Abstract
CXCL8 is a CXC chemokine that recruits leukocytes to sites of inflammation. Expression of CXCL8 in the CNS has been demonstrated in neuroinflammatory diseases, including human immunodeficiency virus (HIV-1) encephalitis, but the mechanism of secretion of this chemokine is not fully understood. CD40 is a 50-kDa protein on the surface of microglia, and we have previously shown that it is increased in expression in HIV-1-infected brain tissue as well as by interferon-γ (IFNγ) in tissue culture. We examined the expression and regulation of CXCL8 in cultured human fetal microglia after ligation of CD40 with soluble trimeric CD40 ligand (sCD40L) as well as the expression of CXCL8 on microglia in HIV encephalitic brain tissue sections. Treatment of cultured microglia with IFNγ + sCD40L resulted in significant induction of CXCL8. This expression was mediated by activation of the ERK1/2 MAPK pathway, as demonstrated by ELISA and Western blot using a specific inhibitor (U0126). Gel shift analyses demonstrated that NFκB and AP-1, but not C/EBPβ, mediate microglial CXCL8 production. We also found increased colocalization of CXCL8 with CD68/CD40-positive cells in HIV encephalitic brain tissue compared with HIV-infected nonencephalitic and normal tissue. Thus, CD40-CD40L interactions facilitate chemokine expression, leading to the influx of inflammatory cells into the CNS. These events can lead to the pathology that is associated with neuroinflammatory diseases.
Keywords: microglia, HIV dementia, chemokines, neuro-AIDS
Microglia, the resident macrophages of the brain, play a critical role in neuroinflammatory diseases. Unactivated microglia express low levels of molecules involved in antigen presentation, including CD40 (Dangond et al., 1997; Aloisi et al., 2000). However, once activated, microglia undergo a morphological change from a ramified cell to a macrophage morphology (Ponomarev et al., 2005) and up-regulate these surface markers (Aloisi et al., 2000; Ponomarev et al., 2006). Data indicate that ligation of CD40 with soluble CD40 ligand (sCD40L) results in the elaboration of various proinflammatory mediators by microglia (D’Aversa et al., 2002), and these mediators may, in turn, enhance the inflammatory disease process. Activated microglia have been demonstrated in a number of neuroinflammatory diseases, including NeuroAIDS.
HIV-1 infection of the CNS occurs early in the course of disease (Ho et al., 1985; Palmer et al., 1994) and produces distinct neuropathological changes that collectively result in NeuroAIDS. NeuroAIDS is characterized by the presence of HIV-1-infected macrophages and microglia in the brain, formation of multinucleated giant cells (Budka et al., 1991; Sharer, 1992; Dickson et al., 1993; Kim et al., 2006), perivascular infiltrates of inflammatory cells, diffuse myelin pallor, activated microglia, astrogliosis, neuronal damage and/or loss, and breakdown of the blood–brain barrier (Wiley et al., 1991; Masliah et al., 1992, 1997; Nath and Geiger, 1998; Albright et al., 2003). One important aspect of this glial cell activation is the production of chemokines. Chemokines are chemotactic cytokines that recruit inflammatory cells to sites of injury (Bornemann et al., 1997; Hesselgesser and Horuk, 1999). Chemokines play an important role in NeuroAIDS, in part because of their ability to chemoattract specific subsets of leukocytes that may be infected and because they are able to activate resident cells within the CNS (Ehrlich et al., 1998; Stoll and Jander 1999; Kaul et al., 2001; Eugenin et al., 2005, 2006b).
One chemokine shown to be important in HIV-1 infection is interleukin-8 (CXCL8). CXCL8 primarily recruits and activates neutrophils (Yoshimura et al., 1987; Baggiolini et al., 1989) but is also chemotactic for T cells (Larsen et al., 1989; Leonard et al., 1990; Wilkinson and Newman 1992) and monocytes (Gerszten et al., 1999), and triggers firm adhesion of monocytes to the endothelium (Gerszten et al., 1999; Casilli et al., 2005). Both CXCL8 mRNA (Xiong et al., 2003) and protein (Sanders et al., 1998) are increased in HIV-1 encephalitic brain tissue compared with uninfected tissue. CXCL8 expression can be induced early after HIV-1 infection of monocytes (Esser et al., 1996), and, after SIV infection of rhesus monkeys, CXCL8 is secreted by microglia (Sopper et al., 1996). In addition, CXCL8 stimulates HIV-1 replication in macrophages and T cells (Lane et al., 2001). It has also been shown that individuals infected with HIV-1 have increased CXCL8 in their serum (Matsumoto et al., 1993), plasma (Carrol et al., 2007), lymphoid tissue (Lane et al., 2001), and lungs (Denis and Ghadirian, 1994). CXCL8 is expressed by astrocytes in HIV-1 encephalitic tissue (Sanders et al., 1998) and is up-regulated in both an astrocytic cell line infected with HIV-1 (Cota et al., 2000) and astrocytes treated with the HIV-1 transactivator protein Tat (Kutsch et al., 2000). Interestingly, it has been demonstrated that CXCL8 can cross-phosphorylate and cross-desensitize both CCR5 and CXCR4 (Richardson et al., 2003), two chemokine receptors that serve as coreceptors for HIV-1 entry into cells. This implies an inhibitory role of CXCL8 in HIV-1 infection or alterations in transmigration of these cells into the brain.
CD40 is a member of the tumor necrosis factor receptor (TNFR) superfamily and is found on a number of cells, including microglia (Armitage et al., 1992a; D’Aversa et al., 2002). The ligand for CD40, CD40L, is a trimeric protein that is expressed on the surface of T cells (Armitage et al., 1992b; Noelle et al., 1992) and monocytes (Mach et al., 1997) as well as other cell types. Interaction between CD40L and CD40 results in the activation of various signaling pathways through the TNFR-associated factor (TRAF) family members (Mukundan et al., 2005; Purkerson et al., 2005) and has been shown to up-regulate costimulatory molecules and secretion of cytokines and chemokines (for review see Benveniste et al., 2004). CD40-CD40L interactions also result in CXCL8 expression in both transfected HEK293 cells (Girouard et al., 2005) and human airway epithelial cells (Cagnoni et al., 2004). We previously demonstrated a dramatic increase in CD40 expression in HIV-1-infected brain tissue compared with uninfected tissue, and this CD40 reactivity colocalized with CD68, a microglial and monocytic/macrophagic marker (D’Aversa et al., 2002). Thus, CD40 up-regulation and ligation may activate microglia to elaborate factors that contribute to inflammatory disease processes.
Neuroinflammation is essential to the pathogenesis of HIV-1 encephalitis. Because CD40 is up-regulated on microglia in HIV-1 encephalitis and CXCL8 is important in this disease process, we addressed the mechanisms by which CXCL8 is up-regulated and contributes to the neuropathogenesis of HIV-1. In this study, we show that microglia secrete CXCL8 after ligation of surface CD40 with sCD40L, and treatment of microglia with specific chemical inhibitors determined that CXCL8 secretion is dependent on the ERK1/2 MAPK pathway and is mediated by nuclear factor-κB (NFκB) and activator protein-1 (AP-1) at the transcriptional level. We also demonstrate that CXCL8 colocalizes with CD68/CD40-positive cells in HIV-1 encephalitic brain tissue. Thus, ligation of CD40 on the surface of microglia by CD40L-expressing T cells and monocytes within the CNS may result in chemokine secretion and therefore may play a vital role in the pathogenesis of neuroinflammatory diseases.
MATERIALS AND METHODS
Cell Culture and Reagents
Human fetal CNS tissue was used as part of an ongoing research protocol approved by the Albert Einstein College of Medicine. Microglia were established as previously described (D’Aversa et al., 2002). Briefly, the tissue was minced and shaken. The slurry was passed through a 250-μm nylon mesh filter, followed by a 150-μm filter, washed once with HBSS, and then with complete DMEM [DMEM plus 25 mM Hepes, 10% fetal calf serum (FCS), 1% penicillin-streptomycin, 1% nonessential amino acids]. Cells were resuspended and seeded at 9 × 107 per 150-cm2 flask for 12 days. The medium, containing microglia, was then removed and centrifuged for 5 min at 220g. The microglia were resuspended in complete DMEM and seeded at 5 × 105 per well of a 24-well plate. The medium was changed after 6–8 hr. Twenty-four hours after plating, microglia were treated with 200 μl DMEM without FCS containing 100 U/ml IFNγ (R&D Systems, Minneapolis, MN), 5 μg/ml soluble trimeric human CD40 ligand (a generous gift from Amgen Corporation, Seattle, WA), or both IFNγ and sCD40L or were left untreated for 24 hr. For inhibitor studies, microglia were pretreated with 10 μM of the MEK1/2 MAPK inhibitor U0126 (Sigma-Aldrich, St. Louis, MO), 10 μM of the p38 MAPK inhibitor SB203580 (Tong et al., 1997; Sigma-Aldrich), or 10 μM of the phosphatidylinositol-3-kinase (PI3K) inhibitor LY294002 for 1 hr, followed by treatment with IFNγ + sCD40L for 24 hr. Supernatants were then collected for ELISA.
Chemokine ELISA
Supernatants were analyzed for CXCL8 protein using a sandwich ELISA according to the manufacturer’s protocol. CXCL8 ELISA antibody pairs were purchased from R&D Systems. The sensitivity for this assay is 2.6 pg/ml.
RNA Extraction and Analysis
Microglia were plated at 1 × 106 cells/100-mm dish for 12 hr. Total RNA was extracted using Tri-Reagent (Molecular Research Center, Cincinnati, OH). Chemokine mRNA expression was analyzed using the human chemokine RPA kit hCK5 from Pharmingen (San Diego, CA). Densitometry was performed using Ambis QuantProbe software, with values normalized to GAPDH.
Western Blot Analysis
Twenty-four hours after plating, cells were pretreated with or without U0126 for 1 hr, after which some cells were left untreated, and others were treated with IFNγ + sCD40L for 10 min. Microglia were washed with 1× PBS and lysed [62.5 mM Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 10% glycerol, 50 mM dithiothreitol (DTT), 0.1% bromophenol blue, phenylarsine oxide 40 μM, protease inhibitor cocktail 50 μl/ml, and okadaic acid 60 nM; Sigma-Aldrich). The slurry was passed through an 18-g needle five times and heated at 95°C for 5 min. Equal amounts of whole-cell lysates (15 μl) were loaded onto each lane of a 12% SDS-PAGE gel. Proteins were transferred electrophoretically to Protran nitro-cellulose (Schleicher and Schuell, Keene, NH). Membranes were blocked with 5% nonfat dry milk in 0.1% Tween-20/TBS and incubated with primary antibodies (p44/42 MAP kinase Ab or phospho-p44/42 MAP kinase Ab; Cell Signaling, Beverly, MA) at a concentration of 1:1,000 overnight at 4°C. After washing, membranes were incubated with anti-rabbit horseradish peroxidase (HRP) secondary antibody (1:2,000; Cell Signaling) for 1 hr at room temperature. Proteins were visualized with an enhanced chemiluminescence detection kit (ECL; Amersham-Pharmacia, Piscataway, NJ).
Gel Shift Analysis
Microglia were serum free for 24 hr and then were treated with IFNγ + sCD40L (added directly to the well without changing media) for 4 hr, after which cells were washed twice with 1× phosphate-buffered saline (PBS), trypsinized, collected in DMEM + 10% FCS, and centrifuged. Cells were resuspended in TBS, centrifuged, resuspended in 1 ml TBS, transferred to an Eppendorff tube, and centrifuged for 15 sec. Supernatant was aspirated, and cells were resuspended in 400 μl of cold buffer A (100 mM Hepes, 1 M KCl, 10 mM EDTA, 10 mM EGTA, 100 mM DTT, and 100 mM PMSF). The pellet was resuspended, and samples were incubated for 15 min on ice. Nonidet P40 (10% solution) was added to the samples, they were vortexed for 10 sec, and centrifuged for 30 sec. After the supernatant was aspirated, the pellet was resuspended in 50 μl of cold buffer C (100 mM Hepes, 4 M NaCl, 10 mM EDTA, 10 mM EGTA, 100 mM DTT, 100 mM PMSF, 1 μg/μl aprotinin, and 1 μg/μl leupeptin), and samples were allowed to rock for 25 min at 4°C. Samples were centrifuged for 5 min at 12,000g and 4°C. Supernatants were collected and protein content was quatitated using the Bradford assay. Oligonucleotides were obtained from Promega (Madison, WI) and were end-labeled with [γ-32P]ATP. The labeled probes were passed through G25 Sephadex TE columns (Roche) and mixed with nuclear protein extracts (3–6 μg of protein) in binding buffer (Promega). Appropriate antibodies were added to some IFNγ + sCD40L treated samples for supershift analysis (anti-p65, anti-p50, anti-p52, ATF2, cFos, and cJun; all from Santa Cruz Biotechnology, Santa Cruz, CA) for 40 min at room temperature. Specific and nonspecific competitors (cold oligonucleotides) were added to other IFNγ + sCD40L treated samples and allowed to interact for 15 min at room temperature. Gels were preelectrophoresed for 1 hr at room temperature in 0.5× TGE buffer. Protein-DNA complexes were resolved in 5% polyacrylamide gels for 2.5 hr. Gels were dried and exposed to radiographic film with an intensifying screen at −70°C.
Immunohistochemistry
Immunohistochemical studies were performed on brain tissue taken at autopsy (kindly provided by Dr. Karen Weidenheim, Albert Einstein College of Medicine). Three individuals with HIV-1 infection, three individuals with HIV-1 encephalitis, and three uninfected control brains with non-CNS pathologies were studied (see Table I). Paraffin-embedded tissue was dehydrated and deparaffinized. After rehydration, the sections were washed in TBS and blocked in 2% normal horse serum/2% fish gelatin/2% IgG-free bovine serum albumin/Tris-buffered saline for 1 hr at room temperature. The sections were then incubated overnight at 4°C in primary antibody (rabbit anti-human CD40, 4 μg/ml; Santa Cruz Biotechnology; goat anti-human CXCL8/IL-8, 2 μg/ml; R&D Systems; and mouse anti-human CD68, EBM11, 8.6 μg/ml; Dako, Carpenteria, CA) or an appropriate isotype-matched antibody or normal serum. The sections were washed and incubated for 1 hr at room temperature with secondary antibody [fluorescein isothiocyanate (FITC)-conjugated sheep anti-rabbit IgG; Cy5-conjugated donkey anti-goat IgG; Immunoresearch; and Cy3-conjugated sheep anti-mouse IgG; Sigma]. Slides were washed and mounted in ProLong Gold antifade reagent with DAPI (Invitrogen, Eugene, OR). To analyze colocalization of these proteins, a Leica confocal microscope was used, as well as Adobe Photoshop and NIH Image J programs.
TABLE I.
Brain Tissue Used for Immunohistochemical Staining
| Case | HIV status | Age/gender | Brain pathology |
|---|---|---|---|
| 1 | − | 22Y/F | Normal |
| 2 | − | 13Y/F | Normal |
| 3 | − | 47Y/F | Normal |
| 4 | + | 20m/F | AIDS, CSTa degeneration, large cell lymphoma |
| 5 | + | 40wk/M | AIDS, HIV leukoencephalopathy |
| 6 | + | 9Y/F | AIDS, septic shock |
| 7 | + | 23m/F | HIVE, b CST degeneration, microglial nodules |
| 8 | + | 2.5Y/F | HIVE, CST degeneration, microglial nodules, HIV leukoencephalopathy, basal ganglia calcification |
| 9 | + | 6m/F | HIVE |
Corticospinal tract.
HIV-1 encephalitis.
Statistical Analysis
The paired, two-tailed Student’s t-test was used to determine statistical significance. P < 0.05 was considered to be significant.
RESULTS
CD40 Ligation Induces CXCL8 Protein and mRNA Expression by Microglia
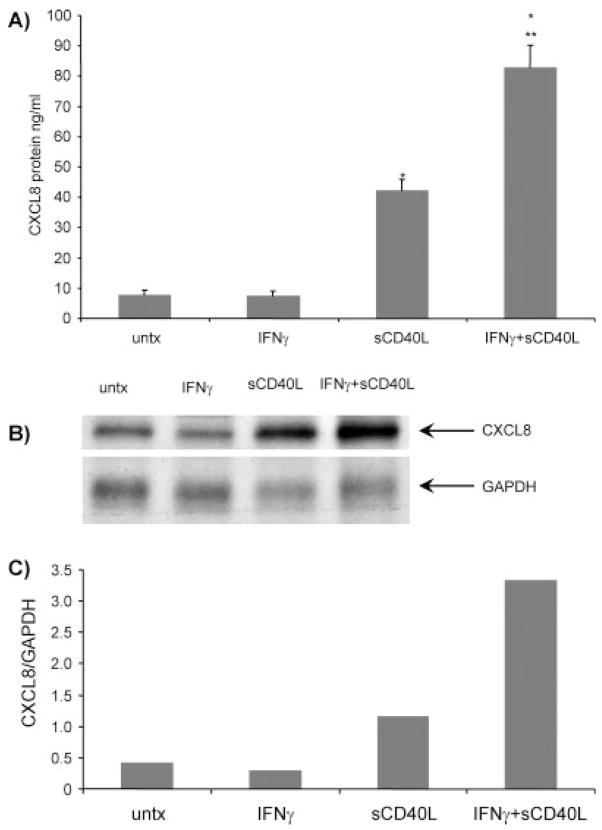
We and others had previously shown that IFNγ treatment increases CD40 expression on the surface of cultured microglia (Nguyen et al., 1998; Tan et al., 1999; Becher et al., 2000; D’Aversa et al., 2002). Microglia treated with 100 U/ml IFNγ and 5 μg/ml sCD40L (referred to as IFNγ + sCD40L) for 24 hr secreted significantly increased amounts of CXCL8 protein compared with untreated cultures (Fig. 1A; *P < 0.0009). Treatment with only 5 μg/ml sCD40L significantly increased CXCL8 protein compared with untreated cells (*P < 0.0009), and there was a synergistic effect when sCD40L was added with IFNγ to the cell cultures compared with sCD40L-treated cells (**P < 0.004). IFNγ treatment alone did not increase CXCL8 protein expression compared with baseline levels. The increase in protein was correlated with up-regulation of CXCL8 mRNA, as IFNγ + sCD40L treatment also induced CXCL8 mRNA after 12 hr (Fig. 1B). Densitometric analysis of IFNγ + sCD40L treatment demonstrated a seven fold increase in CXCL8 mRNA compared to baseline levels (Fig. 1C).
Fig. 1.
IFNγ + sCD40L induces CXCL8 protein and mRNA expression in microglia. A: Microglia were treated with IFNγ alone, sCD40L alone, or IFNγ + sCD40L or were left untreated for 24 hr, after which the supernatants were collected and CXCL8 chemokine ELISAs were performed as described in Materials and Methods. Shown are the means of four separate experiments ± SEM. *P < 0.0009 compared with untreated; **P < 0.004 compared with sCD40L only. B: Microglia were treated with IFNγ alone, sCD40L alone, or both IFNγ + sCD40L or were left untreated for 12 hr. Shown is a representative RPA of two experiments. C: Densitometric analysis comparing CXCL8 to GAPDH expression confirms that IFNγ + sCD40L treatment increases mRNA expression compared with other treatments.
CXCL8 Production by Microglia Is Mediated Through the ERK1/2 Pathway
We examined the signal transduction pathways that are activated in microglia by IFNγ + sCD40L treatment, leading to CXCL8 production. Cotreatment of microglia with IFNγ + sCD40L and 10 μM of the MEK1/2 inhibitor U0126, 10 μM of the p38 inhibitor SB203580, or 10 μM of the PI3K inhibitor LY294002 for 24 hr demonstrated that U0126 significantly inhibited IFNγ + sCD40L-induced CXCL8 protein (reduced by 63%; **P < 0.0008). SB203580 also inhibited CXCL8 protein expression (Fig. 2 reduced by 23%; *P < 0.005), although not to the same extent as U0126. There was no difference in IFNγ + sCD40L-induced CXCL8 protein expression after treatment with LY294002 (Fig. 2). Lysates prepared from microglia treated with IFNγ + sCD40L and U0126 were then examined by Western blot analysis. As demonstrated in Figure 3, IFNγ + sCD40L induced phosphorylation of ERK1/2 (Fig. 3, lane 3) after 10 min, and treatment with U0126 abolished this phosphorylation (Fig. 3, lane 4).
Fig. 2.
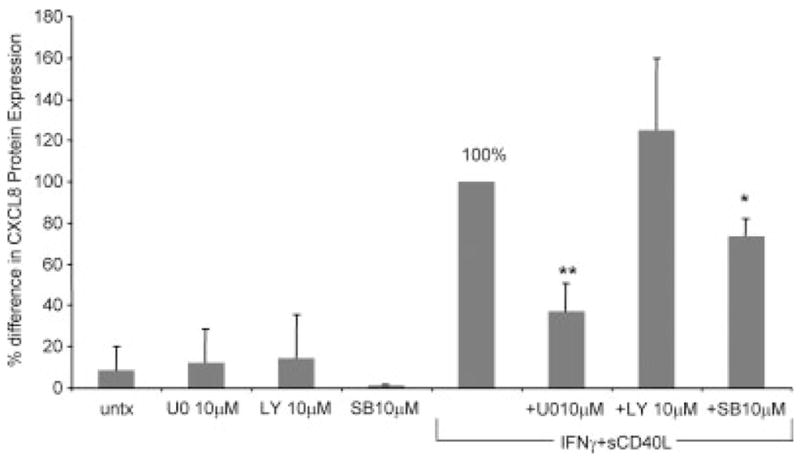
CXCL8 protein expression is mediated by the ERK1/2 MAPK pathway. CXCL8 protein levels were determined as a percentage difference compared with IFNγ + sCD40L (set to 100%). Microglia were pretreated with 10 μM of the MEK1/2 MAPK inhibitor U0126, 10 μM of the p38 MAPK inhibitor SB203580, or 10 μM of the phosphatidylinositol-3-kinase (PI3K) inhibitor LY294002 for 1 hr, then treated with or without IFNγ + sCD40L for an additional 24 hr. Shown are the means of three separate experiments ± SEM. *P < 0.005, **P < 0.0008 compared with IFNγ + sCD40L. U0, U0126; LY, LY294002; SB, SB203580.
Fig. 3.
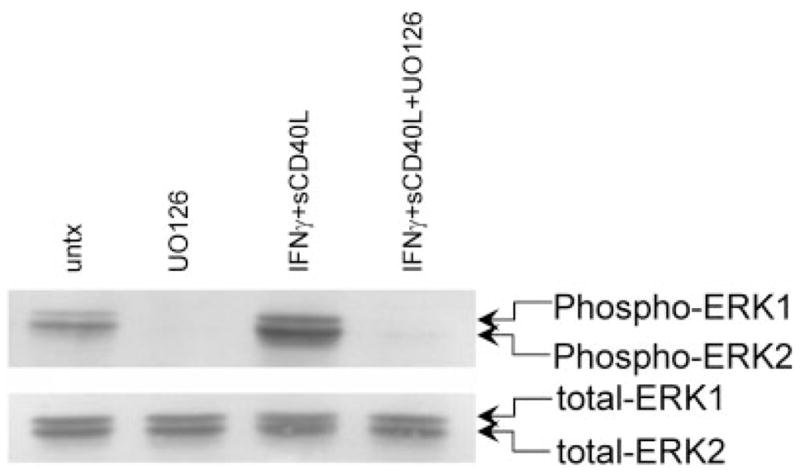
IFNγ + sCD40L induces phosphorylation of ERK1/2, and treatment with U0126 abrogates phosphorylation. Western blot analysis of IFNγ + sCD40L-treated microglia shows phosphorylated ERK1/2 (upper panel) and total ERK1/2 expression (lower panel). Shown is a representative blot of two separate experiments. Lane 1: untreated; lane 2: U0126 10 μM; lane 3: IFNγ + sCD40L; lane 4: IFNγ + sCD40L + U0126.
NFκB and AP-1 Are Necessary Transcription Factors for IFNγ + sCD40L-Induced CXCL8 Secretion by Microglia
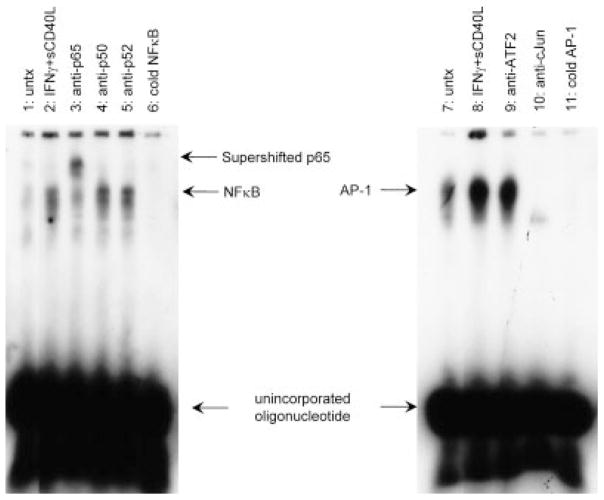
MEK phosphorylates and activates ERK, which can then lead to the induction of various transcription factors. NFκB, AP-1, and C/EBPβ binding sites have been identified within the CXCL8 promoter region (Mukaida et al., 1990). We determined which transcription factor(s) were involved in CD40-CD40L-induced CXCL8 production. With specific probe sequences, gel shift analysis was performed for NFκB (Fig. 4, lanes 1–6), AP-1 (Fig. 4, lanes 7–11), and C/EBPβ (data not shown). Protein-DNA complexes were formed after IFNγ + sCD40L treatment (Fig. 4, lanes 2 and 8) compared with untreated cells (Fig. 4, lanes 1 and 7). Super-shift analysis with IFNγ + sCD40L treated cells demonstrated that the NFκB complex contains p65 (Fig. 4, lane 3) and not p50 (Fig. 4, lane 4) or p52 (Fig. 4, lane 5) and that the AP-1 complex contains cJun (Fig. 4, lane 10) and not ATF2 (Fig. 4, lane 9) or cFos (data not shown). With the AP-1 probe, there was no shift detected, but instead there was a disappearance of the AP-1 band using the cJun antibody, as has been documented previously (Murayama et al., 1997), indicating that the AP-1 complex is composed of cJun. Specific inhibitors (cold oligonucleotides; Fig. 4, lanes 6 and 11) were used to demonstrate that the complex was specific. Unlabelled, nonspecific oligonucleotides had no effect on band intensity (data not shown). These data indicate that NFκB and AP-1 are necessary transcription factors for CXCL8 production by microglia after CD40 activation.
Fig. 4.
IFNγ + sCD40L-induced CXCL8 secretion is dependent on the transcription factors NFκB and AP-1, but not C/EBPβ. Microglia were serum free for 24 hr and then were treated for 4 hr, after which nuclear lysate was prepared. Gel shift analysis with specific probe sequence from NFκB or AP-1 was performed. Both the NFκB and the AP-1 probes demonstrated an increase in DNA-protein binding compared with the untreated. Shown is a representative blot of three separate experiments. Lanes 1 and 7: untreated (untx); lanes 2 and 8: IFNγ + sCD40L; lane 3: IFNγ + sCD40L + anti-p65; lane 4: IFNγ + sCD40L + anti-p50; lane 5: IFNγ + sCD40L + anti-p52; lane 6: IFNγ + sCD40L + cold NFκB oligonucleotide; lane 9: IFNγ + sCD40L + anti-ATF2; lane 10: IFNγ + sCD40L + anti-cJun; lane 11: cold AP-1 oligonucleotide.
CXCL8 Is Increased and Colocalized With CD68/CD40-Positive Cells in HIV Encephalitic Brain Tissue Sections Compared With Control or Nonencephalitic HIV Tissue
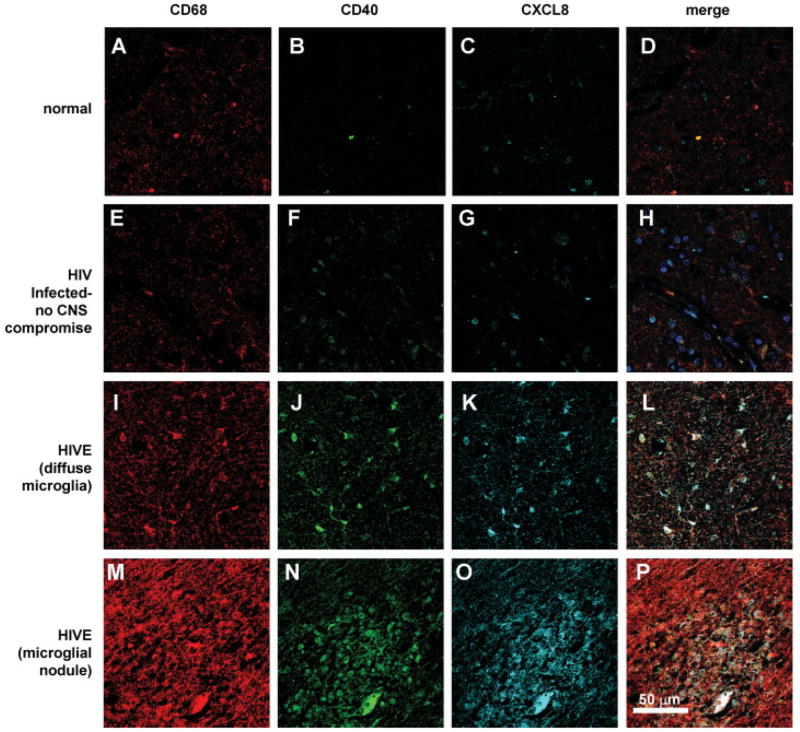
We previously demonstrated that CD40 is increased on microglial cells in brain tissue sections of HIV-1-infected individuals (D’Aversa et al., 2002). Several studies demonstrated the importance of CD40 and CXCL8 in the pathogenesis of HIV-1 encephalitis but had not indicated a relationship between the two. We therefore determined whether CXCL8 is increased and colocalized with CD40 in HIV-1-infected brain tissue. Sections of brain tissue from three uninfected individuals with normal neuropathology (control), three HIV-1-infected people with no neurological disease, and three HIV-1-infected people with encephalitis (HIVE) were analyzed by triple immunohistochemical staining (Table I). Sections were stained with three different antibodies; CD68, CD40, and CXCL-8. A secondary antibody conjugated to Cy3 (red staining) was used to detect anti-CD68 reactivity; a secondary antibody conjugated to FITC (green staining) was used to detect anti-CD40 reactivity; and a secondary antibody conjugated to Cy5 (cyan staining) was used to detect anti-CXCL8 reactivity according to a previously published protocol (Eugenin et al., 2006a). Sections were then examined by confocal microscopy. There was some reactivity with all three antibodies in normal tissue sections (Fig. 5A–D). As shown here, CD40 reactivity was increased in HIV-1-infected tissue with no CNS compromise (Fig. 5F), and there was some colocalization of CXCL8 with CD68-positive/CD40-positive cells (Fig. 5H, white staining). However, HIVE tissue sections analyzed showed increased expression of CD68 (Fig. 5I), CD40 (Fig. 5J), and CXCL8 (Fig. 5K) as well as colocalization of CD68, CD40, and CXCL8 (Fig. 5L, white staining). Compared with non-encephalitic HIV tissue (Fig. 5H), the positive cells appeared diffuse and activated. One hallmark of HIV-1 infection is the presence of microglial nodules. Staining of the nodule demonstrated that there was increased expression of CD68 (Fig. 5M), CD40 (Fig. 5N), and CXCL8 (Fig. 5O) and almost perfect colocalization among all three antibodies (Fig. 5P, white staining). There was little background or nonspecific reactivity with negative control antibodies in all tissues examined (data not shown).
Fig. 5.
CXCL8 colocalizes with CD68/CD40-positive cells in HIV encephalitic brain tissue sections. Immunohistochemical analyses of human brain tissue sections with antibodies to CD68 (red staining), CD40 (green staining), and CXCL8 (cyan staining) were performed and analyzed by confocal microscopy. A–D: In normal brain tissue, there is minimal reactivity with all three antibodies. E–H: HIV tissue with no CNS compromise showed increased reactivity with antibodies, with some colocalization (merge, white staining). HIVE tissue with diffuse activated microglia (I–L) and a microglia nodule (M–P) demonstrated greater staining with antibodies and almost perfect colocalization of CXCL8 with CD68/CD40-positive cells (merge, white staining). Shown are representative figures from three separate tissue sections. Scale bar = 50 μm.
DISCUSSION
Accumulating data support the importance of CXCL8 in NeuroAIDS. CXCL8 has been demonstrated in HIV-1 encephalitic tissue (Sanders et al., 1998) as well as in SIV infection, where there is an increase in microglial CXCL8 expression (Sopper et al., 1996). In addition, HIV-1-infected peripheral blood mononuclear cells produce greater levels of CXCL8 than uninfected cells (Wetzel et al., 2002), and there is an increase in circulating levels of this chemokine in HIV-1-infected individuals (Matsumoto et al., 1993; Thea et al., 1996; Meddows-Taylor et al., 1999). HIV-1-infected (Cota et al., 2000) and Tat-treated astrocytes (Kutsch et al., 2000) produce CXCL8.
We demonstrate that there is an increase in CXCL8 at both the protein and the mRNA levels after ligation of CD40 on microglia with sCD40L. Others have found increased expression of CXCL8 from monocyte-derived macrophages after ligation of CD40 (Chandler et al., 1995; Kiener et al., 1995; Kornbluth et al., 1998; Pearson et al., 2001). By using inhibitors to different MAPK pathways, we demonstrated that CXCL8 production by microglia is dependent on the ERK1/2 MAPK pathway. ERK1/2 MAPK-mediated CXCL8 expression has been demonstrated in islet beta cells (Barbe-Tuana et al., 2006) as well as monocytes and THP-1 cells (Pearson et al., 2001), but activation of p38 MAPK was not detected. We demonstrated previously that CD40-CD40L interactions on microglia activate the p38 MAPK pathway that is critical for CXCL10 secretion (D’Aversa et al., 2002) and now demonstrate that, though not the major pathway, CD40 ligation of microglia induces p38 activity that contributes to CXCL8 secretion.
There are two distinct promoter regions important for the transcriptional regulation of CXCL8, a distal AP-1 element and a proximal site containing NFκB and C/EBPβ (Kunsch and Rosen 1993; Matsusaka et al., 1993; Stein and Baldwin 1993). The AP-1 site, located between −120 and −126, interacts with Jun and Fos family members (Karin et al., 1997) and contains either Jun-Fos heterodimers or Jun-Jun homodimers (Smeal et al., 1989). AP-1 activity is dependent on the phosphorylation of two serine residues in the transactivation domain (Karin et al., 1997). The NFκB and C/EBPβ sites are adjacent on the promoter, with the NFκB site located between −70 and −80, and binding proteins of the Rel family (Barnes, 1997). It has been shown that functional cooperativity among the AP-1, NFκB, and C/EBPβ sites is necessary to achieve optimal CXCL8 activity in a glioblastoma cell line, T98G (Mukaida et al., 1994). We therefore examined the transcription of CXCL8 and found it to be regulated by the cis-regulatory elements NFκB and AP-1 rather than C/EBPβ. Supershift analysis demonstrated that the NFκB complex most likely contains homodimers of p65, insofar as this was the only band that shifted in the presence of antibodies. Also, the AP-1 complex contained homodimers of cJun, because this band disappears after antibody treatment, indicating that there is interference with the complex.
The role of CXCL8 in other neuroinflammatory diseases has been documented. Multiple sclerosis (MS) is an immune-mediated disease of the CNS associated with demyelination of the white matter, perivascular inflammation, oligodendrocyte damage, axonal damage and/or loss, and breakdown of the blood–brain barrier (Trapp et al., 1998; Chang et al., 2002; Minagar and Alexander, 2003). Genetic and environmental factors and autoimmune mechanisms all play an important role in the progression of disease. Activated autoreactive T cells and macrophages are recruited across the damaged blood–brain barrier and are thought to play an essential role in the pathogenesis of MS. The recruitment of these cells is thought to be mediated by various chemokines expressed by CNS cells within MS lesions as well as in lesions of an animal model of MS, experimental autoimmune encephalomylitis (EAE; Simpson et al., 2000; Zhang et al., 2000; Omari et al., 2005).
It has been shown that hypertrophic astrocytes stain strongly for CXCL8 in active MS lesions, but not in silent lesions, and there was no CXCL8 reactivity in tissue obtained from other neurological diseases studied or from normal white matter (Omari et al., 2005). Expression of the CXCL8 receptors CXCL1 and CXCL2 was increased on oligodendrocytes from MS tissue, suggesting a role of the receptor-ligand pairs in remyelination (Omari et al., 2005). Serum and cerebrospinal fluid (CSF) levels in individuals with MS had increased CXCL8 compared with individuals with no or other neurological diseases (Saruhan-Direskeneli et al., 2003; Lund et al., 2004; Bartosik-Psujek and Stelmasiak, 2005), and peripheral blood mononuclear cells from people with MS also secreted high levels of CXCL8 compared with controls (Lund et al., 2004). In addition, CXCL8 induced rapid influx of neutrophils into acute MS lesions, which may result in extensive damage to the CNS (for review see Cuzner and Opdenakker, 1999).
CXCL8 increases the secretion of matrix metalloproteases (MMPs) in a human fetal microglial cell line (Cross and Woodroofe 1999), and it has been demonstrated that CD40L treatment of microglia results in the secretion of high levels of MMPs (Ghorpade et al., 2001), which have been shown to degrade myelin basic protein (MBP; Chandler et al., 1995). Also, monocytes stimulated with MBP have increased mRNA and protein expression of CXCL8 (Baron et al., 1998). MBP has also been shown to activate T cells and is believed to contribute to the pathogenesis of MS. These data, taken with our findings that CD40-CD40L interactions of microglia results in CXCL8 expression, suggest an important role for CXCL8 in inflammatory conditions of the CNS other than directed chemotaxis. For example, CD40 ligation of microglia may induce MMP production and CXCL8 secretion, which may result in further secretion of MMPs from microglia. The secreted MMPs may then facilitate disruption of the blood–brain barrier, which is evident in NeuroAIDS, and allow the influx of inflammatory cells, which may contribute to the pathogenesis of HIV-1 encephalitis.
It has also been shown that CD40 expression in microglia is important for EAE disease progression, in that CD40-deficient microglia do not achieve a full level of activation during EAE, and there was a reduced expression of encephalitogenic T cells and leukocyte infiltration into the CNS (Ponomarev et al., 2006). The importance of CXCL8 and its receptor, CXCR2, have also been demonstrated in other CNS diseases. In a monkey model of ischemia-reperfusion, it was shown that microglia both secreted CXCL8 and expressed CXCR2, allowing for an autocrine loop (Popivanova et al., 2003). A role for CXCL8 in Alzheimer’s disease has been demonstrated, in that brain tissue from demented individuals had intense reactivity for CXCR2 on neurons, which colocalized with amyloid deposits (Horuk et al., 1997). Treatment of THP-1 cells (Giri et al., 2003) or human microglia (Franciosi et al., 2005, 2006) with amyloid beta peptides increased CXCL8 mRNA and protein expression, and treatment of microglia with CXCL8 and amyloid beta leads to an enhancement of mRNA and protein of several other proinflammatory factors compared with peptide alone (Franciosi et al., 2005). In addition, CXCL8 protected mouse hippocampal neurons against amyloid beta-induced death, suggesting that CXCL8 is involved in neuronal survival and maintenance (Watson and Fan, 2005).
In summary, our data suggest a mechanism whereby HIV-1 infected or uninfected inflammatory cells enter the CNS, secrete IFNγ, and up-regulate CD40 expression on microglia. CD40 can also be up-regulated after microglia are exposed to the HIV-1 protein Tat (D’Aversa et al., 2005). Once CD40 is up-regulated, it can be ligated by the infiltrating inflammatory cells. Ligation of CD40 induces CXCL8 chemokine secretion. The increase in CXCL8 may also enhance microglial MMP production. MMPs aid in the breakdown of the blood–brain barrier and invasion of inflammatory cells, thereby enhancing an inflammatory cascade that contributes to a variety of CNS pathologies.
Acknowledgments
Contract grant sponsor: National Institute of Mental Health; Contract grant number: MH075679 (to J.W.B.); Contract grant number: MH070297 (to J.W.B.); Contract grant sponsor: National Institutes of Health; Contract grant number: NS11920 (to J.W.B.); Contract grant sponsor: National Institutes of Health Experimental Neuropathology Training Grant; Contract grant number: NS07098 (to T.G.D.); Contract grant sponsor: Immunology/Pathology Core of the National Institutes of Health Center for AIDS Research (CFAR); Contract grant number: AI051519.
We thank the Fetal Tissue Repository at the Albert Einstein College of Medicine and Dr. Brad Poulos for providing the fetal tissue and Dr. Karen Weidenheim for providing the brain tissue sections. We also thank Amgen for their generous gift of the human soluble CD40L; Dr. Nadia Jahroudi, formerly of AECOM, for help with the gel shift assays; and the Analytical Imaging Facility at the Albert Einstein College of Medicine.
References
- Albright AV, Soldan SS, Gonzalez-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol. 2003;9:222–227. doi: 10.1080/13550280390194073. [DOI] [PubMed] [Google Scholar]
- Aloisi F, De Simone R, Columba-Cabezas S, Penna G, Adorini L. Functional maturation of adult mouse resting microglia into an APC is promoted by granulocyte-macrophage colony-stimulating factor and interaction with Th1 cells. J Immunol. 2000;164:1705–1712. doi: 10.4049/jimmunol.164.4.1705. [DOI] [PubMed] [Google Scholar]
- Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Mac-duff BM, Anderson DM, Gimpel SD, Davis-Smith T, Maliszewski CR, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992a;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Armitage RJ, Sato TA, Macduff BM, Clifford KN, Alpert AR, Smith CA, Fanslow WC. Identification of a source of biologically active CD40 ligand. Eur J Immunol. 1992b;22:2071–2076. doi: 10.1002/eji.1830220817. [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe-Tuana FM, Klein D, Ichii H, Berman DM, Coffey L, Kenyon NS, Ricordi C, Pastori RL. CD40-CD40 ligand interaction activates proinflammatory pathways in pancreatic islets. Diabetes. 2006;55:2437–2445. doi: 10.2337/db05-1673. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Nuclear factor-kappa B. Int J Biochem Cell Biol. 1997;29:867–870. doi: 10.1016/s1357-2725(96)00159-8. [DOI] [PubMed] [Google Scholar]
- Baron P, Constantin G, Meda L, Scarpini E, Scarlato G, Trinchieri G, Monastra G, Rossi F, Cassatella MA. Cultured human monocytes release proinflammatory cytokines in response to myelin basic protein. Neurosci Lett. 1998;252:151–154. doi: 10.1016/s0304-3940(98)00497-2. [DOI] [PubMed] [Google Scholar]
- Bartosik-Psujek H, Stelmasiak Z. The levels of chemokines CXCL8, CCL2 and CCL5 in multiple sclerosis patients are linked to the activity of the disease. Eur J Neurol. 2005;12:49–54. doi: 10.1111/j.1468-1331.2004.00951.x. [DOI] [PubMed] [Google Scholar]
- Becher B, Blain M, Antel JP. CD40 engagement stimulates IL-12 p70 production by human microglial cells: basis for Th1 polarization in the CNS. J Neuroimmunol. 2000;102:44–50. doi: 10.1016/s0165-5728(99)00152-6. [DOI] [PubMed] [Google Scholar]
- Benveniste EN, Nguyen VT, Wesemann DR. Molecular regulation of CD40 gene expression in macrophages and microglia. Brain Behav Immun. 2004;18:7–12. doi: 10.1016/j.bbi.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Bornemann MA, Verhoef J, Peterson PK. Macrophages, cytokines, and HIV. J Lab Clin Med. 1997;129:10–16. doi: 10.1016/s0022-2143(97)90156-6. [DOI] [PubMed] [Google Scholar]
- Budka H, Wiley CA, Kleihues P, Artigas J, Asbury AK, Cho ES, Cornblath DR, Dal Canto MC, DeGirolami U, Dickson D, et al. HIV-associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol. 1991;1:143–152. doi: 10.1111/j.1750-3639.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Cagnoni F, Oddera S, Giron-Michel J, Riccio AM, Olsson S, Dellacasa P, Melioli G, Canonica GW, Azzarone B. CD40 on adult human airway epithelial cells: expression and proinflammatory effects. J Immunol. 2004;172:3205–3214. doi: 10.4049/jimmunol.172.5.3205. [DOI] [PubMed] [Google Scholar]
- Carrol ED, Mankhambo LA, Balmer P, Nkhoma S, Banda DL, Guiver M, Jeffers G, Makwana N, Molyneux EM, Molyneux ME, Smyth RL, Hart CA. Chemokine responses are increased in HIV-infected Malawian children with invasive pneumococcal disease. J Acquir Immune Defic Syndr. 2007;44:443–450. doi: 10.1097/QAI.0b013e31802f8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casilli F, Bianchini A, Gloaguen I, Biordi L, Alesse E, Festuccia C, Cavalieri B, Strippoli R, Cervellera MN, Di Bitondo R, Ferretti E, Mainiero F, Bizzarri C, Colotta F, Bertini R. Inhibition of interleukin-8 (CXCL8/IL-8) responses by repertaxin, a new inhibitor of the chemokine receptors CXCR1 and CXCR2. Biochem Pharmacol. 2005;69:385–394. doi: 10.1016/j.bcp.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Chandler S, Coates R, Gearing A, Lury J, Wells G, Bone E. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett. 1995;201:223–226. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Cota M, Kleinschmidt A, Ceccherini-Silberstein F, Aloisi F, Mengozzi M, Mantovani A, Brack-Werner R, Poli G. Up-regulated expression of interleukin-8, RANTES and chemokine receptors in human astrocytic cells infected with HIV-1. J Neurovirol. 2000;6:75–83. doi: 10.3109/13550280009006384. [DOI] [PubMed] [Google Scholar]
- Cross AK, Woodroofe MN. Chemokine modulation of matrix metalloproteinase and TIMP production in adult rat brain microglia and a human microglial cell line in vitro. Glia. 1999;28:183–189. [PubMed] [Google Scholar]
- Cuzner ML, Opdenakker G. Plasminogen activators and matrix metalloproteases, mediators of extracellular proteolysis in inflammatory demyelination of the central nervous system. J Neuroimmunol. 1999;94:1–14. doi: 10.1016/s0165-5728(98)00241-0. [DOI] [PubMed] [Google Scholar]
- Dangond F, Windhagen A, Groves CJ, Hafler DA. Constitutive expression of costimulatory molecules by human microglia and its relevance to CNS autoimmunity. J Neuroimmunol. 1997;76:132–138. doi: 10.1016/s0165-5728(97)00043-x. [DOI] [PubMed] [Google Scholar]
- D’Aversa TG, Weidenheim KM, Berman JW. CD40-CD40L interactions induce chemokine expression by human microglia: implications for human immunodeficiency virus encephalitis and multiple sclerosis. Am J Pathol. 2002;160:559–567. doi: 10.1016/S0002-9440(10)64875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aversa TG, Eugenin EA, Berman JW. NeuroAIDS: contributions of the human immunodeficiency virus-1 proteins Tat and gp120 as well as CD40 to microglial activation. J Neurosci Res. 2005;81:436–446. doi: 10.1002/jnr.20486. [DOI] [PubMed] [Google Scholar]
- Denis M, Ghadirian E. Dysregulation of interleukin 8, interleukin 10, and interleukin 12 release by alveolar macrophages from HIV type 1-infected subjects. AIDS Res Hum Retroviruses. 1994;10:1619–1627. doi: 10.1089/aid.1994.10.1619. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Lee SC, Mattiace LA, Yen SH, Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease. Glia. 1993;7:75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- Ehrlich LC, Hu S, Sheng WS, Sutton RL, Rockswold GL, Peterson PK, Chao CC. Cytokine regulation of human microglial cell IL-8 production. J Immunol. 1998;160:1944–1948. [PubMed] [Google Scholar]
- Esser R, Glienke W, von Briesen H, Rubsamen-Waigmann H, Andreesen R. Differential regulation of proinflammatory and hematopoietic cytokines in human macrophages after infection with human immunodeficiency virus. Blood. 1996;88:3474–3481. [PubMed] [Google Scholar]
- Eugenin EA, Dyer G, Calderon TM, Berman JW. HIV-1 tat protein induces a migratory phenotype in human fetal microglia by a CCL2 (MCP-1)-dependent mechanism: possible role in neuroAIDS. Glia. 2005;49:501–510. doi: 10.1002/glia.20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Gamss R, Buckner C, Buono D, Klein RS, Schoenbaum EE, Calderon TM, Berman JW. Shedding of PECAM-1 during HIV infection: a potential role for soluble PECAM-1 in the pathogenesis of NeuroAIDS. J Leukoc Biol. 2006a;79:444–452. doi: 10.1189/jlb.0405215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood–brain barrier: a potential mechanism of HIV-CNS invasion and neuroAIDS. J Neurosci. 2006b;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciosi S, Choi HB, Kim SU, McLarnon JG. IL-8 enhancement of amyloid-beta (Abeta 1–42)-induced expression and production of proinflammatory cytokines and COX-2 in cultured human microglia. J Neuroimmunol. 2005;159:66–74. doi: 10.1016/j.jneuroim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Franciosi S, Ryu JK, Choi HB, Radov L, Kim SU, McLarnon JG. Broad-spectrum effects of 4-aminopyridine to modulate amyloid beta1–42-induced cell signaling and functional responses in human microglia. J Neurosci. 2006;26:11652–11664. doi: 10.1523/JNEUROSCI.2490-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- Ghorpade A, Persidskaia R, Suryadevara R, Che M, Liu XJ, Persidsky Y, Gendelman HE. Mononuclear phagocyte differentiation, activation, and viral infection regulate matrix metalloproteinase expression: implications for human immunodeficiency virus type 1-associated dementia. J Virol. 2001;75:6572–6583. doi: 10.1128/JVI.75.14.6572-6583.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri RK, Selvaraj SK, Kalra VK. Amyloid peptide-induced cytokine and chemokine expression in THP-1 monocytes is blocked by small inhibitory RNA duplexes for early growth response-1 messenger RNA. J Immunol. 2003;170:5281–5294. doi: 10.4049/jimmunol.170.10.5281. [DOI] [PubMed] [Google Scholar]
- Girouard J, Reyes-Moreno C, Darveau A, Akoum A, Mourad W. Requirement of the extracellular cysteine at position six for CD40/CD40 dimer formation and CD40-induced IL-8 expression. Mol Immunol. 2005;42:773–780. doi: 10.1016/j.molimm.2004.07.048. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Horuk R. Chemokine and chemokine receptor expression in the central nervous system. J Neurovirol. 1999;5:13–26. doi: 10.3109/13550289909029741. [DOI] [PubMed] [Google Scholar]
- Ho DD, Sarngadharan MG, Resnick L, Dimarzoveronese F, Rota TR, Hirsch MS. Primary human T-lymphotropic virus type III infection. Ann Intern Med. 1985;103:880–883. doi: 10.7326/0003-4819-103-6-880. [DOI] [PubMed] [Google Scholar]
- Horuk R, Martin AW, Wang Z, Schweitzer L, Gerassimides A, Guo H, Lu Z, Hesselgesser J, Perez HD, Kim J, Parker J, Hadley TJ, Peiper SC. Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol. 1997;158:2882–2890. [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kiener PA, Moran-Davis P, Rankin BM, Wahl AF, Aruffo A, Hollenbaugh D. Stimulation of CD40 with purified soluble gp39 induces proinflammatory responses in human monocytes. J Immunol. 1995;155:4917–4925. [PubMed] [Google Scholar]
- Kim WK, Alvarez X, Fisher J, Bronfin B, Westmoreland S, McLaurin J, Williams K. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol. 2006;168:822–834. doi: 10.2353/ajpath.2006.050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth RS, Kee K, Richman DD. CD40 ligand (CD154) stimulation of macrophages to produce HIV-1-suppressive beta-chemokines. Proc Natl Acad Sci U S A. 1998;95:5205–5210. doi: 10.1073/pnas.95.9.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunsch C, Rosen CA. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993;13:6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsch O, Oh J, Nath A, Benveniste EN. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J Virol. 2000;74:9214–9221. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane BR, Lore K, Bock PJ, Andersson J, Coffey MJ, Strieter RM, Markovitz DM. Interleukin-8 stimulates human immunodeficiency virus type 1 replication and is a potential new target for antiretroviral therapy. J Virol. 2001;75:8195–8202. doi: 10.1128/JVI.75.17.8195-8202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CG, Anderson AO, Appella E, Oppenheim JJ, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Leonard EJ, Skeel A, Yoshimura T, Noer K, Kutvirt S, Van Epps D. Leukocyte specificity and binding of human neutrophil attractant/activation protein-1. J Immunol. 1990;144:1323–1330. [PubMed] [Google Scholar]
- Lund BT, Ashikian N, Ta HQ, Chakryan Y, Manoukian K, Groshen S, Gilmore W, Cheema GS, Stohl W, Burnett ME, Ko D, Kachuck NJ, Weiner LP. Increased CXCL8 (IL-8) expression in multiple sclerosis. J Neuroimmunol. 2004;155:161–171. doi: 10.1016/j.jneuroim.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Mach F, Schonbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, Libby P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci U S A. 1997;94:1931–1936. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Ge N, Achim CL, Hansen LA, Wiley CA. Selective neuronal vulnerability in HIV encephalitis. J Neuropathol Exp Neurol. 1992;51:585–593. doi: 10.1097/00005072-199211000-00003. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Miike T, Nelson RP, Trudeau WL, Lockey RF, Yodoi J. Elevated serum levels of IL-8 in patients with HIV infection. Clin Exp Immunol. 1993;93:149–151. doi: 10.1111/j.1365-2249.1993.tb07957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci U S A. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddows-Taylor S, Martin DJ, Tiemessen CT. Dysregulated production of interleukin-8 in individuals infected with human immunodeficiency virus type 1 and Mycobacterium tuberculosis. Infect Immun. 1999;67:1251–1260. doi: 10.1128/iai.67.3.1251-1260.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagar A, Alexander JS. Blood–brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by proinflammatory cytokines. J Biol Chem. 1990;265:21128–21133. [PubMed] [Google Scholar]
- Mukaida N, Morita M, Ishikawa Y, Rice N, Okamoto S, Kasahara T, Matsushima K. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-kappa B is target for glucocorticoid-mediated interleukin 8 gene repression. J Biol Chem. 1994;269:13289–13295. [PubMed] [Google Scholar]
- Mukundan L, Bishop GA, Head KZ, Zhang L, Wahl LM, Suttles J. TNF receptor-associated factor 6 is an essential mediator of CD40-activated proinflammatory pathways in monocytes and macrophages. J Immunol. 2005;174:1081–1090. doi: 10.4049/jimmunol.174.2.1081. [DOI] [PubMed] [Google Scholar]
- Murayama T, Ohara Y, Obuchi M, Khabar KS, Higashi H, Mukaida N, Matsushima K. Human cytomegalovirus induces interleukin-8 production by a human monocytic cell line, THP-1, through acting concurrently on AP-1- and NF-kappaB-binding sites of the interleukin-8 gene. J Virol. 1997;71:5692–5695. doi: 10.1128/jvi.71.7.5692-5695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Geiger J. Neurobiological aspects of human immunodeficiency virus infection: neurotoxic mechanisms. Prog Neurobiol. 1998;54:19–33. doi: 10.1016/s0301-0082(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Walker WS, Benveniste EN. Post-transcriptional inhibition of CD40 gene expression in microglia by transforming growth factor-beta. Eur J Immunol. 1998;28:2537–2548. doi: 10.1002/(SICI)1521-4141(199808)28:08<2537::AID-IMMU2537>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omari KM, John GR, Sealfon SC, Raine CS. CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis. Brain. 2005;128:1003–1015. doi: 10.1093/brain/awh479. [DOI] [PubMed] [Google Scholar]
- Palmer DL, Hjelle BL, Wiley CA, Allen S, Wachsman W, Mills RG, Davis LE, Merlin TL. HIV-1 infection despite immediate combination antiviral therapy after infusion of contaminated white cells. Am J Med. 1994;97:289–295. doi: 10.1016/0002-9343(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Pearson LL, Castle BE, Kehry MR. CD40-mediated signaling in monocytic cells: up-regulation of tumor necrosis factor receptor-associated factor mRNAs and activation of mitogen-activated protein kinase signaling pathways. Int Immunol. 2001;13:273–283. doi: 10.1093/intimm/13.3.273. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Novikova M, Maresz K, Shriver LP, Dittel BN. Development of a culture system that supports adult microglial cell proliferation and maintenance in the resting state. J Immunol Methods. 2005;300:32–46. doi: 10.1016/j.jim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Shriver LP, Dittel BN. CD40 expression by microglial cells is required for their completion of a two-step activation process during central nervous system autoimmune inflammation. J Immunol. 2006;176:1402–1410. doi: 10.4049/jimmunol.176.3.1402. [DOI] [PubMed] [Google Scholar]
- Popivanova BK, Koike K, Tonchev AB, Ishida Y, Kondo T, Ogawa S, Mukaida N, Inoue M, Yamashima T. Accumulation of microglial cells expressing ELR motif-positive CXC chemokines and their receptor CXCR2 in monkey hippocampus after ischemia-reperfusion. Brain Res. 2003;970:195–204. doi: 10.1016/s0006-8993(03)02343-6. [DOI] [PubMed] [Google Scholar]
- Purkerson JM, Smith RS, Pollock SJ, Phipps RP. The TRAF6, but not the TRAF2/3, binding domain of CD40 is required for cytokine production in human lung fibroblasts. Eur J Immunol. 2005;35:2920–2928. doi: 10.1002/eji.200526219. [DOI] [PubMed] [Google Scholar]
- Richardson RM, Tokunaga K, Marjoram R, Sata T, Snyderman R. Interleukin-8-mediated heterologous receptor internalization provides resistance to HIV-1 infectivity. Role of signal strength and receptor desensitization. J Biol Chem. 2003;278:15867–15873. doi: 10.1074/jbc.M211745200. [DOI] [PubMed] [Google Scholar]
- Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–1026. [PubMed] [Google Scholar]
- Saruhan-Direskeneli G, Yentur SP, Akman-Demir G, Isik N, Serdaroglu P. Cytokines and chemokines in neuro-Behcet’s disease compared with multiple sclerosis and other neurological diseases. J Neuroimmunol. 2003;145:127–134. doi: 10.1016/j.jneuroim.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Sharer LR. Pathology of HIV-1 infection of the central nervous system. A review. J Neuropathol Exp Neurol. 1992;51:3–11. doi: 10.1097/00005072-199201000-00002. [DOI] [PubMed] [Google Scholar]
- Simpson J, Rezaie P, Newcombe J, Cuzner ML, Male D, Woodroofe MN. Expression of the beta-chemokine receptors CCR2, CCR3 and CCR5 in multiple sclerosis central nervous system tissue. J Neuroimmunol. 2000;108:192–200. doi: 10.1016/s0165-5728(00)00274-5. [DOI] [PubMed] [Google Scholar]
- Smeal T, Angel P, Meek J, Karin M. Different requirements for formation of Jun:Jun and Jun:Fos complexes. Genes Dev. 1989;3:2091–2100. doi: 10.1101/gad.3.12b.2091. [DOI] [PubMed] [Google Scholar]
- Sopper S, Demuth M, Stahl-Hennig C, Hunsmann G, Plesker R, Coulibaly C, Czub S, Ceska M, Koutsilieri E, Riederer P, Brinkmann R, Katz M, ter Meulen V. The effect of simian immunodeficiency virus infection in vitro and in vivo on the cytokine production of isolated microglia and peripheral macrophages from rhesus monkey. Virology. 1996;220:320–329. doi: 10.1006/viro.1996.0320. [DOI] [PubMed] [Google Scholar]
- Stein B, Baldwin AS., Jr Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-kappa B. Mol Cell Biol. 1993;13:7191–7198. doi: 10.1128/mcb.13.11.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Tan J, Town T, Paris D, Placzek A, Parker T, Crawford F, Yu H, Humphrey J, Mullan M. Activation of microglial cells by the CD40 pathway: relevance to multiple sclerosis. J Neuroimmunol. 1999;97:77–85. doi: 10.1016/s0165-5728(99)00053-3. [DOI] [PubMed] [Google Scholar]
- Thea DM, Porat R, Nagimbi K, Baangi M, St Louis ME, Kaplan G, Dinarello CA, Keusch GT. Plasma cytokines, cytokine antagonists, and disease progression in African women infected with HIV-1. Ann Intern Med. 1996;124:757–762. doi: 10.7326/0003-4819-124-8-199604150-00009. [DOI] [PubMed] [Google Scholar]
- Tong L, Pav S, White DM, Rogers S, Crane KM, Cywin CL, Brown ML, Pargellis CA. A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nat Struct Biol. 1997;4:311–316. doi: 10.1038/nsb0497-311. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Watson K, Fan GH. Macrophage inflammatory protein 2 inhibits beta-amyloid peptide (1–42)-mediated hippocampal neuronal apoptosis through activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Mol Pharmacol. 2005;67:757–765. doi: 10.1124/mol.104.004812. [DOI] [PubMed] [Google Scholar]
- Wetzel MA, Steele AD, Henderson EE, Rogers TJ. The effect of X4 and R5 HIV-1 on C, C-C, and C-X-C chemokines during the early stages of infection in human PBMCs. Virology. 2002;292:6–15. doi: 10.1006/viro.2001.1249. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Masliah E, Morey M, Lemere C, DeTeresa R, Grafe M, Hansen L, Terry R. Neocortical damage during HIV infection. Ann Neurol. 1991;29:651–657. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- Wilkinson PC, Newman I. Identification of IL-8 as a locomotor attractant for activated human lymphocytes in mononuclear cell cultures with anti-CD3 or purified protein derivative of Mycobacterium tuberculosis. J Immunol. 1992;149:2689–2694. [PubMed] [Google Scholar]
- Xiong H, Boyle J, Winkelbauer M, Gorantla S, Zheng J, Ghorpade A, Persidsky Y, Carlson KA, Gendelman HE. Inhibition of long-term potentiation by interleukin-8: implications for human immunodeficiency virus-1-associated dementia. J Neurosci Res. 2003;71:600–607. doi: 10.1002/jnr.10503. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Matsushima K, Oppenheim JJ, Leonard EJ. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)- stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL1) J Immunol. 1987;139:788–793. [PubMed] [Google Scholar]
- Zhang GX, Baker CM, Kolson DL, Rostami AM. Chemokines and chemokine receptors in the pathogenesis of multiple sclerosis. Mult Scler. 2000;6:3–13. doi: 10.1177/135245850000600103. [DOI] [PubMed] [Google Scholar]