Summary
Differentially expressed genes in various benign and malignant salivary gland tumours were identified by use of cDNA microarrays containing 19,000 human expressed sequence tags. Samples were derived from 5 patients with pleomorphic adenoma, 4 with Warthin’s tumour, one with clear cell carcinoma, and 2 with mucoepidermoid carcinoma. Tumours were classified by using a subset of 486 genes. Benign Warthin’s tumour and pleomorphic adenoma showed very distinctive gene expression patterns. A total of 133 genes differentiated the single malignant clear cell carcinoma from non-tumour salivary glands (p < 0.01), whereas only 16 genes separated it from the highly related benign pleomorphic adenoma (p < 0.01). A total of 57 cDNAs were associated with mucoepidermoid carcinoma (p < 0.01). The results show gene expression alterations common to all tumours and unique to each benign and malignant tumour. The numerous Expressed Sequence Tags of unknown function we identified could also become useful as tumour markers and represent a set of novel tumour-associated genes.
Keywords: Parotid tumours, genetic expression, microarrays
Riassunto
Sono stati identificati con l’uso di cDNA microarrays contenenti 19.000 sequenze geniche, differenti geni espressi in tumori benigni e maligni delle ghiandole salivari. I campioni sono stati ricavati da 5 pazienti affetti da adenoma pleomorfo, 4 da tumori di Warthin, 1 con carcinoma a cellule chiare e 2 con carcinoma muco-epidermoidale. Questi tumori sono stati classificati usando un subset di 486 geni. Il tumore di Warthin e l’adenoma pleomorfo hanno mostrato un pattern di espressione genica estremamente tipico. Il campione di carcinoma a cellule chiare ha dimostrato 133 geni differenti rispetto al tessuto salivare normale (p < 0.01), solo 16 geni lo differenziano dall’adenoma pleomorfo (p < 0.01), mentre 57 geni erano associati al carcinoma muco epidermoidale (p < 0.01). Questi risultati dimostrano che esistono alterazioni dell’espressione genica comuni a tutti i tumori e altre esclusive per i tumori benigni e/o maligni. Sono stati anche identificati numerosi Espressed Sequence Tags, la cui funzionalità è ancora sconosciuta e che potrebbero essere usati come marker tumorali e rappresentare un nuovo gruppo di geni tumore associati.
Introduction
Pleomorphic adenoma is the most common benign tumour of major salivary glands. Cytogenetic analyses have recognized, in addition to a subgroup with normal karyotypes, recurrent chromosomal translocations, with breakpoints at 8q12, 3p21, and 12q13-15 1 and corresponding to PLAG1 2, β-catenin (CTNNB1) 3, and HMGIC 4 genes, respectively. The second most common benign lesion of the salivary glands is Warthin’s tumour. It consists of lymphoid stroma and oncocytic epithelium that form cystic or papillary structures. The epithelial component represents the neoplastic proliferation of salivary ducts that have been entrapped within the lymph nodes associated with the salivary gland. Mucoepidermoid carcinoma is the most common malignant, locally invasive tumour of the salivary glands, especially of the parotid gland. Some markers of tumour progression, invasiveness, and prognosis are p21(Kip1), a cyclin-dependent kinase inhibitor, the oncoproteins Bcl-2 and Bax, the tumour suppressor gene product p53, terminal deoxynucleotidyltransferase-mediated nick end labelling staining, and the cell cycle antigen Ki-67 5 6. Clear cell carcinoma is a rare form of malignant tumour occurring more often in pleomorphic adenoma. This tumour shows definite areas characteristic of pleomorphic adenoma combined with areas revealing evidence of malignancy. With the technology of cDNA microarrays, it is possible to make a comparative analysis of mRNA expression of thousands of genes in parallel 7. Several studies have already demonstrated the usefulness of this technique for identifying novel cancer-related genes and for classifying human cancer at molecular level 8–10. In this study, a cDNA microarray has been used containing 19,200 different human probes corresponding to different Unigene clusters to characterize benign and malignant salivary gland tumours.
Material and Methods
Patients and tissue specimens
Samples were obtained from surgically resected parotid tissue from 5 patients with pleomorphic adenoma, 4 with Warthin’s tumour, one with clear cell carcinoma, and two with mucoepidermoid carcinoma. Informed consent was obtained from patients to use the surgical specimens for research purposes. Part of each sample was frozen in liquid nitrogen and stored at -80°C until RNA extraction, and the remainder was used for histopathological analysis. Tumour paired control, non-tumour, tissues were obtained from a clinically unaffected site and were histologically normal.
Microarray assays
Total RNA was extracted from the biopsies using RNAzol. Ten μg of total RNA were used for each sample. cDNA was synthesized using Superscript II (Life Technologies, Inc.) and amino-allyl dUTP (Sigma). Monoreactive Cy3 and Cy5 esters (Amersham Pharmacia) were used for indirect cDNA labelling. A pool of 8 non-tumour salivary gland RNA was labelled with Cy3 and used as a control against the Cy5-labelled tumour cDNA. Human 19.2 K DNA microarrays, containing ESTs3 corresponding to at least 15,448 different Unigene clusters, were used (Ontario Cancer Institute) 3. One hundred μl of the sample and control cDNA in DIG Easy hybridization solution (Roche) were used in a sandwich hybridization of the two slides constituting the 19.2 K set at 37 °C overnight. Washing was performed 3 times for 10 min with 1-SSC, 0.1% SDS at 42 °C and 3 times for 5 min with 0.1-SSC at room temperature. Slides were dried by centrifugation for 2 min at 2,000 rpm. Hybridized slides were scanned using the GenePix 4,000A scanner (Axon Instruments, Foster City, CA, USA). Arabidopsis RNA was used as a reference for RNA labelling. Images were analysed using GENEPIX PRO 3.0 (Axon Instruments). Spots showing no signal or obvious defects were accordingly flagged by GENEPIX PRO and excluded from the analysis. Local background was subtracted from the remaining spots, and then spots with fluorescence intensity in both channels of < 1,000 were stringently flagged as absent. The background level for a typical array was of 67.5 (SD, 145.2) for the Cy5 channel and of 182.7 (SD, 305.4) for Cy3 channel. Maximum fluorescence intensity is at about 65,500 in both channels, and arrays were scanned ensuring that < 1% of the total number of spots measured on the slide had median intensities close to maximal. Ratios of net fluorescence from the Cy5-specific channel to the net fluorescence from the Cy3-specific channel were calculated for each spot, representing tumour mRNA expression related to the corresponding non-tumour salivary gland tissue. A normalization factor was estimated from ratios of median by GENEPIX PRO. Cy5:Cy3 expression ratios were then logtransformed (base 2) and normalized, for each different array, by adding the log2 of the respective normalization factor to the log2 of the ratio of medians for each spot within the array, so that the average log-transformed ratio was equal to zero. The threshold for significant RNA expression changes (3.0-fold; i.e, ~ 1.5 on the log2 scale) was established as 3 times the SD of an assay, where the same RNA sample was independently retrotranscribed and labelled with both cyanines. DNA spots present in at least 75% of the arrays (5,681 spotted cDNAs) and with expression ratios higher than the above-defined threshold, in at least one array, were selected for the clustering analysis (4,434 spotted cDNAs). To select the probes that distinguish between two sample groups (e.g, pleomorphic adenoma versus Warthin’s tumour), we developed a programme performing two statistical tests, t test and log likelihood. Log likelihood was defined as the difference between the mean of each probe in the two groups. To perform multiple t testing correction, the Benjamini and Hochberg procedure, as modified by Storey and Tibshirani 11, was applied. Briefly, it controls the pFDR, defined as the proportion of probes expected to be identified by chance (assuming probes are independent) relative to the total number of probes called significant. This procedure provides a good balance between discovery of significant spots and protection against false positives, because occurrence of the latter is held to a small proportion of the list. A balanced bootstrap analysis with 10,000 permutations was, therefore, applied to the tests to define the number of expected false positives by chance. Expression tables were analysed by using hierarchical clustering, PCA, and similarity searches tools in JExpress v.2.1.5 Variance normalization across the expression table was performed only prior to similarity search to identify probes with a similar expression pattern and was obtained by normalizing the mean and then multiplying each vector by a scalar so that its variance becomes unity.
Results
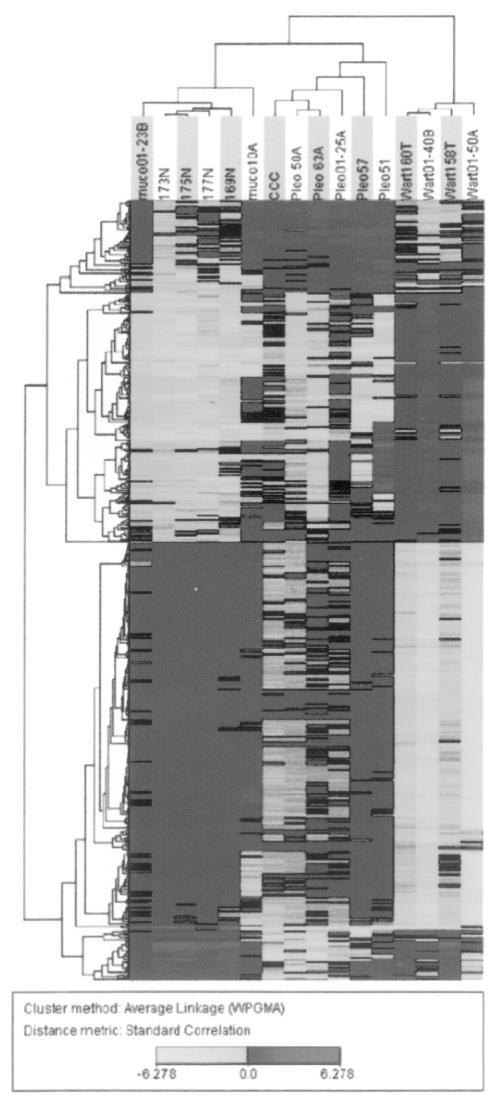
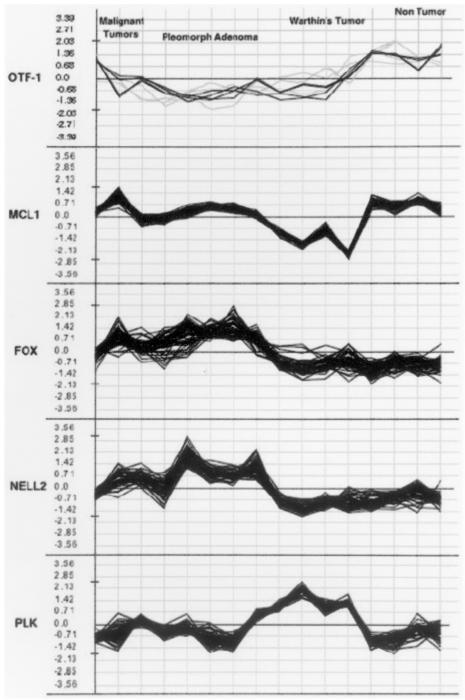
We compared the expression profiles of 12 salivary gland tumours and four normal tissue samples by hybridization to microarrays containing 19,200 cDNAs. As the arrays shared a common normal tissue reference made of a pool of 8 non-tumour salivary glands, different from those used as test samples, the samples could be compared to identify gene expression patterns correlated with clinical features of the tumours. We used hierarchical clustering 12 to look at the variation in gene expression between tumours. A clustering analysis was performed by using the selected 4,434 spotted cDNAs, as described in Materials and Methods, corresponding to 2,333 distinct Unigene clusters present in 75% of the arrays and with Cy5:Cy3 expression ratios that varied at least 3.0-fold in at least one array. Using the t test, we then attempted to identify gene expression alterations differentiating the tumours from normal samples. t test selected 713 spotted cDNA probes (p < 0.01, average t test significant spots in bootstrap, 91.82; pFDR, 0.05) corresponding to 486 distinct Unigene clusters. Clustering, based on the 486-probe set, correlated very well with the tumour histotypes, with the sole exception of the mucoepidermoid carcinoma (Fig. 1). Warthin’s tumours were the most distant branch, with the non-tumour salivary glands in a separate branch from that of the pleomorphic adenoma. The single case of cell carcinoma, developed from a pleomorphic tumour, correctly clustered within the pleomorphic adenoma group. Differentially expressed cancer-associated genes, with the most highly significant t test values 5 are outlined in Table I. Using a similarity search with Euclidean distances on the variance normalized expression table, as obtained using the corresponding JExpress plugin, we identified genes with coherent expression patterns to those of some genes selected by the t test (Fig. 2). Interestingly, among gene subsets that display expression patterns coherent with FOXO1A, there are the PAX1, homologous to the FOX fused gene described above, a novel Bcl-2 homologue (MIL1 or Bcl-rambo), and the 7b and 7c subunits of cytochrome c oxidase (COX7B, COX6C). Using semiquantitative reverse transcription, we confirmed the expression levels of OTF1, Wee1, and EMP2 (data not shown). Furthermore, we identified a number of up- and down-regulated Expressed Sequence Tags (ESTs), which suggest the involvement of a large number of genes that are not yet functionally characterized in salivary gland tumours. Defined subsets of clones that most strongly defined the division of biopsies by histotype were identified using the t test. When we applied this test to compare each tumour to non-tumour samples, we selected 599 cDNA elements differentially expressed (p < 0.01, average t test significant spots in bootstrap, 49.95; pFDR, 0.04) in pleomorphic adenoma and 2586 elements (p < 0.01, average t test significant spots in bootstrap, 5.73; pFDR, p < 0.001) specifically expressed in Warthin’s tumours. A total of 1198 clones were differentially expressed (p < 0.001, average t test significant spots in bootstrap, 0.13; pFDR, p < 0.001) in pleomorphic adenoma when compared with Warthin’s tumour, of which 479 are over-expressed in Warthin’s, whereas 719 are over-expressed in pleomorphic denoma. The specificity of clone expression is shown in Table I. Finally, we selected a subset of clones that most strongly defined the division between malignant and benign lesions. Using the log likelihood test, and as log2 threshold a value of 1.5, corresponding to 3X SD from mean, we identified 16 Unigene clusters differentiating this malignant clear cell carcinoma from its related benign pleomorphic adenoma (p < 0.01, as determined by bootstrap distribution). Because, as shown by hierarchical clustering (Fig. 1), Warthin’s tumour was very divergent from the other biopsies, we then compared the mucoepidermoid carcinoma to all the other samples after the exclusion of Warthin’s tumour samples. Using the t test, we selected 57 cDNA probes (p < 0.01). Among the relevant genes repressed in mucoepidermoid carcinoma, there is the PSG11 member of the carcinoembryonic antigen family and two genes involved in apoptosis, CIDEB and ADORA1. CIDEB or cell death-inducing DFFAlike effector b, is involved in induction of apoptosis by DNA damage 19, and ADORA1, adenosine A1 receptor, is involved in induction of apoptosis by extracellular signals. Interestingly, these genes are repressed only in mucoepidermoid carcinoma. FLJ10355, which is over-expressed in the mucoepidermoid carcinomas, is also over-expressed in the other malignant tumour clear cell carcinoma and not expressed in the benign tumours. FLJ10355 has some homology to Bcl9, a gene translocated in some B-cell malignancies with abnormalities of 1q21 18 and with no recognizable protein motifs or significant homologies to any other known proteins.
These data have been partially already published on ref. 20.
Discussion and conclusions
The list of up- and down-regulated genes in most salivary gland tumours include known genes involved in neoplasia, members of signal transduction pathways, transcription factors, oncogenes, and tumour suppressor genes, cell cycle regulator, and genes that encode protein involved in cellular metabolism and adhesion. Forkhead box O1A (FOXO1A) and PAX1 are present among up-regulated transcription factors. Fusion of forkhead box O1A with PAX3, a PAX1-related gene, is associated with solid tumour rhabdomyosarcoma. Over-expression of the fusion protein activates the transcription of anti-apoptotic protein Bcl-XL 12, capable of suppressing cell death pathways by blocking the release of cytochrome c from mitochondria and the caspase protease cascade. Cytochrome c oxidase down-regulation, alongside cytochrome c release to the cytosol, was found to be present in Bax-induced growth arrest 13. Over-representation of this gene subset suggests that the activation of mechanisms that block apoptosis is a major characteristic of tumour progression in salivary glands. One of the very few genes consistently different in every tumour is OTF1, highly expressed in non-tumour salivary glands and under-expressed in all tumours. OTF1 protein binds specifically its POU domain to octamer DNA motifs present in the promoters of several genes and regulates their expression. In addition, OTF1 participates in the cellular response to DNA damage and is involved in the regulation of stress-inducible genes 14. The identification of a number of up- and down-regulated ESTs suggests the involvement of a large number of genes that remain to be functionally characterized in salivary gland tumours. The many clones associated with Warthin’s tumour are probably related to the presence of lymphoid stroma in the affected glands, because some of the selected genes are T-cell specific (i.e, NFATC3 and ITGAE, an integrin preferentially expressed on intraepithelial lymphocytes). Plag1, a gene that is often rearranged in pleomorphic tumour 19, surprisingly, is significantly over-expressed in Warthin’s tumour (p < 0.01). In the Plag1 expression pattern group (Fig. 2), other genes present are: PLK1, progranulin, the elevated expression of which confers a transformed phenotype on epithelial cells, including anchorage independence in vitro and growth as tumours in nude mice 15; hypothetical protein FLJ13605 containing a tetra-tricopeptide repeat TPR domain, which forms scaffolds to mediate protein-protein interactions and often the assembly of multi-protein complexes and has a spatial arrangement of α-helices similar to those within 14-3-3 proteins; αL subunit of integrin leukocyte function antigen-1, a membrane glycoprotein that functions in cell-cell adhesion by heterophilic interaction with intercellular adhesion molecule 1; and polo-like serine/threonine kinase PLK1 and TXK, a member of the Tec nonreceptor subfamily of Src protein tyrosine kinases. We identified only 16 Unigene clusters differentiating the probe of malignant clear cell carcinoma from its related benign pleomorphic adenoma. On the contrary, this clear cell carcinoma differed from the Warthin’s tumours on account of a much larger number of cDNA elements (1,315), as expected for these two non-related tumours, whereas 133 elements were different from the non-tumour reference pool. Genes differentiating this clear cell carcinoma from its related benign tumour pleomorphic adenoma include myelin transcription factor 1-like, huntingtin, ubiquitin specific protease 9, and FLJ10355 5.
In summary, the results show gene expression alterations common to all tumours and unique to each benign and malignant tumour. A larger epidemiological study is necessary to determine whether expression levels of the selected genes can be used as prognostic indicators or whether additional genes need to be included in the analysis. Finally, the numerous ESTs of unknown function we identified could also become useful as tumour markers and represent a set of novel tumour-associated genes.
Fig. 1.
Clustering of 713 spotted cDNA probes corresponding to 486 Unigene clusters, selected by t test, that were differentially expressed in salivary gland tumours (p < 0.01, mean t test significant spots in bootstrap, 80.4; pFDR, 0.04). Rows, cDNA probes; columns, individual patient samples. Dark grey: probe over–expression; light grey: under–expression.
Fig. 2.
Clones with expression patterns coherent to prominent tumour–associated genes, selected by using similarity search with Euclidean distances on variance normalized expression patterns.
Table I.
Differentially expressed genes in salivary gland tumours.
After t test selection, as explained in Result and Discussion, the gene graphs were individually confirmed by using JExpress. In this table, a selection of genes with the most highly significant t test values is reported. The complete tables can be found at the web site.
| Clone | Gene bank | Name | Sample groupsa | t test |
| Genes up–regulated in salivary gland tumours | ||||
| 279762 | N48348 | CEBPD | pleo vs. wart | 14.86 |
| 297460 | W03635 | RER1 | pleo vs. wart | 14.4 |
| 298792 | W05091 | KIAAO105 | pleo vs. wart | 12.72 |
| 259563 | N41755 | MYO7A | pleo vs. nt | 11.96 |
| 320698 | W32039 | DJ37E16.5 | pleo vs. nt | 11.41 |
| 309842 | N94626 | SNRPD2 | pleo vs. wart | 11.15 |
| 280943 | N47576 | TSLP | pleo vs. wart | 10.71 |
| 469566 | AA027104 | SLC3A1 | pleo vs. wart | 10.53 |
| 281588 | N47992 | MTP | pleo vs. wart | 10.42 |
| 114869 | T87398 | C5 | pleo vs. wart | 10.37 |
| 154604 | R55493 | PLVAP | pleo vs. nt | 10.2 |
| 322111 | W37664 | SLC25A20 | pleo vs. wart | 10.04 |
| 286970 | N67453 | CDKN1A | pleo vs. wart | 9.92 |
| 285638 | N66473 | HNRPA3 | pleo vs. nt | 9.82 |
| 109931 | T84450 | LOC51651 | wart vs. nt | 9.59 |
| 112468 | T85888 | DKFZP564O043 | pleo vs. nt | 9.46 |
| 32194 | R17332 | KIAA0564 | pleo vs. nt | 9.07 |
| 297460 | W03635 | RER1 | pleo vs. nt | 8.89 |
| 322111 | W37664 | SLC25A20 | pleo vs. nt | 8.08 |
| 114869 | T87395 | C5 | pleo vs. nt | 7.95 |
| 503523 | AA131279 | LMOD1 | pleo vs. nt | 7.69 |
| 212698 | H69644 | LOC51266 | wart vs. nt | 7.49 |
| 27679 | R13006 | GDA | wart vs. nt | 7.44 |
| 162251 | H25971 | DKFZp434G171 | wart vs. nt | 7.33 |
| 195560 | R91819 | PIK3R2 | wart vs. nt | 7.11 |
| 116851 | T93608 | STAM2 | wart vs. nt | 6.93 |
| 146355 | R79518 | MCAM | wart vs. nt | 6.9 |
| 41067 | R56098 | FREQ | wart vs. nt | 6.8 |
| 207669 | H62271 | LGP1 | wart vs. nt | 6.68 |
| 109863 | T88721 | EMP2 | pleo vs. wart | 5.74 |
| 271009 | N42817 | COX6C | pleo vs. nt | 5.72 |
| 321607 | W32908 | FOXO1A | at vs. nt | 5.6 |
| 308119 | N92360 | NFATC3 | wart vs. nt | 5.5 |
| 180257 | R84726 | ADORA1 | pleo vs. wart | 5.48 |
| 201990 | R99333 | KIAA1733 | at vs. nt | 5.23 |
| 501817 | AA127953 | HHEX | at vs. nt | 5.18 |
| 116844 | T93783 | FLJ13605 | wart vs. nt | 5.04 |
| 278649 | N66214 | NPEPPS | at vs. nt | 4.91 |
| 321269 | W52940 | MIL1 | at vs. nt | 4.75 |
| 446261 | AA203732 | CHP | at vs. nt | 4.7 |
| 358848 | W94646 | ITGAE | wart vs. nt | 4.24 |
| 109863 | T88721 | EP2 | pleo vs. nt | 4.16 |
| Genes down–regulated in salivary gland tumours | ||||
| 42553 | R61223 | CAMK2 | wart vs. nt | –12.13 |
| 242058 | H93330 | SLC9A3R1 | wart vs. nt | –11.59 |
| 152489 | R62399 | MGC11352 | wart vs. nt | –9.43 |
| 33862 | R19859 | EST | pleo vs. wart | –8.84 |
| 46743 | H10327 | RAP1B | wart vs. nt | –8.74 |
| 132026 | R24904 | PP1665 | wart vs. nt | –8.64 |
| 29961 | R14696 | FLJ12438 | wart vs. nt | –8.52 |
| 111650 | T84578 | CDC37 | wart vs. nt | –8.16 |
| 133326 | R26844 | EST | wart vs. nt | –8.13 |
| 265082 | N30528 | PPARD | wart vs. nt | –8.11 |
| 143341 | R74281 | PPP4C | wart vs. nt | –8.06 |
| 289002 | N59818 | HRIHFB2436 | wart vs. nt | –8.04 |
| 33862 | R19859 | EST | pleo vs. nt | –7.98 |
| 148698 | H12850 | EST | pleo vs. nt | –7.97 |
| 129794 | R16987 | SEC8 | wart vs. nt | –7.94 |
| 207992 | H60458 | ACOX2 | pleo vs. wart | –7.64 |
| 488555 | AA047136 | EST | pleo vs. wart | –7.56 |
| 49961 | H29294 | FLJ20364 | pleo vs. wart | –7.54 |
| Genes up–regulated in salivary gland tumours | ||||
| 36318 | R21064 | KIAAO118 | pleo vs. nt | –7.52 |
| 241224 | H91140 | FLJ179 | pleo vs. wart | –7.32 |
| 66513 | T66987 | EST | pleo vs. wart | –7.28 |
| 39241 | R51610 | EST | pleo vs. nt | –7.14 |
| 136872 | R36627 | 11–Oct | pleo vs. wart | –7.06 |
| 195560 | R91819 | PIK3R2 | pleo vs. wart | –7.05 |
| 236338 | H61357 | TP53 | pleo vs. wart | –7.01 |
| 132550 | R25899 | HNRPD | pleo vs. nt | –6.87 |
| 130426 | R21806 | EST | pleo vs. nt | –6.86 |
| 129794 | R16987 | SEC8 | pleo vs. nt | –6.65 |
| 380794 | AA054151 | OXA1L | pleo vs. nt | –6.41 |
| 146858 | R80974 | WEE1 | wart vs. nt | –6.26 |
| 178468 | H47026 | MGAT3 | pleo vs. nt | –6.21 |
| 140878 | R67235 | BAT1 | pleo vs. nt | –6.17 |
| 286050 | N64281 | EST | at vs. nt | –6.01 |
| 180257 | R84726 | ADORA1 | wart vs. nt | –5.73 |
| 150673 | H02088 | EST | at vs. nt | –5.57 |
| 36318 | R21064 | KIAAO118 | at vs. nt | –5.52 |
| 152489 | R62399 | MGC11352 | at vs. nt | –5.49 |
| 46743 | H10327 | RAP18 | at vs. nt | –5.41 |
| 178468 | H47026 | MGAT3 | at vs. nt | –5.36 |
| 380794 | AA054151 | OXA1L | at vs. nt | –5.35 |
| 375693 | AA033736 | AD037 | at vs. nt | –5.28 |
| 179383 | H50403 | FLJ20793 | at vs. nt | –5.19 |
| 134887 | R32270 | EMP2 | wart vs. nt | –4.61 |
| 147839 | R81839 | TXK | at vs. nt | –3.16 |
at: all tumours; nt: non–tumor; pleo: pleomorphic adenoma; wart: Warthin’s tumour.
References
- 1.Bullerdiek J, Wobst G, Meyer-Bolte K, Chilla R, Haubrich, J, Thode B, et al. Cytogenetic subtyping of 220 salivary gland pleomorphic adenomas: correlation to occurrence, histological subtype, and in vitro cellular behavior. Cancer Genet Cytogenet 1993;65:27-31. [DOI] [PubMed] [Google Scholar]
- 2.Astrom A, Voz M, Kas K, Roijer E, Wedell B, Mandahl N, et al. Conserved mechanism of PLAG1 activation in salivary gland tumors with and without chromosome 8q12 abnormalities: identification of SII as a new fusion partner gene. Cancer Res 1999;59:918-23. [PubMed] [Google Scholar]
- 3.Kas K, Voz M, Roijer E, Astrom A, Meyen E, Stenman G, et al. Promoter swapping between the genes for a novel zinc finger protein and B-catenin in pleiomorphic adenomas with t(3;8)(p21;q12) translocation. Nat Genet 1997;15:170-4. [DOI] [PubMed] [Google Scholar]
- 4.Schoenmakers EF, Wanschura S, Mols, R, Bullerdiek J, Van den Berghe H, Van de Ven WJ. Recurrent rearrangements in the high mobility group protein gene, HMGI-C, in benign mesenchymal tumours. Nat Genet 1995;10:436-44. [DOI] [PubMed] [Google Scholar]
- 5.Choi CS, Choi G, Jung KY, Choi JO, Chae YS. Low expression of p27(Kip1) in advanced mucoepidermoid carcinomas of head and neck. Head Neck 2001;23:292-7. [DOI] [PubMed] [Google Scholar]
- 6.Yin HF, Okada N, Takagi M. Apoptosis and apoptotic-related factors in mucoepidermoid carcinoma of the oral minor salivary glands. Pathol Int 2000;50:603-9. [DOI] [PubMed] [Google Scholar]
- 7.Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science 1999;286:531-7. [DOI] [PubMed] [Google Scholar]
- 8.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 2000;406:536-40. [DOI] [PubMed] [Google Scholar]
- 9.Kahn J, Simon R, Bittner M, Chen Y, Leighton SB, Pohida T, et al. Gene expression profiling of alveolar rhabdomyosarcoma with cDNA microarrays. Cancer Res 1998;58:5009-13. [PubMed] [Google Scholar]
- 10.Luo J, Duggan DJ, Chen Y, Sauvageot J, Ewing CM, Bittner ML, et al. Human prostate cancer and benign prostatic hyperplasia: molecular dissection by gene expression profiling. Cancer Res 2001;61:4683-8. [PubMed] [Google Scholar]
- 11.Storey JD, Tibshirani R. Estimating the positive false discovery rate under dependence, with applications to DNA microarrays. Technical report number 2001-28, 2001. Stanford, CA: Department of Statistics, Stanford University, 2001. [Google Scholar]
- 12.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 1998;95:14863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margue CM, Bernasconi M, Barr FG, Schafer BW. Transcriptional modulation of the anti-apoptotic protein BCL-XL by the paired box transcription factors PAX3 and PAX3/FKHR. Oncogene 2000;19:2921-9. [DOI] [PubMed] [Google Scholar]
- 14.Manon S, Chaudhuri B, Guerin M. Release of cytochrome c and decrease of cytochrome c oxidase in Bax-expressing yeast cells, and prevention of these effects by co-expression of Bcl-XL. FEBS Lett 1997;415:29-32. [DOI] [PubMed] [Google Scholar]
- 15.Jin S, Fan F, Fan W, Zhao H, Tong T, Blanck P, et al. Transcription factors Oct-1 and NF-YA regulate the p53-independent induction of the GADD45 following DNA damage. Oncogene 2001;20:2683-90. [DOI] [PubMed] [Google Scholar]
- 16.He Z, Bateman A. Progranulin gene expression regulates epithelial cell growth and promotes tumor growth in vivo. Cancer Res 1999;59:3222-9. [PubMed] [Google Scholar]
- 17.Chen Z, Guo K, Toh SY, Zhou Z, Li P. Mitochondria localization and dimerization are required for CIDE-B to induce apoptosis. J Biol Chem 2000;275:22619-22. [DOI] [PubMed] [Google Scholar]
- 18.Willis TG, Zalcberg IR, Coignet LJ, Wlodarska I, Stul M, Jadayel DM, et al. Molecular cloning of translocation t(1;14)(q21;q32) defines a novel gene (BCL9) at chromosome 1q21. Blood 1998;91:1873-81. [PubMed] [Google Scholar]
- 19.Voz M, Mathys J, Hensen K, Pendeville H, Van Valckenborgh I, Van Huffel C, et al. Microarray screening for target genes of the proto-oncogene PLAG1. Oncogene 2004;23:179-91. [DOI] [PubMed] [Google Scholar]
- 20.Franciosoo F, Carinci F, Tosi L, Scapoli L, Pezzetti F, Passerella E, et al. Identification of differentially expressed genes in human salivary gland tumors by DNA microarrays. Mol Cancer Ther 2002;1:533-8. [PubMed] [Google Scholar]