Abstract
Intracellular transport along microtubules is often bidirectional, employing multiple plus- and minus-end directed motors. How cells regulate such transport in time and space is a fundamental but unsolved question in cell biology. A recent paper presents a new modeling approach to predict how much of transport can be understood just from our knowledge of the motors involved. The model can generate strikingly complex patterns of motion, mimicking key aspects of cargo transport in vivo. Previous studies had inferred that plus-end motors on bidirectional cargoes are usually turned off when the minus-end motors are engaged (and vice versa). In the model, such motor coordination can arise from motors competing in a tug-of-war, without help from additional regulators. This new theoretical framework should stimulate much research that will help unravel whether regulation of intracellular transport is dominated by higher-order control mechanisms or is achieved simply by tuning basic properties of the motors themselves.
One hallmark of eukaryotic cells is their intricate spatial organization. Building this complex order and adapting it to changing environments requires delivery of proteins, RNAs, and organelles to well-defined subcellular locations at specific times. Much of this active trafficking occurs along microtubule tracks, powered by molecular motors from the kinesin and dynein superfamilies (Vale, 2003). Not surprisingly, motor proteins play central roles in development and neurobiology, and when they fail to function properly, severe diseases result (Goldstein, 2001).
In the cell, these motors work together with adaptor proteins, various signaling cascades, and only dimly understood cargo-bound regulators to bring about precisely choreographed intracellular transport. Of all those components, the motors are by far the best understood. Because they can be studied in vitro by single-molecule biochemistry, their physical and chemical properties have been defined in great detail. The motor Kinesin-1, for example, walks along microtubules in a hand-over-hand fashion, moves 8 nm per ATP hydrolyzed, and goes through approximately 100 enzymatic cycles per second resulting in a mean velocity of about 800 nm∕s. The tiny force kinesin produces has been measured, and we even know how Kinesin-1 slows down when it has to walk against an opposing force (called an “applied load” in the jargon of the field).
A long-term dream of molecular biologists is to understand cellular processes based entirely on the properties and interactions of the underlying components. Motor-based transport is one of the leading candidates for realizing this dream first, because molecular motors are among the best-characterized cellular machines. However, because the other components required for intracellular transport remain quite mysterious, implementing lifelike models of intracellular transport may still be far off.
Even before good quantitative information on those other components becomes available, it is, of course, possible to ask which aspect of intracellular trafficking can be understood from the properties of the motors alone. A priori, this question might seem exceedingly bold, such as taking apart the engine from a Ferrari, measuring combustion rates, and asking if it explains the traffic pattern in Los Angeles. Yet, a recent paper (Müller et al., 2008) suggests that capturing the intricacies of intracellular transport may already be within reach. This study introduces a quantitative model of cellular cargo transport that is based predominantly on known properties of motors in vitro. The model predicts surprisingly intricate cargo behavior that mimics the complexities observed in vivo.
THE COMPLEXITIES OF INTRACELLULAR TRANSPORT
One hurdle to applying insights from single-molecule characterizations to transport in vivo is that motion in cells is probably rarely due to the action of single motors. First, individual cargoes may engage multiple copies of the same motor (Gross et al., 2007), and it has been proposed that such multiple motors allow cargoes to move with higher velocities and for longer distances than achievable by single motors (Kural et al., 2005). Second, many cargoes undergo bidirectional motion (Gross, 2004; Welte, 2004): after a short spurt (“run”) driven by a plus-end motor, such cargoes move in the opposite direction, driven by a minus-end motor, then switch back to plus-end motion, and so on. Runs in the two directions are interspersed with occasional periods of no motion (“pauses”).
At first sight, bidirectional motion appears to be a chaotic, pointless dance of cargoes constantly moving back and forth along microtubules. Yet, this stochastic process at the level of individual cargoes leads to predictable, highly controlled order at the cellular level. The distances traveled in individual runs are indeed stochastic, but they do follow predictable distributions, with characteristic average run lengths for plus- and minus-end travel. If these averages are different, individual cargoes undergo a biased random walk, but the population of all cargoes displays net transport with defined directionality and predictable speed. In vivo, these run lengths are tuned in response to extracellular or intrinsic signals, e.g., hormonal stimulation or developmental cues. Unraveling the mechanisms of run-length control is key to understanding net transport.
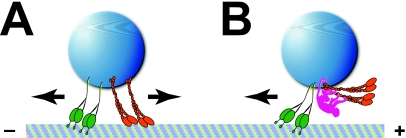
If single cargoes can have multiple copies of both plus- and minus-end motors attached to them, we need to know the rules that govern which of those motors are actively pulling the cargo at any particular moment. A cargo pulled simultaneously by two plus- and two minus-end motors [Fig. 1A] will move very differently from one in which those plus-end motors are not engaged [Fig. 1B]. In the case of motor competition [tug-of-war, Fig. 1A], the motors will likely interfere with each other. If these opposing motors are of similar strength [as has been seen in vivo, (Welte et al., 1998)] and engage and disengage with microtubules completely independently of each other, then cargoes with multiple copies of both motors might be expected to be stuck motionless on their tracks most of the time. In vivo, in contrast, periods of no motion are typically rare, and there is good evidence that during long runs in one direction there is little, if any, competition from the opposite-polarity motors (Gross et al., 2002; Kural et al., 2005).
Figure 1. Tug-of-war versus coordination.
Cargoes (blue) carrying two minus-end (green) and two plus-end (orange) motors. (A) Tug-of-war: All four motors are engaged on the microtubule, pulling the cargo into opposite directions simultaneously. This motor competition allows very little overall motion. (B) Coordination: A cargo-bound regulator (pink) pushes the plus-end motors away from the tracks, effectively turning them off. As a result, the minus-end motors can freely move the cargo.
Based on biophysical analysis of lipid-droplet motion in Drosophila and its response to mutations in motor subunits, we concluded previously that during bidirectional transport in vivo a tug-of-war is usually avoided (Gross et al., 2002). We therefore proposed that opposite-polarity motors are not indiscriminately active and that mechanisms exist to coordinate them, i.e., to turn one set of motors off when the motors for the other direction of motion are active (Gross et al., 2002). Motor coordination was later also invoked by others to explain apparently unhindered motion of other cellular organelles (Kural et al., 2005). The mechanisms responsible for this postulated motor coordination have remained mysterious. Speculations include steric interference and reversible posttranslational modifications (Welte, 2004). In the former case, coordination factors might prevent one set of motors from contacting the tracks [as whimsically depicted in Fig. 1B]; in the latter, modified motors may have a low microtubule-binding affinity.
MODELING MULTIMOTOR CARGO TRANSPORT
Given the complexities introduced by multiple motors, it is not at all clear which in vivo aspects of intracellular transport can be understood from the properties of the individual motors. The paper by Müller and colleagues (Müller et al., 2008) developed a quantitative model of cargoes with multiple motors engaging and disengaging stochastically, predominantly using known properties of microtubule motors and invoking no additional levels of regulation.
Despite the rather basic assumptions, the predicted cargo motion is anything but simple. For certain combinations of parameter values, the model predicts complex cargo behavior that mimics many crucial aspects of in vivo motion. The authors even succeed in identifying parameter values in biologically reasonable ranges to quantitatively explain the published characterization of one particularly well-studied cargo, lipid droplets in Drosophila embryos. One especially interesting prediction is that the orderly, apparently coordinated behavior of motors on cargoes may emerge from stochastic properties of the individual motors. Thus, just as tightly controlled net transport arises from biased random bidirectional motion, long runs characterized by cooperating motors could possibly emerge from random (but biased) binding and detaching of motors to and from their tracks.
KEY ASSUMPTIONS OF THE MODEL
The new model is an extension of a previous description of how multiple kinesin motors function together (Klumpp and Lipowsky, 2005). One of the key conclusions of this previous work was that the ensemble function of multiple kinesin motors working together was relatively sensitive to a force opposing their forward motion. For instance, if the critical force where a single kinesin is unable to advance is 5.7 pN, the theory predicts that the similar critical forces for two, three, and five motors are 8.8, 10.6, and 13.8 pN, respectively (Klumpp and Lipowsky, 2005)—much less than multiples of the single-motor critical force. As we will see, this sensitivity to the opposing load allows interesting dynamics of multiple interacting motors.
The new model assumes that cargoes have a fixed number of plus- (N+) and minus-end (N−) motors stably attached to them. As each of these motors may or may not be bound to the microtubule, there are a total of (N++1)⋅(N−+1) possible combinations (“states”).
For each state, we need two types of information. First, how does the cargo move in this state? For example, inFig. 2A, two minus-end and three plus-end motors compete on the same cargo. Does that result in an entirely stalled cargo or are some of these motors forced to walk backward under the load applied by the opposing motors? Second, what is the probability for transitions from this state to others? For instance, given the sensitivity to load discussed above, perhaps one set of motors will detach almost immediately, which would be reflected in a high probability of leaving the state. If initially the system was as shown in Fig. 2A, it would have some probability per unit time of a minus-end motor detaching, and thus transitioning to the situation shown in Fig. 2B. If two minus-end motors detached, it could also transition to the situation diagrammed in Fig. 2C. How frequently does each transition occur? These transitions are computed in the model based on the rates with which the motors bind to and detach from the microtubules. As in the previous model, the detachment rates increase drastically when the motors function under load, and thus in principle the engagement of multiple opposing motors can in some cases induce the rapid detachment of the initially engaged motors.
Figure 2. Competition breeds coordination.
Possible scenario for how a tug-of-war might resolve into cooperative motion. (A) Initially, two minus-end and three plus-end motors compete against each other, resulting in little overall motion. The load applied by the opposing motors increases the probability for motors to detach from the microtubule. (B) Once one minus-end motor has detached, the load on the remaining minus-end motor increases drastically, (C) inducing its detachment. As a result, only plus-end motors remain active.
Overall, the current model uses six parameters to describe each motor. Two of these characterize the force a motor can produce (Fs) and the force that induces its detachment from the tracks (Fd); the ratio of these turns out to be very important. In addition, the model uses the rates of binding to and unbinding from the microtubule (at no applied load) and the maximal velocities during (unconstrained) forward and (forced) backward motion. As these parameters can in principle be determined for any processive motor by single-molecule experiments in vitro, the model has the potential to generate quantitative predictions for any bidirectional transport for which the plus- and minus-end motors have been identified.
COORDINATION WITHOUT COORDINATORS
To assess the performance of the model, the authors systematically varied the 12 input parameters and quantified how frequently various states occur. Depending on the parameters chosen, the outcomes were qualitatively very different. In some cases, the most likely situation was motors in severe competition, such as in Fig. 1A, resulting in no or very slow motion, as expected from the naive tug-of-war idea. However, in other cases, motion was characterized by fast plus- and minus-end runs, with immediate switches in direction and negligible no-motion states. Other outcomes were also observed, such as essentially unidirectional motion with occasional pauses. Finally, the situation observed in vivo was also represented: a mix of plus- and minus-end motion interspersed with pauses.
Some of the results are not easily derived intuitively, such as how plus- and minus-end motion depend on each other. For example, when the authors varied parameters of the minus-end motors, combinations that impaired minus-end motion could have qualitatively distinct consequences for plus-end motion. In some causes, plus-end motion was enhanced, a result easily rationalized if motors are in competition. But plus-end motion could also be entirely unaffected or could even be impaired.
The most startling outcome was that under some parameter combinations the most probable states were those that have only one type of motor active, i.e., only plus-end motors or only minus-end motors. How is this possible if cargoes carry multiple motors for both directions that bind and rebind randomly? It seems that under such conditions states with only one type of motor active should not be generated very often. However, it turns out that the states with competing motors are inherently unstable.
A rationale is provided in Fig. 2. Here a cargo starts out [Fig. 2A] with two minus-end motors and three plus-end motors engaged. (For the sake of this discussion, we assume that the properties of the plus- and minus-end motors are very similar, other then that they go in different directions). Because the minus-end motors feel more than twice the applied load (here just two motors share the force of three opposing motors, i.e., each motor feels 3⋅Fc∕2) that the plus-end motors experience (three motors share the force of two opposing motors, i.e., 2⋅Fc∕3), the minus-end motors are considerably more likely to detach from their tracks. If one does, this generates the situation in Fig. 2B, which is even more lopsided. Each plus-end motor has to work against even less load (Fc∕3) and therefore becomes even less likely to detach, while the load (3⋅Fc) and detachment rate of the lone minus-end motor double, resulting in its unbinding [Fig. 2C]. Similar cascades of detachment ensure that most cases of tug-of-war get quickly resolved, such that only one motor type remains active.
This intriguing result suggests an elegant way to avoid nonproductive tug-of-war states and achieve motor coordination, i.e., situations where motors for one direction are entirely off when motors for the other direction are on. This mechanism does not rely on distinct coordinator molecules separate from the motors [e.g., the pink monkey in Fig. 1B], but represents an emergent property that arises from the activity of individual motors and their response to applied load, in particular reflecting the above-mentioned sensitivity to applied load. If such sensitivity turns out to be true, or can be achieved via accessory proteins, apparent coordination will emerge as a natural outcome of tug-of-wars that resolve very quickly.
HOW GOOD IS THE MODEL?
Müller et al. make a good case that the general pattern of intracellular transport observed in vivo might be largely explained with the properties of individual motors. This possibility does, of course, not prove that transport regulation in vivo does follow these rules. The history of biology is littered with elegant models that had to be abandoned in the light of later research. For example, some of the early proposals for how the information present in mRNA is translated into a protein sequence are truly ingenious models, simple and beautiful (Hayes, 1998); in comparison, the real translation process seems as inelegant as a Rube Goldberg machine. Those models, even if ultimately incorrect, were nevertheless a crucial stepping stone because they made clear predictions how changes in the RNA sequence would alter the protein and experiments designed to test those predictions eventually uncovered the real rules nature follows.
To determine how well their model describes actual cargo transport, Müller et al. modeled the transport of lipid droplets in Drosophila embryos. Here, a large number of quantitative data is available that characterizes cargo motion at different times of embryogenesis and in distinct genotypes (Welte et al., 1998; Gross et al., 2000; Gross et al., 2002); in particular, force measurements with optical tweezers have made it possible to estimate the number of motors active on these cargoes.
Because the single-motor parameters for the droplet motors are either unknown or not well defined, the authors varied their 12 parameters to find the combination that provides the best fits to observed droplet motion. In total, they examined five different datasets and in each instance obtained good (though not perfect) quantitative fits. They even recovered certain features of droplet motion that were not explicitly put into the model or the fitting procedure.
These good quantitative fits have two important implications. First, they demonstrate that the model can describe real-life data. Second, they identify which motor parameters need to be altered to explain observed in vivo behavior. For example, when they modeled motion in wild-type embryos versus embryos expressing mutant versions of dynein, the best fit requires that the mutant dynein unbinds from microtubules at twice the rate observed for the wild type. Such a change is in principle detectable in future in vitro experiments, and thus the model identifies critical tests that will help decide how well it captures in vivo transport.
A NEW FRAMEWORK FOR TRANSPORT STUDIES?
There are two fundamentally different perspectives how regulation of intracellular transport might come about. In one view, motors are just like the car engines mentioned in the Introduction. Although essential for the car to move, their properties are not terribly informative when it comes to understanding how a car negotiates city traffic and why it pauses at Stop signs. For that, higher levels of regulation (drivers, traffic cops, traffic lights) are more important, and we and others have proposed the existence of such regulatory mechanisms that control how and when motors are active (Welte et al., 1998; Gross et al., 2000; Welte et al., 2005). Since these regulators [e.g., the coordinator of Fig. 1B] dominate motion, they are likely the targets of the extrinsic and intrinsic signals that tune transport. Multiple signaling pathways would be integrated at the levels of these regulators to yield coherent, unified responses of the motors.
In a contrasting view, such higher-order regulators are at best ancillary, and it is the properties of the motors themselves that dominate cargo motion. In this case, signaling pathways will likely target the motors directly. Multiple pathways would converge on the motors, each altering specific motor properties. If this view is correct, it might be possible to add purified regulators to single-motor assays in vitro and determine which exact motor property they modulate. Such analyses will not require recreating bidirectional motion.
Until the work by Müller et al., it was hard to imagine that simply manipulating a few fundamental properties of motors would be sufficient to generate the complexity of cargo motion observed in vivo. Their paper establishes that regulating the properties of individual motors has the potential to make a major and possibly overwhelming contribution to the control of intracellular transport.
In the next few years, there will likely be increased dialogue between researchers studying the properties of single motors in vitro and those interested in transport in vivo. The model by Müller et al. suggests that observations in either field may be much more relevant for the other than previously suspected. In the long run, such dialogue should get us closer to the goal of explaining the inner workings of the intracellular transport machinery from the properties of its constituents, be it motors, coordinators, or other as yet unimagined regulators. Not only would that be a major intellectual breakthrough, it will also make it possible to understand disease processes at an entirely new level and lead to the molecular-level design of new medicines to correct them.
References
- Goldstein, L S (2001). “Kinesin molecular motors: transport pathways, receptors, and human disease.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.111145298 98, 6999–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, S P (2004). “Hither and yon: a review of bi-directional microtubule-based transport.” Phys. Biol. 10.1088/1478-3967/1/2/R01 1, R1–R11. [DOI] [PubMed] [Google Scholar]
- Gross, S P, Vershinin, M, and Shubeita, G T (2007). “Cargo transport: two motors are sometimes better than one.” Curr. Biol. 17, R478–486. [DOI] [PubMed] [Google Scholar]
- Gross, S, Welte, M, Block, S, and Wieschaus, E (2000). “Dynein-mediated cargo transport in vivo: a switch controls travel distance.” J. Cell Biol. 10.1083/jcb.148.5.945 148, 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, S P, Welte, M A, Block, S M, and Wieschaus, E F (2002). “Coordination of opposite-polarity microtubule motors.” J. Cell Biol. 10.1083/jcb.200109047 156, 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, B (1998). “The invention of the genetic code.” Am. Sci. 86, 8. [Google Scholar]
- Klumpp, S, and Lipowsky, R (2005). “Cooperative cargo transport by several molecular motors.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0507363102 102, 17284–17289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kural, C, Kim, H, Syed, S, Goshima, G, Gelfand, V I, and Selvin, P R (2005). “Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement?” Science 10.1126/science.1108408 308, 1469–1472. [DOI] [PubMed] [Google Scholar]
- Müller, M J, Klumpp, S, and Lipowsky, R (2008). “Tug-of-war as a cooperative mechanism for bidirectional cargo transport by molecular motors.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0706825105 105, 4609–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale, R D (2003). “The molecular motor toolbox for intracellular transport.” Cell 112, 467–480. [DOI] [PubMed] [Google Scholar]
- Welte, M A (2004). “Bidirectional transport along microtubules.” Curr. Biol. 10.1016/j.cub.2004.06.045 14, R525–R537. [DOI] [PubMed] [Google Scholar]
- Welte, M A, Cermelli, S, Griner, J, Viera, A, Guo, Y, Kim, D H, Gindhart, J G, and Gross, S P (2005). “Regulation of lipid-droplet transport by the perilipin homolog LSD2.” Curr. Biol. 15, 1266–1275. [DOI] [PubMed] [Google Scholar]
- Welte, M A, Gross, S P, Postner, M, Block, S M, and Wieschaus, E F (1998). “Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics.” Cell 10.1016/S0092-8674(00)80947-2 92, 547–557. [DOI] [PubMed] [Google Scholar]