Summary
Sjörgen’s syndrome (SS) is an autoimmune exocrinopathy characterized by lymphocyte infiltration of salivary and lacrimal glands that leads to progressive xerostomia and xerophtalmia. One-third of patients suffer of systemic manifestations including arthritis, fever, fatigue and mucosal dryness whereas those with major salivary involvement show an increased risk to develop low-grade non-Hpdgkin lymphomas. In addition, a minority of patients show symptoms related to progressive hearing loss whose pathogenesis remains undefined. Both deposition of autoantibodies to antigens of the inner-ear structures and infiltration by autoreactive T-cells have been implicated in its pathogenesis. In this context, high levels of autoantibodies to both cardiolipin and M3 muscarinic receprtors as well as to ciliar epitopes of the cochlear cells have been recently described. Here we review recent advances on the pathodgenesis of SS with a particular focus to otolaryngological manifestations.
Keywords: Salivary glands, Sjögren’s syndrome, Autoimmunity, Non-Hodgkin’s lymphoma
Riassunto
La sindrome di Sjörgen (SS) è una esocrinopatia autoimmune prevalentemente caratterizzata da progressiva xerostomia e xeroftalmia. La malattia si manifesta in circa un terzo dei pazienti con sintomi sistemici tra cui artriti, febbre, astenia e secchezza delle mucose. Tuttavia, i pazienti con prevalente interessamento delle ghiandole salivari maggiori presentano aumentato rischio di sviluppo di malattie di tipo linfoproliferativo, tra cui il linfoma non-Hodgkin. In una minoranza di pazienti è presente coinvolgimento di tipo neurosensoriale e vasculitico delle strutture cocleari con progressiva riduzione delle capacità uditive. Dal punto di vista patogentico elevati livelli di anticorpi anticardiolipina, anti-recettori muscarinici ed anti-epitopi ciliari delle cellule cocleari potrebbero svolgere un ruolo significativo nella perdita della funzione neurosensoriale uditiva. In questa rassegna vengono rivisitati alcuni tra i meccanismi immunopatologici principali responsabili della cmparsa di manifestazioni di interesse otorinolaringologico nei pazienti con SS.
Introduction
Sjögren’s syndrome (SS) is a chronic autoimmune disease characterized by lymphocyte infiltration of both salivary and lacrimal glands resulting in xerostomia and xerophthalmia. It may also occur as a systemic disease involving kidneys, lungs, liver, vessels and lymph nodes. As a primary syndrome, SS predominantly affects women in the fourth-fifth decade of life and the initial clinical picture is characterized both by oral and ocular symptoms. Chronic fatigue, fever, non-erosive arthritis and Raynaud’s phenomenon are features of the systemic disease, which also involves extraglandular manifestations such as small-vessel vasculitis, glomerulonephritis and peripheral neuropathy. In addition, kidney failure may also be provoked by interstitial or glomerular damage. Uncommon signs include palpable purpura, normochromic-normocytic anaemia, pleuritis and lung fibrosis.
Most patients display the typical mucosal dryness. Ear lesions, leading to severe sensorineural hearing loss may be an early manifestation of SS, even if it is also a less common feature of other autoimmune disorders such as systemic lupus erythematosus (SLE) and rheumatoid arthritis. In this context, autoantibodies both to cardiolipin and M3 muscarinic receptors (mAchRs) in the sera of SS patients are suspected to play a pathogenetic role in the occurrence of both progressive hearing loss and neurological complications. Patients with major salivary lymphocytic infiltration may also develop lymphoid tumours of the parotid or submandibular glands, with progressive involvement of other lymph nodes as usually observed in non-Hodgkin’s lymphoma.
Here, a review is made of some clinical and immunopathogenetic events of SS with respect to the otolaryngological manifestations and recent approaches to the use of immunotherapy.
Otolaryngological manifestations
As already pointed out, typical findings are dryness of the mouth with progressive difficulty both in swallowing and fluent speech, oral burning sensation and recurrence of dental caries, as well as chronic periodontitis. Physical examination reveals a dry, erythematosus, sticky oral mucosa with little saliva. Impaired salivary secretion frequently leads to atrophy of the tongue mucosa and mycosis. Although the sicca syndrome prevails, at clinical presentation, a bilateral parotid swelling induced by progressive lymphocyte infiltration leads both to ductal inflammation and acinar destruction in about 50% of patients. Uncommon events include early and progressive hearing loss and symptoms related to neuropathy of the eighth cranial nerve. Approximately one-fourth patients suffers from high frequency hearing loss of cochlear origin, as detected by impedance audiometry or auditory brainstem procedures. Deposition of autoantibodies in the antigenic sites of the inner-ear structures and autoreactive T-cells has been implicated in the pathogenesis of this hearing loss. Several issues, however, remain to be elucidated including the relative predominance of B and T cells in the inner-ear structures as well as the identity of the putative inner-ear self-antigen(s).
Early SLE studies suggested that anticardiolipin antibodies exert a pathogenic role in the sudden hearing loss, albeit the molecular mechanisms involved in the progressive neuronal damage are, as yet, poorly understood. Since autoantibodies to β2-glycoprotein are responsible for thromboembolic events, slight vasculitis of the inner-ear arterial microvessels due to those antibodies may lead to the hearing loss. However, other studies suggest that autoantibodies to the ciliar epitopes of cochlear cells may be pathogenic in patients with a genetic background of increased susceptibility to autoimmunity and sudden deafness 1 2. However, studies in humans have failed to define the antigen(s) involved in the autoimmune mechanisms hypothesized, whereas animal models have provided evidence of the involvement of a 68 kDa heat shock protein, the synthesis of which is greatly enhanced in cochlear tissues. Moreover, despite its presence in other organs, type-II collagen has also been proposed as a potential molecular target on account of its ability to induce otospongiotic changes in the osseous labyrinth or atrophy of both the cochlear nerve and the stria vascularis. Furthermore, MRL/lpr mice which spontaneously develop hearing loss, secondary to a systemic lymphoproliferative disorder, show high levels of circulating autoantibodies directed to vessels of the stria vascularis, breakdown of the endothelial tight junctions and corticosteroid-responsive auditory dysfunction, whereas thickening of the capillary basement membrane, as an effect of IgM and IgG immune complex deposition has been demonstrated in autoimmune-prone NZB mice. The first compelling evidence that autoreactive T-cells may be implicated in deafness has been provided by recent studies showing that activated T cells from patients with autoimmune disorders inhibit leukocyte migration in response to stimulation in vitro by an inner-ear homogenate 3. It should also be pointed out that increased production of either gamma interferon (IFN-γ) or other inflammatory cytokines by inner-ear cells indicates that pro-inflammatory effector cells, specific for inner-ear antigens, may play a role in the development of hearing failure in SS 4. The use of an epitope mapping peptide series derived from inner-ear specific proteins will hopefully lead to identification of the candidate self-antigen(s).
SS-related lymphomas
SS patients are at risk of developing a non-Hodgkin’s lymphoma (NHL) and, therefore, regarded as a natural model of evolution from polyclonal B lymphocyte activation to oligo/monoclonal B-cell expansion, which may lead to a lymphoproliferative disease. There is a prevalence of marginal zone B-cell lymphomas, though other variants such as mucosa-associated lymphoid tissue (MALT) and monocytoid B-cell variants have been reported 5. Although controversy exists concerning the mechanisms underlying lymphoproliferation, expansion of antigen-driven activated IgM-positive B cell clones has been hypothesised, as suggested for HCV-related lymphoma-genesis. Previous reports 6 support a potential pathogenetic linkage of SS with HCV-related infections, but direct involvement of the virus in triggering the progression to lymphoma has not been clearly demonstrated. In the salivary glands, infiltrating T cells are the prevalent population 7 and contribute to tissue destruction by promoting a persistent inflammatory state. However, recent studies on patients with systemic SS associated with NHL have shown characteristic monoclonal B-cell expansion both in major salivary glands and lymph nodes prior to clinical and histological evidence of glandular enlargement 8. Furthermore, an increased intra-glandular accumulation of hypermutated memory CD27+ polyclonal B-cells has been described, suggesting that chronic stimulation of B-cells is an early molecular event that prompts the oligo/monoclonal transformation and hence lymphomagenesis 9.
Immunopathogenesis
Experimental and human studies have provided controversial data on the pathogenesis of SS on account of the heterogeneous clinical picture. The following events are variably involved: a) susceptibility to autoimmunity; b) potential lymphocyte activation by viruses; c) autoantibody production; d) acinar destruction by immunopathogenetic mechanisms.
Susceptibility to autoimmunity
Susceptibility to SS and a peculiar association with selected HLA-class II antigens have been definitely proved in the past few years 10. Haplotype HLA-DR3 is recurrent in 70% of patients and a linkage dysequilibrium between the alleles DRBI**1101/DRBI*1104and DRBI*0301/DQA1*0501 is detectable in many groups of patients. This suggests that the presence of the DQA1*0501 allele indicates an increased risk of SS irrespective of the ethnic background 11. Moreover, co-expression of HLA class I A-24 with class-II antigens is evidence of greater susceptibility, while polymorphism of the interleukin (IL)-10 promoter gene (GCC haplotype) is associated with a worse prognosis in the primary syndrome 12 13.
Potential lymphocyte activation by viruses
Several viruses, such as Epstein-Barr (EBV), hepatitis C (HCV), T-cell leukemia (HTLV)-1 and human immunodeficiency (HIV)-1, have been suspected to trigger lymphocyte activation in SS 14. EBV genome has been found both in salivary tissues and cultured acinar cells from patients with active disease, while anti-EBV antibodies are suspected to activate the immune system and perpetuate the autoimmune response. Furthermore, HCV induces a spontaneous chronic lymphocytic sialoadenitis in transgenic mice carrying the HCV envelope genes, and many HCV-RNA copies are found in the lymphatic foci of salivary glands from patients with chronic HCV infection 15. However, a direct link between HCV infection and lymphoproliferation has not been clearly elucidated in SS, though the participation of B-cells in infiltration of the salivary glands and the occurrence of cryoglobulinaemia suggest a role for HCV in activating both lymphocyte replication and development of SS 16. Other viruses are suspected to be involved in the chronic sialoadenitis observed in SS. In this context, transgenic mice bearing the tax gene of the HTLV-1 have been shown to develop an autoimmune exocrinopathy resembling human SS, with acinar cell proliferation followed by progressive lymphocyte and plasma cell infiltration.
Autoantibody production
Several autoantibodies have been related both to the extent and the severity of SS. Antibodies reacting with salivary ducts, gastric mucosa and nerve cells have been reported, though they are not essential for diagnosis. By contrast, other autoantibodies including rheumatoid factor, anti-histones, anti-centromere, anti-cytokeratin and anti-ribonucleoproteins (RNPs) are useful both for diagnostic and prognostic purposes. Anti-RNPs antibodies are detectable in 85% of patients with primary SS and bind 52 kDa, 60 kDa and 48 kDa SSA/Ro and SSB/La antigens. The occurrence of anti-Ro antibodies correlates with systemic clinical features as well as with specific alleles of HLA and T-cell receptor genes. SSA-60 and SSA-48 proteins are predominantly located in the nucleus, whereas the SSA-52 antigen accumulates primarily in the cytoplasm. Their function is not fully understood, though SSA-60 regulates the translational fate of ribosomal proteins and SSA-52 acts as a transcription factor binding the DNA. These proteins are useful in the diagnosis and management of SS, since serum titres correlate with its activity 17. Other antibody specificities include anti-cardiolipin, anti-endothelial cells and anti-neutrophil cytoplasmic antibodies that may correlate with clinical manifestations such as vasculitis, glomerulonephritis and hearing loss. Finally, anti-muscarinic acetycholine receptor autoantibodies may contribute to the failure of the parasympathetic neurotransmission that leads to a loss of secretory function by altering the water channel protein of acinar cells 18.
Immunopathogenetic mechanisms
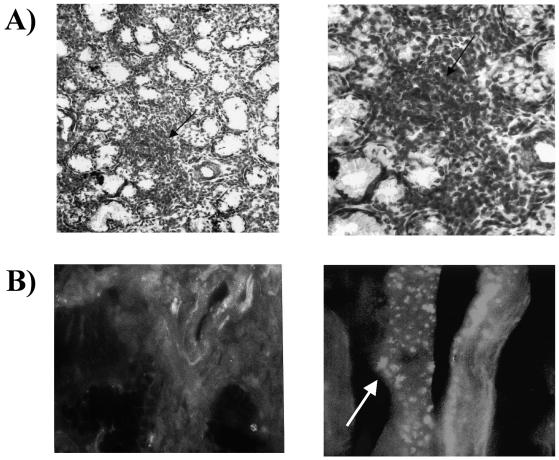
Several studies support the role both of T and B cells in the pathogenesis of glandular damage in SS. T cells contribute to the formation of lymphoid follicles within salivary glands (Fig. 1A). However, oligo/monoclonal B-cell proliferation has been identified as a parallel event which contributes to the progressive lymphocyte accumulation and propensity to proliferate within exocrine tissues. Early acinar destruction of exocrine glands is primed by activated CD45RO+/CD4+ T-cells clustered around the epithelium that induce cell cytotoxicity by either T helper-1 cytokines, such as IL-12, IL-18, tumour necrosis factor-alpha (TNF-α) and IFN-γ, or induction of apoptosis 19. In addition, an increased production of T-helper 2 cytokines (such as IL-6 and IL-10), as a result of polyclonal B-cell hyperactivity, has been described in advanced SS. Additional features include overexpression of several chemokines and adhesion molecules that allow B cells to migrate towards peripheral tissues and undergo monoclonal restriction. This mechanism may partly explain the characteristic decrease in peripheral memory CD27+/CD5+ B cells and their intraglandular accumulation in systemic SS, although the original defect leading to CD27+ cell migration towards lymphoid areas of salivary glands remains unclear 20.
Fig. 1.
A) Biopsy of minor salivary gland from SS patient. Left panel: haematoxylin/eosin staining reveals typical infiltration in form of large foci of both lymphocytes and plasma cells (arrow) adjacent to acinar and ductal structures of gland (x20). Right panel: lymphoid foci (x100). B) Apoptosis of acinar and ductal cells by immunofluorescence. Left panel: Fas expression (green fluorescence) on acinar cells of minor salivary gland from patient with active SS. Right panel: epithelial cell apoptosis is confirmed by presence of apoptotic TUNEL-positive bodies (arrow).
An accelerated apoptosis both of epithelial and glandular cells has recently emerged as an alternative mechanism responsible for the progressive exhaustion of exocrine glands in SS. Experimental models of the human disease have demonstrated that autocrine activation of the Fas/Fas-L system leads to increased apoptosis, as detected by the enhancement of nucleosome production and membrane translocation of autoantigens by apoptotic cells, resulting in progressive tissue damage and impaired secretory function. Apoptosis has been demonstrated by several methods, including the measurement of cleaved caspases and detection of TUNEL-positive cells (Fig. 1B). Apoptosis predominantly affects epithelial cells in primary SS, whereas most infiltrating T cells become resistant through overexpression of the inhibitory molecule bcl-2 21, which allows their survival and autocrine production of inflammatory cytokines (such as IFN-γ and TNF-α). Moreover, they may induce glandular damage through a cytotoxic mechanism operated by their own Fas-L.
Enhanced apoptosis, however, leads to major antigen exposure, activation of MHC class II molecules, including both ICAM-1 and VCAM-1, which lead to progressive impairment of acinar and ductal structures 22. Finally, cytotoxic T cells may also prime apoptosis by releasing proteases such as granzyme and perforin, whereas epithelial cells may upregulate the MHC class-II antigenic presentation as well as IFN-γ production and Fas/Fas-L activation, while reducing the expression of TGF-β 23 24. In conclusion, death of epithelial cells by deregulated apoptosis may be due to: a) their intrinsic activation and suicide by membrane folding through an autocrine Fas/Fas-L interaction; b) their targeting by infiltrating T cells and apoptosis by activation of their own Fas pathway; c) the release by autoreactive T cells of proteases and cytotoxic cytokines that upregulate the susceptibility of epithelial cells to apoptosis by intrinsic activation of caspases.
Therapeutic implications
Several drugs have been shown to have a beneficial effect on SS. Albeit, none induces a substantial reduction of the mononuclear cell infiltrate within the salivary glands. Drugs improving salivation such as pilocarpine and cevimeline, namely muscarinic agonists, are the treatment of choice for patients with residual functional exocrine tissue. Combined administration of corticosteroids (CS) and non-steroidal anti-inflammatory drugs are useful in the treatment of systemic complications. Local irrigation of parotid glands with CS reduces both inflammation and swelling while improving the salivary flow rate and reducing the xerostomia.
Recent trials have focused on the immunological treatment of systemic SS. The accumulation of TNF-α and IFN-γ or other inflammatory molecules within the salivary glands suggests that inhibition of TNF-α levels could reduce tissue destruction, as proved by recent in vitro studies. It has also been shown that inhibition of TNF-α by either anti-TNF-α monoclonal antibody or the soluble TNF-α receptor may induce a variable improvement in the salivary flow rate and glandular enlargement 25.
IFN-α increases saliva production and reduces inflammation, though its mechanisms of action remain to be defined. Finally, high-dose intravenous immunoglobulins have been usefully employed in the treatment of severe neurological complications, but had no effect on other systemic manifestations 26 27. Most lymphoproliferative disorders associated with SS are “low-grade lymphomas” expressing large amounts of the membrane antigen CD20. Treatment with a chimeric monoclonal antibody anti-CD20 (Rituximab) may lead to stable remission due to the inhibition of B-cell proliferation. Furthermore, a variable improvement in several symptoms, such as purpura, neuropathy and arthralgias, has been obtained in parallel with a marked decrease in circulating immunecomplexes and autoantibody levels.
References
- 1.Moysan JF, Jouquan J, Gerard C, Pennec Y, Le Goff P, Youinou P. Is the target of anti-cardiolipin antibodies the same in Gougerot-Sjögren syndrome and lupus erythematosus disseminatus? Rev Med Intern 1987;8:163-8. [DOI] [PubMed] [Google Scholar]
- 2.Novoselov VI, Peshenko IV, Evdokimov VJ, Nikolaev JV, Matveeva EA, Fesenko EE. Water-soluble GTP-binding protein from rat olfactory epithelium. FEBS Lett 1994;353:286-8. [DOI] [PubMed] [Google Scholar]
- 3.Sone M, Nariuchi H, Saito K, Yanagita N. A substrain of NZB mouse as an animal model of autoimmune inner ear disease. Hear Res 1995;83:26-36. [DOI] [PubMed] [Google Scholar]
- 4.Trune DR. Cochlear immunoglobulin in the C3H/lpr mouse model for autoimmune hearing loss. Otolaryngol Head Neck Surg 1997;117:504-8. [DOI] [PubMed] [Google Scholar]
- 5.Voulgarelis M, Dafni UG, Isenberg DA, Moutsopoulos HM. Malignant lymphoma in primary Sjögren’s syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjögren’s Syndrome. Arthritis Rheum 1999;42:1765-72. [DOI] [PubMed] [Google Scholar]
- 6.De Re V, De Vita S, Gasparotto D, Marzotto A, Carbone A, Ferraccioli G, et al. Salivary gland B cell lymphoproliferative disorders in Sjögren’s syndrome present a restricted use of antigen receptor gene segments similar to those used by hepatitis C virus-associated non-Hodgkins’s lymphomas. Eur J Immunol 2002;32:903-10. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjögren’s syndrome. Arthritis Rheum 2002;46:2730-41. [DOI] [PubMed] [Google Scholar]
- 8.Gellrich S, Rutz S, Borkowski A, Golembowski S, Gromnica-Ihle E, Sterry W, et al. Analysis of V(H)-D-J(H) gene transcripts in B cells infiltrating the salivary glands and lymph node tissues of patients with Sjögren’s syndrome. Arthritis Rheum 1999;42:240-7. [DOI] [PubMed] [Google Scholar]
- 9.Dong HY, Shahsafaei A, Dorfman DM. CD148 and CD27 are expressed in B cell lymphomas derived from both memory and naive B cells. Leuk Lymphoma 2002;43:1855-8. [DOI] [PubMed] [Google Scholar]
- 10.Arnett FC, Bias WB, Reveille JD. Genetic studies in Sjögren’s syndrome and systemic lupus erythematosus. J Autoimmun 1989;2:403-13. [DOI] [PubMed] [Google Scholar]
- 11.Reveille JD, Macleod MJ, Whittington K, Arnett FC. Specific amino acid residues in the second hypervariable region of HLA-DQA1 and DQB1 chain genes promote the Ro (SS-A)/La (SS-B) autoantibody responses. J Immunol 1991;146:3871-6. [PubMed] [Google Scholar]
- 12.Loiseau P, Lepage V, Djelal F, Busson M, Tamouza R, Raffoux C, et al. HLA class I and class II are both associated with the genetic predisposition to primary Sjögren syndrome. Hum Immunol 2001;62:725-31. [DOI] [PubMed] [Google Scholar]
- 13.Font J, Garcia-Carrasco M, Ramos-Casals M, Aldea AI, Cervera R, Ingelmo M, et al. The role of interleukin-10 promoter polymorphisms in the clinical expression of primary Sjögren’s syndrome. Rheumatology (Oxford) 2002;41:1025-30. [DOI] [PubMed] [Google Scholar]
- 14.James JA, Harley JB, Scofield RH. Role of viruses in systemic lupus erythematosus and Sjögren syndrome. Curr Opin Rheumatol 2001;13:370-6. [DOI] [PubMed] [Google Scholar]
- 15.Hansen A, Feist E, Hiepe F, Burmester GR, Scholze J. Diffuse infiltrative lymphocytosis syndrome in a patient with anti-52-kd Ro/SSA and human immunodeficiency virus type 1. Arthritis Rheum 1999;42:578-80. [DOI] [PubMed] [Google Scholar]
- 16.Ramos-Casals M, Cervera Segura R. Sjögren syndrome and hepatitis C virus: casual or etiopathogenic relationship. Rev Clin Esp 2001;201:515-7. [DOI] [PubMed] [Google Scholar]
- 17.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beroukas D, Goodfellow R, Hiscock J, Jonsson R, Gordon TP, Waterman SA. Up-regulation of M3-muscarinic receptors in labial salivary gland acini in primary Sjögren’s syndrome. Lab Invest 2002;82:203-10. [DOI] [PubMed] [Google Scholar]
- 19.Fox PC, Brennan M, Di Sun P. Cytokine expression in human labial minor salivary gland epithelial cells in health and disease. Arch Oral Biol 1999;44(Suppl 1):S49-52. [DOI] [PubMed] [Google Scholar]
- 20.Agematsu K, Nagumo H, Oguchi Y, Nakazawa T, Fukushima K, Yasui K, et al. Generation of plasma cells from peripheral blood memory B cells: synergistic effect of interleukin-10 and CD27/CD70 interaction. Blood 1998;91:173-80. [PubMed] [Google Scholar]
- 21.Kong L, Ogawa N, Nakabayashi T, Liu GT, D’Souza E, McGuff HS, et al. Fas ligand expression in the salivary glands of patients with primary Sjögren’s syndrome. Arthritis Rheum 1997;40:87-97. [DOI] [PubMed] [Google Scholar]
- 22.Nakabayashi T, Letterio JJ, Geiser AG, Kong L, Ogawa N, Zhao W, et al. Up-regulation of cytokine mRNA, adhesion molecule proteins, and MHC class II proteins in salivary glands of TGF-beta1 knockout mice: MHC class II is a factor in the pathogenesis of TGF-beta1 knockout mice. J Immunol 1997;158:5527-35. [PubMed] [Google Scholar]
- 23.Darmon AJ, Bleackley RC. Proteases and cell-mediated cytotoxicity. Crit Rev Immunol 1998;18:255-73. [DOI] [PubMed] [Google Scholar]
- 24.Kizu Y, Sakurai H, Katagiri S, Shinozaki N, Ono M, Tsubota K, et al. Immunohistological analysis of tumour growth factor beta 1 expression in normal and inflamed salivary glands. J Clin Pathol 1996;49:728-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sankar V, Brennan MT, Kok MR, Leakan RA, Smith JA, Manny J, et al. Etanercept in Sjögren’s syndrome: a twelve-week randomized, double-blind, placebo-controlled pilot clinical trial. Arthritis Rheum 2004;50:2240-5. [DOI] [PubMed] [Google Scholar]
- 26.Taguchi Y, Takashima S, Takata M, Dougu N, Asaoka E, Inoue H. [High-dose intravenous immunoglobulin in the treatment of sensory ataxic neuropathy with Sjögren’s syndrome: a case report]. No To Shinkei 2004;56:421-4. [PubMed] [Google Scholar]
- 27.Cummins MJ, Papas A, Kammer GM, Fox PC. Treatment of primary Sjögren’s syndrome with low-dose human interferon alfa administered by the oromucosal route: combined phase III results. Arthritis Rheum 2003;49:585-93. [DOI] [PubMed] [Google Scholar]