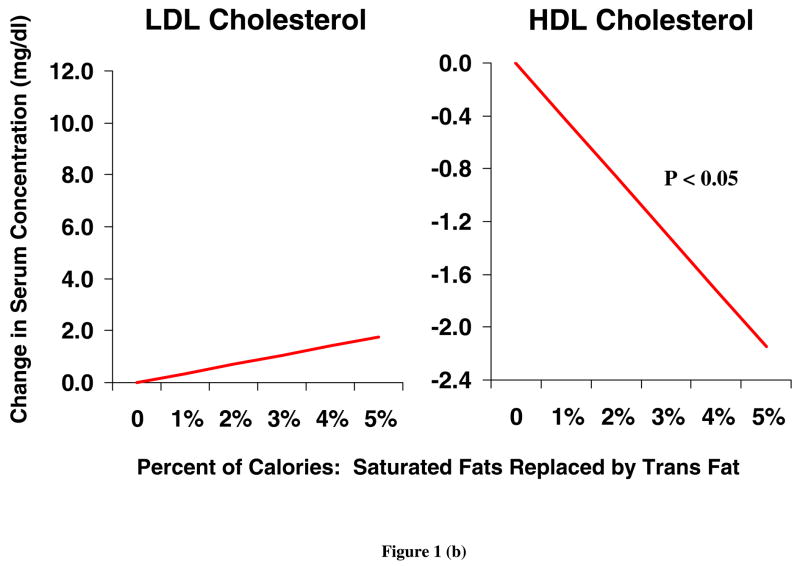
TFA have a wide range of physiologic effects, including both lipid and non lipid effects. The unique adverse effects of TFA on serum lipids have been documented in numerous human metabolic studies. A recent meta-analysis of 13 randomized controlled trials indicated that isocaloric replacement of TFA with either polyunsaturated fatty acids (PUFA), monounsaturated fatty acids (MUFA), or saturated fatty acids (SFA) increased the total cholesterol to high density lipoprotein cholesterol (HDL-C) ratio, increased the ratio of apolipoprotein B (apo-B) to apolipoprotein A (apo-A), and increased lipoprotein(a) levels [18]. Isocaloric replacement of either saturated or cis unsaturated fats with TFA also raises LDL-C and reduces HDL-C [19] (Figure 1). These lipid effects seen in short-term RCTs are similar to differences seen in observational studies of habitual TFA consumption. In one study, higher TFA consumption (habitual intake of 2.5 to 3.6 g/day) was associated with higher LDL-C, lower HDL-C and a higher LDL-C to HDL-C ratio [20]. Compared with unsaturated fats, dietary TFA also increase serum triglycerides and reduce LDL-C particle size [18]. Each of these changes in serum lipids are independently associated with higher CHD risk. However, prospective studies investigating the associations between TFA and CHD endpoints (reviewed below) demonstrate an incidence of CHD attributable to TFA intake that is greater than that predicted by changes in serum lipids alone [21, 22]. Thus, documented effects of TFA on other, non-lipid risk factors are likely to be relevant.
Figure 1.
Changes in LDL and HDL cholesterol levels, when cis unsaturated (a) or saturated (b) fatty acids are isocalorically replaced with TFA, based on a meta-analysis of 12 randomized controlled trials in humans [19]. LDL, high density lipoprotein; HDL, high density lipoprotein; TFA, trans fatty acids.
TRANS FATTY ACIDS – NON LIPID EFFECTS
Trans fats have been implicated in systemic inflammation, endothelial dysfunction, adiposity, and insulin resistance.
Systemic inflammation
Inflammation is an independent risk factor for atherosclerosis, sudden death, diabetes, and heart failure [23–26]. TFA have pro-inflammatory effects, seen in both observational studies and randomized control trials. In observational studies, higher TFA intake has been associated with increased activity of the tumor necrosis factor (TNF) system among otherwise healthy women [27], and higher levels of interleukin-6 (IL-6) and C-reactive protein (CRP) among overweight women [27, 28]. In patients with established heart disease, erythrocyte membrane TFA concentrations (a biomarker of dietary intake) were independently associated with higher levels of IL-6, TNF-α, TNF-receptors, and monocyte chemoattractant protein [27].
Consistent with these observational results, in a randomized cross-over trial of 50 healthy men, consumption of TFA for five weeks (8% of energy intake) increased plasma levels of IL-6 and CRP, compared with consumption of oleic acid (cis 18:1), and plasma levels of CRP, compared with consumption of carbohydrate [29]. In another randomized trial among hypercholesterolemic subjects, consuming a one-month diet with 6.7% of energy from TFA (partially hydrogenated soybean oil), compared with 0.6% of energy from TFA (nonhydrogenated soybean oil), increased macrophage production of both IL-6 and TNF-α [30].
The pro-inflammatory effects of dietary TFA are likely to account, at least in part, for harmful effects on CVD outcomes. For example, based on the associations of CRP levels with CVD risk [31], the difference in CRP levels seen for a median TFA intake of 2.1% versus 0.9% of energy would correspond to ~30% higher CHD risk [28].
Endothelial function
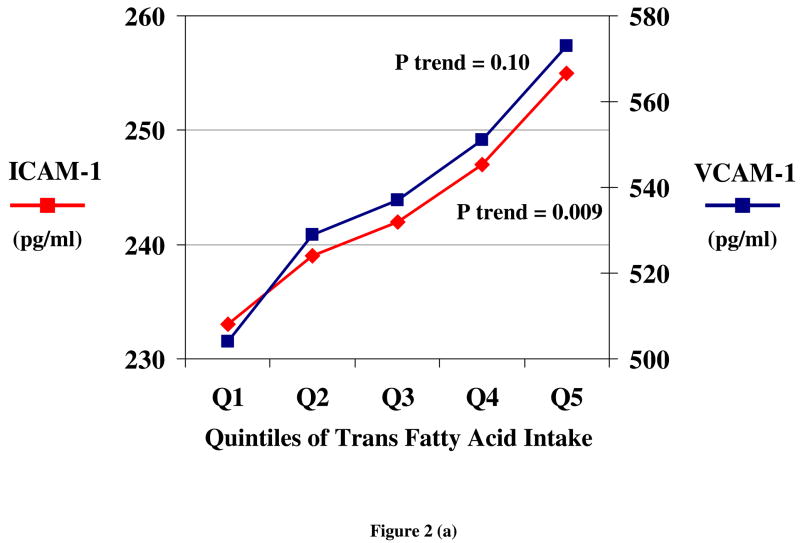
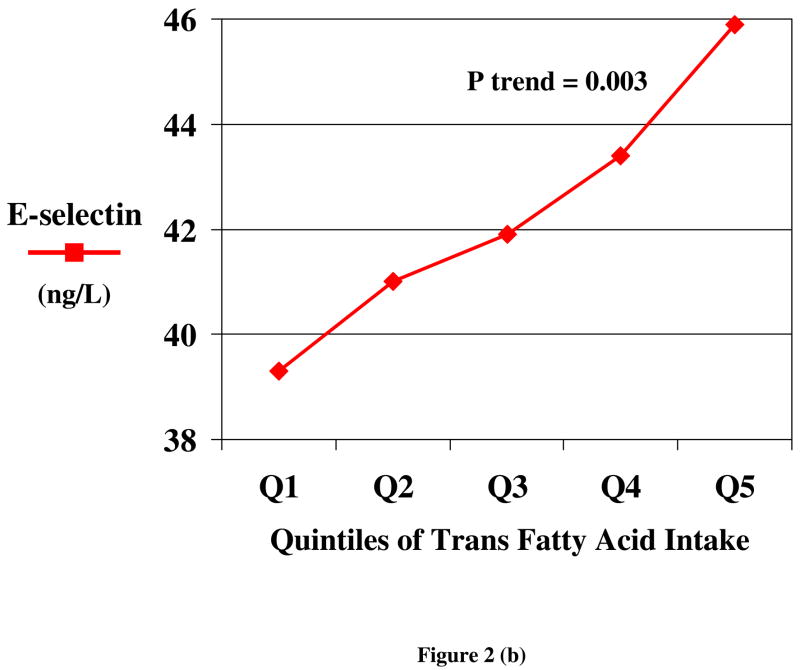
Both observational studies and randomized trials indicate that TFA consumption produces endothelial dysfunction. Among overweight women, greater TFA intake was associated with higher levels of circulating markers of endothelial dysfunction, including soluble cell adhesion molecules (ICAM-1 and VCAM 1) and E-selectin [28], after adjusting for other risk factors (Figure 2). Similarly, in a randomized trial, E-selectin levels were increased by TFA consumption, compared with either carbohydrate or oleic acid [29]. In addition to effects on these circulating markers of dysfunction, TFA consumption affects functional measures of endothelial dysfunction. In a randomized trial among 39 subjects, brachial flow mediated vasolidation was reduced by 29% following 4 weeks of TFA intake (9% of energy), compared with SFA intake [32].
Figure 2.
Circulating markers of endothelial dysfunction, including soluble vascular adhesion molecules (ICAM 1 and VCAM 1) (a) and E-selectin (b) according to quintiles of habitual TFA consumption, after adjustment for other risk factors and lifestyle habits, among 730 overweight but otherwise generally healthy women [28].
Adiposity
Emerging evidence suggests that TFA consumption may increase weight gain and fat accumulation, particularly of visceral fat. In two large prospective cohort studies, increases in TFA intake were associated with increases in abdominal circumference in men [33] and increases in body weight in women [34]; other types of fat (SFA, MUFA, PUFA) and total fat consumption were not associated with weight gain. Consistent with these observational findings in humans, a 6-year RCT in nonhuman primates (green monkeys) demonstrated that consumption of TFA (trans 18:1, 8% energy), compared with cis MUFA, increased body weight (7% versus <2%, respectively), largely due to increased deposition of intra-abdominal fat [35]. Because this trial was calorie-controlled, these findings suggest that TFA consumption affects metabolism and adipocyte growth or adipogenesis.
Insulin resistance
Both duration of TFA exposure and underlying predisposition to insulin resistance appear to play important roles in the effects of TFA on glucose-insulin homeostasis. Short-term feeding trials in humans that compared TFA with oleic acid in lean healthy individuals found little effect on insulin sensitivity [36, 37]. Conversely, among moderately overweight individuals, TFA consumption increased markers of insulin resistance [37]. In the RCT among green monkeys, 6 years of TFA consumption adversely affected glucose metabolism, as demonstrated by markedly reduced phosporylation of protein kinase B (PKB) in both adipose and muscle tissue, postprandial hyperinsulinemia, and elevated fructosamine levels [35]. Experimental evidence suggests that the timing of exposure may also be relevant. In rats fed TFA during gestation, the offspring had fewer insulin receptors and insulin receptor substrate-1 [38]. Considering that TFA is transported across the human placenta and is present in breast milk [8], additional studies are needed to investigate potential effects of maternal TFA consumption on glucose-insulin homeostasis in early life.
TRANS FATTY ACIDS – A UNIQUE CARDIOMETABOLIC IMPRINT?
The combination of effects of TFA consumption on both lipid and non lipid risk factors suggest that TFA produce a unique cardiometabolic imprint. Lipid effects include lowering of HDL-C and apo-A levels, increases in LDL-C and apo-B levels, decreases in LDL particle size (i.e. more dense particles), and increased triglycerides. Non lipid effects include activation of inflammatory processes, endothelial dysfunction, and possible increases in abdominal adiposity and exacerbation of insulin resistance. Thus, it has been proposed that this constellation of effects suggests an impact of TFA on pathways linked to the metabolic or insulin resistance syndrome [39].
TRANS FATTY ACIDS – BIOLOGIC PATHWAYS
The pluripotent effects of TFA appear to be linked to biological effects on hepatocytes (e.g., lipid metabolism), monocytes/macrophages (e.g., systemic inflammation), endothelial cells (e.g., endothelial dysfunction) and adipocytes (e.g., adiposity, glucose-insulin homeostasis). Each of these tissues are central to CVD risk. Although the molecular pathways whereby TFA influence these tissues require further investigation, pathways of effect of other dietary fatty acids suggest that effects on both cell membranes and gene transcription are likely to be important. Dietary fatty acids are key structural elements of cell membranes, altering membrane fluidity and the responses of receptors [40–42]. Dietary fatty acids also regulate gene transcription by binding to and modulating nuclear receptors, such as peroxisome-proliferator-activated receptors (PPAR), liver X receptor, sterol regulatory element-binding protein-1 [43], and membrane G-protein receptors [44]. In hepatocytes, TFA alter the secretion, lipid composition, and size of apolipoprotein B-100 particles [45, 46], and increase the cellular accumulation and secretion of free cholesterol and cholesterol esters [45]. TFA increase monocyte production of TNF-α and IL-6 [30] and levels of monocyte chemoattractant protein-1 [27]. TFA also affect endothelial cells by increasing biomarker levels of endothelial dysfunction [28, 29] and impairing nitric oxide-dependent arterial dilatation [32]. In adipocytes, TFA alter fatty acid metabolism by reducing triglyceride uptake and esterification of newly synthesized cholesterol, and by increasing production of free fatty acids [47]. In adipocytes, TFA also alter the expression of genes for PPAR-γ, resistin and lipoprotein lipase [44], all of which play central roles in fatty acid and glucose metabolism [43]. Some evidence suggests that adiposity may modify the degree to which TFA consumption affects IL-6 and CRP levels, further supporting a role of adipose tissue in the pro-inflammatory effects of TFA [27].
TRANS FATTY ACIDS – RELATIONS WITH CORONARY HEART DISEASE EVENTS
In a meta-analysis of four prospective cohort studies totaling ~140,000 participants, each 2% increase in energy intake from TFA (~4 g per day, or 40 calories per day on a 2000 kcal diet) was associated with a 23% higher incidence of myocardial infarction and CHD death (pooled RR=1.23, 95% CI=1.11–1.37, p<0.001) [19]. Adding to this meta-analysis the results from three retrospective case-control studies, the risk of CHD was even greater, with a pooled RR of 1.29 (95% CI, 1.11–1.49; p<0.001) for each 2% increase in energy intake from TFA [19]. Calorie for calorie, TFA have stronger relationships with CHD risk than seen for any other macronutrient. In a meta-analysis of 4 prospective cohorts (for TFA) and 2 prospective cohorts (for SFA, MUFA, and PUFA), the risk of CHD for isocaloric replacement of carbohydrate with each of the different dietary fats was far higher for TFA [48].
POLICY AND LEGISLATION
In 2005, the US Dietary Guidelines Advisory Committee recommended that every individual’s consumption of TFA should be “as low as possible,” below 1% of total energy intake [7]. This level of consumption could be achieved on a population level by removal of TFA from foods, or on an individual level by strict avoidance by consumers of foods that contain partially hydrogenated oils.
As of Dec 12, 2005, in Canada [49], and Jan 1, 2006, in the US [50], nutrition labels for all conventional foods and supplements were required to list the content of TFA. Foods with less than 0.5 g (0.2 g in Canada) of TFA per serving can be listed as having zero content. These labeling changes, together with publicity surrounding harmful effects of TFA [51], prompted some food manufacturers to reformulate their products to reduce or eliminate TFA; for example, Kraft reformulated most of their brands, including Oreo cookies, to eliminate TFA [52]. Thus, at least in the U.S. and Canada, many manufactured foods are being reformulated to reduce TFA, and those that still contain TFA have mandatory labels so that consumers can identify TFA content. In contrast, food labeling is not mandatory in food service establishments, including foods served at restaurants, fast food chains, coffee and donut shops, grocery stores, bakeries, schools, workplace cafeterias, and other retail food outlets. Consequently, at these sites, labeling is not to present to facilitate avoidance of TFA by consumers, and recognition of which foods contain TFA would be very difficult without detailed personal knowledge of the types of fats and oils used in food preparation at each site. Thus, following the lead of Denmark [53] and New York City [54], many other countries and US cities, counties, and states are considering or have passed legislation to reduce the use of TFA (partially hydrogenated oils) in food service establishments. For example, in New York City, food service establishments can no longer fry with fats that contain more than 0.5 g of TFA per serving (as of July 1st, 2007) or serve foods that contain more than 0.5 g of TFA per serving (as of July 1st, 2008). In New York, premanufactured foods with food labels are exempt from this legislation, as such food labels allow informed choice by the consumer regarding the consumption of TFA.
From a nutritional standpoint, TFA have no intrinsic health value, whereas based on their lipid and non lipid adverse effects and their strong relationship with clinical CHD events, TFA are likely to have substantial adverse effects on health. Because industrially-produced TFA (i.e., partially hydrogenated oils) represent an additive to food that have no health benefit and substantial potential risk, there is no compelling reason to continue their use in foods. Theoretical concerns have been raised about sufficient supply of healthy alternatives to replace partially hydrogenated oils, but such concerns have not been borne out in experiences in Denmark or NYC [55, 56]. Indeed, a nationwide survey of recent product reformulations to reduce TFA in Canada demonstrated that, in nearly all cases, manufacturers are taking advantage of the up-front cost and effort of reformulation to not only eliminate TFA but also increase the content of cis unsaturated fats [49].
What type of alternative fats/oils are optimal to replace partially hydrogenated vegetable oils? For frying applications, many choices are available, including cottonseed, soybean, canola and high oleic sunflower oil [54]. For many baking applications, liquid vegetable oils (e.g., soybean) can also be used. However, some particular products (e.g., donuts) can require solid or semi-solid fats to maintain product consistency and feel. Choices of solid or semi-solid fats include tropical oils (e.g., palm or coconut), butter, lard, fully hydrogenated vegetable oil, and blends or interesterified oil. In an analysis evaluating (a) effects of TFA and other fats on CVD risk factors in RCTs, and (b) relationships of TFA and other fats with clinical CHD endpoints in prospective studies, replacement of 7.5% of energy from partially hydrogenated vegetable oils (containing 35% TFA or more) with any of the alternatives examined, including nonhydrogenated vegetable oils, tropical oils, butter, and lard, would result in decreased CHD risk [18]. The magnitude of the benefit was greatest when partially hydrogenated vegetable oils were replaced by nonhydrogenated vegetable oils (highest in unsaturated fats), intermediate for tropical oils or lard, and smallest – but not negligible – for butter.
CONCLUSIONS
TFA adversely affect a wide range of lipid and nonlipid cardiovascular risk factors, indicating a unique cardiometabolic imprint among dietary fats. These adverse effects, together with the strong positive relationships with CHD events in observational studies, indicate substantial potential for harm. Experiences in both North America and Europe indicate that eliminating industrially-produced TFA from foods, and substituting these largely with cis unsaturated fats, is both feasible and practical. TFA consumption can be reduced by appropriate guidance and education of consumers, by voluntary adoption of alternative fats and oils by the food industry, or by labeling, legislation, and other policies aimed at reducing TFA use. In Western countries, a combination of these strategies has proven to be most effective. Less is known about patterns of TFA consumption in developing countries; consumption in the form of partially hydrogenated cooking fat may be very high in some regions. Reducing TFA consumption in these populations will require attention by multiple stakeholders [57], but is critically important given that TFA consumption appears to be highest (i.e., in the form of partially hydrogenated cooking oil) in the more socioeconomically disadvantaged segments of these societies. A comprehensive strategy to eliminate use of industrial TFA in both developed and developing countries would likely prevent tens of thousands of CHD events worldwide each year.
Footnotes
No conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Heart Association. [Acessed 4/28/2004, 2004];Heart Disease and Stroke Statistics - 2004 Update. :25–26. www.americanheart.org.
- 2.Mozaffarian D, et al. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation. 2008;117(9):1130–7. doi: 10.1161/CIRCULATIONAHA.107.732826. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D. Effects of dietary fats versus carbohydrates on coronary heart disease: A review of the evidence. Curr Atheroscler Rep. 2005;7(6):435–445. doi: 10.1007/s11883-005-0060-y. [DOI] [PubMed] [Google Scholar]
- 4.Allison DB, et al. Estimated intakes of trans fatty and other fatty acids in the US population. J Am Diet Assoc. 1999;99(2):166–74. doi: 10.1016/S0002-8223(99)00041-3. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, et al. Consumption of trans fats and estimated effects on coronary heart disease in Iran. Eur J Clin Nutr. 2007;61:1004–10. doi: 10.1038/sj.ejcn.1602608. [DOI] [PubMed] [Google Scholar]
- 6.Stender S, Dyerberg J, Astrup A. High levels of industrially produced trans fat in popular fast foods. New England Journal of Medicine. 2006;354(15):1650–2. doi: 10.1056/NEJMc052959. [DOI] [PubMed] [Google Scholar]
- 7.Dietary Guidelines Advisory Committee. 2005 Dietary Guidelines Advisory Committee Report. 2005 [cited Sep 12, 2005]; Available from: http://www.health.gov/dietaryguidelines/dga2005/report/
- 8.Innis SM, King DJ. Trans fatty acids in human milk are inversely associated with concentrations of essential all-cis n-6 and n-3 fatty acids and determine trans, but not n-6 and n-3, fatty acids in plasma lipids of breast-fed infants. Am J Clin Nutr. 1999;70(3):383–90. doi: 10.1093/ajcn/70.3.383. [DOI] [PubMed] [Google Scholar]
- 9.Lock AL, Bauman DE. Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids. 2004;39(12):1197–206. doi: 10.1007/s11745-004-1348-6. [DOI] [PubMed] [Google Scholar]
- 10.Riserus U, et al. Effects of cis-9, trans-11 conjugated linoleic acid supplementation on insulin sensitivity, lipid peroxidation, and proinflammatory markers in obese men. Am J Clin Nutr. 2004;80(2):279–83. doi: 10.1093/ajcn/80.2.279. [DOI] [PubMed] [Google Scholar]
- 11.Moloney F, et al. Conjugated linoleic acid supplementation, insulin sensitivity, and lipoprotein metabolism in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2004;80(4):887–95. doi: 10.1093/ajcn/80.4.887. [DOI] [PubMed] [Google Scholar]
- 12.Smedman A, Vessby B, Basu S. Isomer-specific effects of conjugated linoleic acid on lipid peroxidation in humans: regulation by alpha-tocopherol and cyclo-oxygenase-2 inhibitor. Clin Sci (Lond) 2004;106(1):67–73. doi: 10.1042/CS20030105. [DOI] [PubMed] [Google Scholar]
- 13.Nugent AP, et al. The effects of conjugated linoleic acid supplementation on immune function in healthy volunteers. Eur J Clin Nutr. 2005;59(6):742–50. doi: 10.1038/sj.ejcn.1602132. [DOI] [PubMed] [Google Scholar]
- 14.Oomen CM, et al. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population-based study. Lancet. 2001;357(9258):746–51. doi: 10.1016/s0140-6736(00)04166-0. [DOI] [PubMed] [Google Scholar]
- 15.Pietinen P, et al. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Epidemiol. 1997;145(10):876–87. doi: 10.1093/oxfordjournals.aje.a009047. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, et al. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet. 1993;341(8845):581–5. doi: 10.1016/0140-6736(93)90350-p. [DOI] [PubMed] [Google Scholar]
- 17.Jakobsen MU, et al. Intake of ruminant trans fatty acids and risk of coronary heart disease-an overview. Atheroscler Suppl. 2006;7(2):9–11. doi: 10.1016/j.atherosclerosissup.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr. 2008 doi: 10.1038/sj.ejcn.1602976. In press. [DOI] [PubMed] [Google Scholar]
- 19.Mozaffarian D, et al. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354(15):1601–13. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 20.Sun Q, et al. A prospective study of trans fatty acids in erythrocytes and risk of coronary heart disease. Circulation. 2007;115(14):1858–65. doi: 10.1161/CIRCULATIONAHA.106.679985. [DOI] [PubMed] [Google Scholar]
- 21.Mensink RP, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77(5):1146–55. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 22.Ascherio A, et al. Trans fatty acids and coronary heart disease. N Engl J Med. 1999;340(25):1994–8. doi: 10.1056/NEJM199906243402511. [DOI] [PubMed] [Google Scholar]
- 23.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 24.Albert CM, et al. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105(22):2595–9. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 25.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27(3):813–23. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 26.Vasan RS, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107(11):1486–91. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 27.Mozaffarian D, et al. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr. 2004;79(4):606–12. doi: 10.1093/ajcn/79.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Garcia E, et al. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr. 2005;135(3):562–6. doi: 10.1093/jn/135.3.562. [DOI] [PubMed] [Google Scholar]
- 29.Baer DJ, et al. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79(6):969–73. doi: 10.1093/ajcn/79.6.969. [DOI] [PubMed] [Google Scholar]
- 30.Han SN, et al. Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J Lipid Res. 2002;43(3):445–52. [PubMed] [Google Scholar]
- 31.Ridker PM, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 32.de Roos NM, Bots ML, Katan MB. Replacement of dietary saturated fatty acids by trans fatty acids lowers serum HDL cholesterol and impairs endothelial function in healthy men and women. Arterioscler Thromb Vasc Biol. 2001;21(7):1233–7. doi: 10.1161/hq0701.092161. [DOI] [PubMed] [Google Scholar]
- 33.Koh-Banerjee P, et al. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16 587 US men. Am J Clin Nutr. 2003;78(4):719–27. doi: 10.1093/ajcn/78.4.719. [DOI] [PubMed] [Google Scholar]
- 34.Field AE, et al. Dietary fat and weight gain among women in the Nurses’ Health Study. Obesity (Silver Spring) 2007;15(4):967–76. doi: 10.1038/oby.2007.616. [DOI] [PubMed] [Google Scholar]
- 35.Kavanagh K, et al. Trans fat diet induces abdominal obesity and changes in insulin sensitivity in monkeys. Obesity (Silver Spring) 2007;15(7):1675–84. doi: 10.1038/oby.2007.200. [DOI] [PubMed] [Google Scholar]
- 36.Louheranta AM, et al. A high-trans fatty acid diet and insulin sensitivity in young healthy women. Metabolism. 1999;48(7):870–5. doi: 10.1016/s0026-0495(99)90221-4. [DOI] [PubMed] [Google Scholar]
- 37.Lefevre M, et al. Comparison of the acute response to meals enriched with cis- or trans-fatty acids on glucose and lipids in overweight individuals with differing FABP2 genotypes. Metabolism. 2005;54(12):1652–8. doi: 10.1016/j.metabol.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Albuquerque KT, et al. Intake of trans fatty acid-rich hydrogenated fat during pregnancy and lactation inhibits the hypophagic effect of central insulin in the adult offspring. Nutrition. 2006;22(7–8):820–9. doi: 10.1016/j.nut.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Mozaffarian D, Willett WC. Trans fatty acids and cardiovascular risk: a unique cardiometabolic imprint? Curr Atheroscler Rep. 2007;9(6):486–93. doi: 10.1007/s11883-007-0065-9. [DOI] [PubMed] [Google Scholar]
- 40.Clandinin MT, et al. Dietary fat: exogenous determination of membrane structure and cell function. Faseb J. 1991;5(13):2761–9. doi: 10.1096/fasebj.5.13.1916101. [DOI] [PubMed] [Google Scholar]
- 41.Feller SE, Gawrisch K. Properties of docosahexaenoic-acid-containing lipids and their influence on the function of rhodopsin. Curr Opin Struct Biol. 2005;15(4):416–22. doi: 10.1016/j.sbi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Roach C, et al. Comparison of cis and trans fatty acid containing phosphatidylcholines on membrane properties. Biochemistry. 2004;43(20):6344–51. doi: 10.1021/bi049917r. [DOI] [PubMed] [Google Scholar]
- 43.Vanden Heuvel JP. Diet, fatty acids, and regulation of genes important for heart disease. Curr Atheroscler Rep. 2004;6(6):432–40. doi: 10.1007/s11883-004-0083-9. [DOI] [PubMed] [Google Scholar]
- 44.Saravanan N, et al. Differential effects of dietary saturated and trans-fatty acids on expression of genes associated with insulin sensitivity in rat adipose tissue. Eur J Endocrinol. 2005;153(1):159–65. doi: 10.1530/eje.1.01946. [DOI] [PubMed] [Google Scholar]
- 45.Dashti N, et al. Trans polyunsaturated fatty acids have more adverse effects than saturated fatty acids on the concentration and composition of lipoproteins secreted by human hepatoma HepG2 cells. J Nutr. 2002;132(9):2651–9. doi: 10.1093/jn/132.9.2651. [DOI] [PubMed] [Google Scholar]
- 46.Mitmesser SH, Carr TP. Trans fatty acids alter the lipid composition and size of apoB-100-containing lipoproteins secreted by HepG2 cells. J Nutr Biochem. 2005;16(3):178–83. doi: 10.1016/j.jnutbio.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Matthan NR, et al. Hydrogenated fat consumption affects acylation-stimulating protein levels and cholesterol esterification rates in moderately hypercholesterolemic women. J Lipid Res. 2001;42(11):1841–8. [PubMed] [Google Scholar]
- 48.Mozaffarian D, Aro A, Willett WC. Health effects of trans-fatty acids: Experimental and observational evidence. Eur J Clin Nutr. 2008 doi: 10.1038/sj.ejcn.1602973. In press. [DOI] [PubMed] [Google Scholar]
- 49.Health Canada. Government response to the interim recommendations of the trans fat task force. 2005 [cited 2005 20 September]; Available from: http://www.hc-sc.gc.ca/fn-an/nutrition/gras-trans-fats/government_response_reponse_gouvernement_e.html.
- 50.Food and Drug Administration. Federal Register. Department of Health and Human Services, Editor; 2003. Food labeling: Trans fatty acids in nutrition labeling, nutrient content claims, and health claims. [PubMed] [Google Scholar]
- 51.Ban Trans Fats. The campaign to ban partially hydrogenated oils. 2003 [cited 2008 16 August]; Available from: http://www.bantransfats.com/index.html.
- 52.Kraft Foods. Kraft Foods Reformulates Hundreds of U.S. Products as Part of Voluntary Trans Fat Reduction Efforts. 2005 [cited 2008 16 August]; Available from: http://www.kraft.com/MediaCenter/country-press-releases/us/2005/us_pr_12202005.html.
- 53.Leth T, et al. The effect of the regulation on trans fatty acid content in Danish food. Atheroscler Suppl. 2006;7(2):53–6. doi: 10.1016/j.atherosclerosissup.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 54.New York City Department of Health and Mental Hygiene. Healthy Heart - Avoid Trans Fat. 2007 [cited 2008 26 January]; Available from: http://www.nyc.gov/html/doh/html/cardio/cardio-transfat.shtml.
- 55.Mozaffarian D. Trans fatty acids - Effects on systemic inflammation and endothelial function. Atheroscler Suppl. 2006;7(2):29–32. doi: 10.1016/j.atherosclerosissup.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 56.New York City Department of Health and Mental Hygiene. Press Release: 94% of inspected restaurants in compliance with first phase of trans fat regulation. 2007 [cited 2007 15 October]; Available from: http://www.nyc.gov/html/doh/html/pr2007/pr080-07.shtml.
- 57.Colon-Ramos U, et al. Translating research into action: a case study on trans fatty acid research and nutrition policy in Costa Rica. Health Policy Plan. 2007;22(6):363–74. doi: 10.1093/heapol/czm030. [DOI] [PubMed] [Google Scholar]