Abstract
β-catenin acts as a critical regulator of gastrointestinal homeostasis through its control of the Wnt signaling pathway, and genetic or epigenetic lesions which activate Wnt signaling are the primary feature of colon cancer. β-catenin is also a key element of mechanotranscription pathways, leading to upregulation of master developmental gene expression during Drosophila gastrulation, or regulating mammalian bone development and maintenance. Here we investigate the impact of mechanical stimulation on the initiation of colon cancer. Myc and Twist1, two oncogenes regulated through β-catenin, are expressed in response to transient compression in APC deficient (APC1638N∕+) colon tissue explants, but not in wild-type colon explants. Mechanical stimulation of APC1638N∕+ tissue leads to the phosphorylation of β-catenin at tyrosine 654, the site of interaction with E-cadherin, as well as to increased nuclear localization of β-catenin. The mechanical activation of Myc and Twist1 expression in APC1638N∕+ colon can be prevented by blocking β-catenin phosphorylation using Src kinase inhibitors. Microenvironmental signals are known to cooperate with genetic lesions to promote the nuclear β-catenin accumulation which drives colon cancer. Here we demonstrate that when APC is limiting, mechanical strain, such as that associated with intestinal transit or tumor growth, can be interpreted by cells of preneoplastic colon tissue as a signal to initiate a β-catenin dependent transcriptional program characteristic of cancer.
In early Drosophila embryos, the expression of patterning genes like twist and snail can be induced by exogenous mechanical deformation. The mechanosensitivity of Twist expression is involved in the primitive anterior gut in response to the endogenous morphogenetic movements of convergent extension at the onset of gastrulation (Farge, 2003). β-catenin is a key element of these exogenous and endogenous mechanotranscription events, by acting both as a component of a mechanosensitive adherens junction complex, and as a direct transcriptional co-activator when translocated into the nucleus (Farge, 2003). The role of β-catenin in mechanotranscription processes is also found during mammalian bone development and homeostasis, in which endogenous mechanical strains are recurrent (Hens et al., 2005; Robinson et al., 2006). β-catenin may therefore represent the critical regulatory protein of a general mechanotransduction pathway.
Inappropritate β-catenin transcriptional activity, most often due to truncating mutations in the adenomatous polyposis coli (APC) gene, is known to be the principal cause of colon cancer development (Andreu et al., 2005; Gregorieff and Clevers, 2005), yet loss of APC expressions appears to be necessary but not sufficient in itself to trigger neoplastic transformation. Environmental signals, in addition to loss of both APC alleles, were suggested to be required for both initiation of tumorigenesis (Luebeck and Moolgavkar, 2002) and for tumor progression, based on the multiple thresholds of β-catenin activity required to initiate transcription of different sets of target genes, with secondary waves of nuclear β-catenin being microenvironmentally triggered (Brabletz et al., 2005; Fre et al., 2008). Understanding the source of these additional environmental cues which promote tumor initiation and progression will allow the development of alternative approaches to colon cancer prevention and treatment. Because the gastrointestinal tract is naturally submitted to significant endogenous mechanical strains (Basson, 2003), here we address the participation of mechanical cues in the initiation of colon cancer. We tested the ability of colon explants from normal or APC deficient mice to respond to mechanical strain by analyzing changes in the distribution of β-catenin and expression of its target genes.
RESULTS
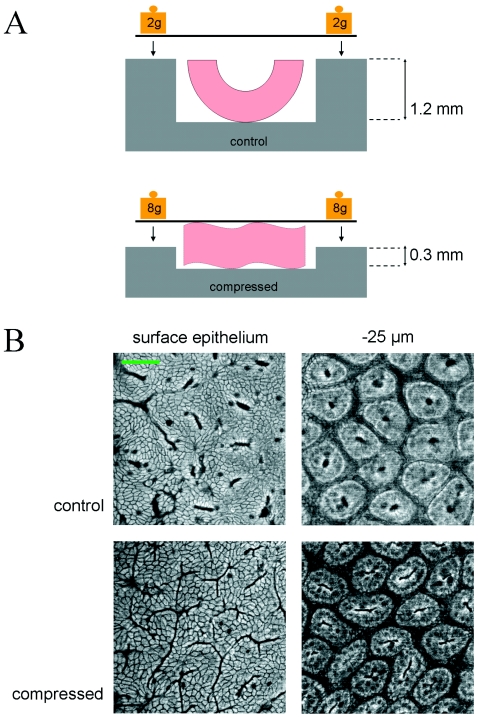
To apply mechanical strains, short segments of colon tissue were opened lengthwise and placed into the mechanical deformation box, a device of 0.2–1.2 mm depth, in culture medium at 37 °C, and then compressed by the application of a weighted coverslip for 20–40 min [Figure 1A]. A tissue box with 1.2 mm depth weighted by 4 g simply pins the tissue open and was used for control experiments, in order to control for possible effects due to cutting and handling of the tissues. Compressed tissues were placed at a depth of 0.2–0.4 mm and deformed to this thickness (approximately half of their relaxed thickness) once weighted by 16 g. Deformation from the luminal face of the tissue was used in order to mimic the in vivo effects of pressure and contact due to intestinal transit.
Figure 1. Mechanical deformation of colon explants and visualization by 2PEF microscopy.
(A) Schematic of tissue compression devices. Colon explants were pinned open (control; 1.2 mm depth) or compressed (0.2–0.4 mm depth) with a weighted coverslip while maintained in medium. (B) 2PEF microscopy was used to visualize unlabeled colon explants within the mechanical deformation apparatus. The wild-type colon is pictured, showing the surface epithelium and the crypts 25 μm deep into the tissue. Scale bar: 50 μm.
The deformation of the tissue induced by compression was observed by two-photon excitation (2PEF) microscopy of the endogenous tissue fluorescence, which allowed us to visualize the explants under compression and verify their overall structural integrity [Fig. 1B]. At a depth of 25 μm below the surface epithelium, the crypt openings were almost round in uncompressed tissues (average length/width ratio 1.7; n=21 crypts), whereas tissue compressed to 0.3 mm showed elongated crypt openings (average length/width ratio 6.6; n=26 crypts), indicating shape changes at the cellular level throughout the tissue, and not restricted to the surface in contact with the coverslip.
Tissues were either fixed immediately following compression or incubated in culture medium for a further 4 h before fixing, then embedded and cryosectioned. No significant increase in number of cells expressing the apoptosis marker cleaved caspase 3 was observed in the colon tissues under these conditions (not shown), thus verifying the health of the explants after mechanical stimulation and incubation, despite the deformation of the crypt structure due to the mechanical perturbation.
Expression of Myc and Twist1 are mechanically induced in APC deficient but not wild-type colon
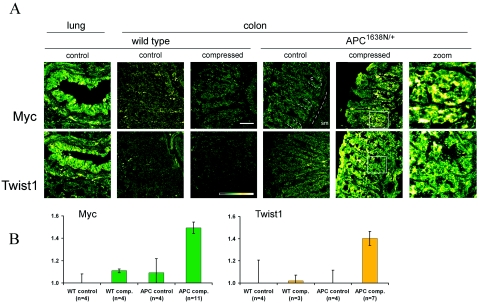
In gastrointestinal tissues, β-catenin acts as the major gatekeeper of tumorigenesis because it targets genes involved in both cell cycle regulation and invasivity. The role of mechanical cues in the induction of β-catenin dependent cell cycle regulation was thus investigated, by analyzing the expression of the Myc proto-oncogene by immunofluorescence (He et al., 1998). As seen in Fig. 2A, Myc is not significantly expressed either in normal colon crypts or after a 20 min mechanical deformation followed by 4 h incubation to allow for transcription and translation, in tissues from the wild-type mouse [lung is shown as positive control, as determined by the UniGene EST database (Wheeler et al., 2003)]. In contrast, mechanical deformation of the preneoplastic colon of APC1638N∕+ mice, which have effective loss of one APC allele (Fodde et al., 1994; Smits et al., 1999), triggered the expression of Myc in both the cytoplasm and some nuclei of epithelial crypt cells.
Figure 2. Mechanical induction of Myc and Twist1 expression in APC deficient but not wild-type colon.
(A) Immunofluorescent analysis of Myc and Twist1 expression in response to mechanical strain. No significant expression of Myc or Twist1 is seen in control or compressed wild-type colon, as compared to the lung epithelium. Strong expression of both Myc and Twist1 is induced in APC1638N∕+ colon by 20 min compression followed by 4 h incubation. Magnification of APC1638N∕+ crypts are shown to the right. Insets: Intensity gradient color scheme and 50 μm scale bar. C: crypt; SM: submucosa. (B) Quantification of immunofluorescent signal, calculated as intensity ratio of crypt epithelium to underlying submucosa (Myc) or as ratio of crypt epithelium to background (Twist1), normalized to the expression level in the wild-type uncompressed control. Myc and Twist1 were mechanically activated in APC1638N∕+ colon in 64% (Myc) and 71% (Twist1) of the compressed tissues observed, with the associated mean value of positive expression represented in the histogram. Bars show the standard error. WT: wild type; APC: APC1638N∕+.
Next, the role of mechanical cues in the expression of the β-catenin target gene Twist1 was analyzed. Twist expression is mechanosensitive in Drosophila in an Armadillo/β-catenin dependent manner (Farge, 2003), and it is a potent regulator of invasivity in mammalian breast and gastric cancers (Howe et al., 2003; Ma et al., 2007; Yang et al., 2007). As with Myc, a mechanical deformation of 20 min in APC deficient colon tissues induced the expression of Twist1 in both the cytoplasm and some nuclei of crypt cells. Wild-type tissues showed no significant expression under these conditions [Fig. 2A; lung shown as positive control (Wheeler et al., 2003)].
The immunofluorescent signal intensity representing Myc and Twist1 expression was quantified using ImageJ [Fig. 2B; see Materials and Methods]. As compared to the background in wild-type uncompressed controls, Myc and Twist1 expression in compressed APC deficient tissues showed a 50% and 40% increase, respectively. This remarkable mechanosensitive expression of both a cell cycle regulator and an invasivity regulator suggests a role for APC in preventing β-catenin dependent early tumor gene expression in response to mechanical cues.
β-catenin nuclear translocation is mechanically induced in APC deficient but not wild-type colon
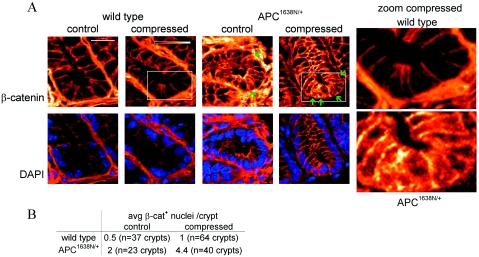
The effect of mechanical stimulation on β-catenin in the crypts was analyzed, using explants from the distal third of the colon, fixed immediately after deformation and labeled with an antibody recognizing the total pool of β-catenin. In wild-type tissues, β-catenin is strictly localized to the basolateral junctions, while in the APC deficient colon there is some additional enrichment of β-catenin in the apical cytoplasm [Fig. 3A]. Almost no nuclear β-catenin was detected in the wild-type colon epithelium, as expected (Munne et al., 1999; Iwamoto et al., 2000; Hao et al., 2001; Anderson et al., 2002; Sena et al., 2006), with an average of only 0.5 and 1 β-catenin positive nuclei visible per crypt in control and compressed tissues, respectively. Not surprisingly, nuclear β-catenin staining is relatively weak when detected by immunofluorescence without amplification and on frozen rather than paraffin embedded sections, but was consistent across experiments. The APC deficient colon showed an average of 2 β-catenin positive nuclei per crypt, and in response to mechanical strain, this increased to 4.4 [Figure 3B; sections were counterstained with DAPI to verify localization of β-catenin within the nucleus]. Thus the APC deficiency which leads to a slight constitutive accumulation of β-catenin in the cytoplasm and nuclear localization in occasional crypt cells, also permits further nuclear localization on mechanical induction.
Figure 3. Mechanical induction of nuclear β-catenin in APC deficient but not wild-type colon.
(A) β-catenin is localized to the basolateral junctions of distal colon crypts in the wild-type, both in control tissues and following 20 min compression. In APC1638N∕+ crypts, β-catenin is primarily junctional but also accumulates in the apical cytoplasm and is visible in the nucleus of an average of 2 cells per crypt (green arrows). After 20 min compression, a mean value of 4.4 cells per crypt stain positive with β-catenin in the APC deficient distal colon (green arrows). DAPI nuclear counterstain (blue) is shown below. Insets: intensity gradient color scheme and 20 μm scale bar. (B) Summary of nuclear β-catenin staining.
Mechanical induction of Y654 phosphorylated β-catenin, and of Myc and Twist1 expression, are blocked by Src kinase inhibitors
The effects of mechanical strain on β-catenin and on expression of β-catenin dependent oncogenes suggest a potential role for mechanical strains as cues participating in the initiation of the neoplastic process in the colon. The stability of β-catenin at adherens junctions is regulated by tyrosine phosphorylation: Y654-phosphorylated β-catenin has a greatly reduced affinity for E-cadherin, which can release it from the junctions (Roura et al., 1999), and also an increased affinity for TATA-binding protein in the nucleus, leading to more efficient transcriptional activation of target genes (Roura et al., 1999; Piedra et al., 2001). Y654 of β-catenin is a target of activated Src kinase in both Drosophila embryos and cultured mammalian cells (Takahashi et al., 1991; Roura et al., 1999). Src is also known to be mechanosensitive, or to be involved in mechanotransduction pathways (Wang et al., 2005; Sawada et al., 2006), and is thus a priori a candidate for involvement in the upstream events leading to the mechanical induction of transcriptionally competent β-catenin in the cytoplasm, and translocation of β-catenin to the nucleus in APC deficient tissues. With a view towards therapeutic intervention to block mechanical activation of the β-catenin pathway, we focused on a pharmacological approach targeting the Src kinase family, using the drugs PP1, PP2 and SU6656 (Bain et al., 2003). With varying specificities, these drugs inhibit the tyrosine autophosphorylation of Src family kinases, thus blocking phosphorylation of target proteins.
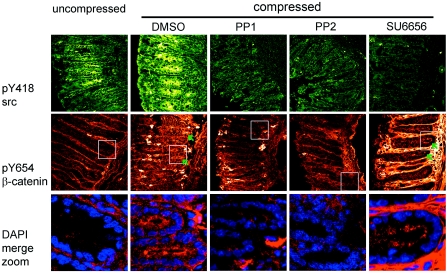
First, the phosphorylation state of tyrosine 418 of Src was followed to monitor the efficiency of the drug treatment. Activation of Src (pY418 Src) in healthy tissues is seen in restricted areas of the distal portion of both wild-type and APC deficient colon. Tissues mechanically strained for 40 min showed a significant increase in both the level and extent of pY418 Src (Fig. 4). The activation of Src in mechanically strained colon was completely blocked by adding the wide spectrum Src-family kinase inhibitors PP1 or PP2 to the medium prior to and during mechanical strain, while the vehicle alone showed no blocking effect. The chemically unrelated Src inhibitor SU6656 reduced activated Src in 67% of experiments (Fig. 4). In these experiments, pY418 Src expression was reduced from 85% of areas in untreated tissues to only 20% of areas in SU6656 treated tissues.
Figure 4. Mechanical induction ofβ-catenin tyrosine 654 phosphorylation in APC deficient colon is impaired by Src-family kinase inhibition.
Src is activated (Y418 phosphorylated) in restricted areas of uncompressed preneoplastic APC1638N∕+ distal colon, and similarly when compressed for 40 min. pY418 Src expression is lost when compressed in the presence of PP1 (n=3), PP2 (n=3) or SU6656 (n=9) Src-family kinase inhibitors. pY654 β-catenin is absent in uncompressed APC deficient colon but can be mechanically induced to accumulate in the apical cytoplasm (green arrows; mesenchymal staining with the β-catenin antibody was variable but uncorrelated with epithelial cell staining or inhibitor treatment). Treatment with PP1 or PP2 blocks mechanical induction of Y654 phosphorylation, but treatment with SU6656 does not. Merged images of pY654 β-catenin (red) and DAPI nuclear counterstain (blue) are shown magnified below. Untreated tissues shown here have been compressed in the presence of an equivalent volume of DMSO (n=2), which is used as a vehicle for the kinase inhibitors, in order to control for possible side effects. The same result is obtained without DMSO (not shown).
Next the effect of the inhibitors on β-catenin phosphorylation was tested using a phospho-tyrosine 654 specific antibody. Y654 phosphorylated β-catenin is not detected in uncompressed APC control tissues but is induced in APC deficient colon mechanically strained for 40 min, where it is weakly distributed throughout the cytoplasm and often concentrated apically. Phosphorylated β-catenin is not visible in the nuclei by immunofluorescence. Y654 phosphorylation of β-catenin was blocked by Src inhibitors PP1 and PP2, but not by SU6656 (Fig. 4).
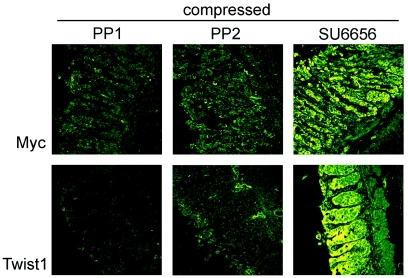
Consistent with this blockade of phosphorylated β-catenin by PP1 and PP2, mechanically induced Myc was reduced to very weak and localized expression, and Twist1 expression was entirely eliminated, by both PP1 and PP2, in samples incubated for 4 h after compression. SU6656 treatment was not able to inhibit mechanically activated gene expression, and in fact resulted in a consistently increased intensity of cytoplasmic and nuclear Myc and Twist1 expression in 100% of experiments (Fig. 5). These results together show that β-catenin can be phosphorylated on tyrosine 654 in response to mechanical stimulation, leading to transcription of Myc and Twist1, and that this process is dependent on PP1- and PP2-sensitive members of the Src kinase family.
Figure 5. Mechanical induction of Myc and Twist1 expression in APC deficient colon is impaired by Src-family kinase inhibition.
APC1638N∕+ colon compressed for 40 min in the presence of inhibitors and incubated a further 4 h shows that treatment with PP1 (n=2) and PP2 (n=2), but not SU6656 (n=4), blocks mechanical induction of Myc and Twist1. Some disorganization of the junctions is apparent on treatment with inhibitors, as well as with vehicle only.
DISCUSSION
Physical environmental cues, such as substrate nature or rigidity, have been demonstrated to be intimately linked to tissue integrity, with information about such properties of the extracellular matrix contributing to regulation of gene expression via cell shape determination, cytoskeletal dynamics, nuclear membrane interactions, and alterations in chromatin structure (Chen et al., 2003; Le Beyec et al., 2007). These environmental cues can even act dominantly to genetic parameters, as cells with oncogenic lesions can give rise to healthy tissues when placed in a normalizing tissue environment, while disruption of tissue structure can induce genetic instability, leading to tumor formation (Bissell et al., 2005; Radisky et al., 2005).
Physical cues such as macroscopic mechanical strain have been shown to be involved in cell cycle regulation in the healthy gastrointestinal tract and in colon cancer cell lines (Hirokawa et al., 1997; Hirokawa et al., 2001; Walsh et al., 2004). Increased proliferation and induction of Myc expression in an intestinal cell line has been observed in response to pressure (Hirokawa et al., 2001). Conversely, colon-derived cell culture experiments have shown downregulation of β-catenin, inhibition of proliferation, and loss of Myc expression on application of pressure (Avvisato et al., 2007). However, these experiments were carried out in a high grade adenocarcinoma cell line showing high nuclear β-catenin expression. As such, this response might represent a negative feedback signal which follows the primary mechanical activation of β-catenin. Mechanosensing is also known to act on β-catenin in endothelial cells, which show a transient reduction in junctional β-catenin and an increase in tyrosine phosphorylation of β-catenin in response to fluid shear stress, which is involved in adherens junction redistribution in response to mechanical stress (Noria et al., 1999; Ukropec et al., 2002).
Human gastrointestinal tumors occur primarily in the colon, in contrast to mice which develop tumors predominantly in the small intestine, yet the Wnt pathway represents the primary regulatory control of tissue homeostasis in both species, and its deregulation drives tumorigenesis in both organs. Because the APC deficient mouse colon remains largely healthy, unlike the intestine, the use of this model provides a morphologically normal tissue despite the constitutive mutation, thus facilitating the study of the earliest events in tumorigenesis by minimizing the probability of additional genetic lesions having already accumulated. This system therefore provides a physiologically relevant model for preclinical investigations into the initiation of human colon cancer.
The in situ experiments described here allow investigation of the role of mechanical cues in the earliest stages of tumor development in preneoplastic tissues, in a system closely representing the in vivo context. Even though mechanical stimulation likely provokes a wide range of transcriptional and posttranscriptional effects in the tissue, here we focused specifically on the β-catenin dependent aspects of the response, which are relevant to both mechanotransduction and colon cancer. Within the integrated tissue context, we find mechanical induction of expression of both a cell cycle regulator and a transcription factor involved in invasivity, in the APC deficient colon. This effect is specifically inhibited by pharmacological treatment with PP1 or PP2 to block activation of β-catenin via tyrosine 654 phosphorylation, thus preventing subsequent expression of genes involved in tumor progression.
The family of closely related Src kinases is extensive, and while SU6656 is specifically effective against Src, Fyn, Lyn, and Yes kinases (Blake et al., 2000), PP1 and PP2 have a wider range of targets (Bain et al., 2003). Thus, lack of sensitivity to SU6656 shows that activated Src itself is not necessary for β-catenin Y654 phosphorylation, but that other members of the Src family are involved. While the details of the pathway remain to be deciphered, specific pharmacological inhibition of mechanical induction of Myc and Twist1 in APC deficient tissues is nevertheless possible using the existing drugs PP1 and PP2.
The multifunctional nature of β-catenin, which depends on its conformation, binding partners, subcellular localization, and phosphorylation state at several key residues (Harris and Peifer, 2005), suggests the partitioning of β-catenin into various pools, which may be dynamically interchanged to an unknown degree. Here we have shown that tyrosine 654 phosphorylation can be mechanically induced and can lead to target gene activation, yet the dynamics of β-catenin redistribution under these conditions remain to be elucidated. Phosphorylation could be carried out at the junctional pool of β-catenin, promoting its dissociation from E-cadherin and release into the cytoplasm. The phosphorylated β-catenin could then either reach the nucleus directly, or increase the cytoplasmic concentration of β-catenin such that the existing nonphoshorylated cytoplasmic pool may be translocated into the nucleus. Alternatively, cytoplasmic β-catenin could be the phosphorylation target, thus preventing its subsequent association into junctional complexes, and favoring transcription via TATA-binding protein association once it reaches the nucleus. The observed accumulation of pY654 β-catenin in the apical cytoplasm (see Fig. 4), as opposed to the vicinity of the basolateral junctions, provides support for the latter option, while the lack of visible pY654 β-catenin in the nuclei supports the former.
The β-catenin dependent Twist mechanical induction pathway of the early Drosophila embryo was proposed to parallel that involved in mammalian tumor progression (Brouzes et al., 2004). Here we find that not only β-catenin activation and Twist1 expression, but also Myc expression, are triggered in response to mechanical stimulation in healthy (preneoplastic) APC deficient tissues. In contrast, wild-type tissues are not genetically sensitized to mechanical strains, indicating that a level of robustness is conferred by APC in the normal context, most likely via efficient degradation of any β-catenin accumulating in the cytoplasm due to the mechanical microenvironment. Interestingly, in the Drosophila embryo, β-catenin dependent mechanical induction of Twist at gastrulation may be causative in defining the future anterior gut cells, with little expression of APC at this stage of development (Hayashi et al., 1997). Therefore, mechanical cues in APC nonexpressing tissues are potentially signaling the activation of the embryonic gut cell genetic cascade, which might then be reactivated by endogenous mechanical cues in APC deficient adult gut cells, thus participating in the initiation of tumorigenesis.
By extension, one can imagine that once initiated, tumor progression may be further accelerated by mechanical signals resulting from the tumor growth itself, such as inappropriate proliferation, elevated interstitial fluid pressure, fibrosis, or loss of elasticity (Padera et al., 2004; Paszek et al., 2005). Interestingly, the 16 g weight applied to the tissue through a 2 cm2 coverslip leads to a 6 mm Hg pressure applied to the tissue. Such pressure is physiologically relevant, because it is on the order of magnitude of 10 mm Hg of pressure associated with colon transit in the rat (Tsukamoto et al., 2007). It is also softer than the pressure of 20–50 mm Hg associated with tumor interstitial fluid pressure (Boucher and Jain, 1992). Microenvironmental signals are known to be necessary contributors to the β-catenin nuclear accumulation seen at the invasive front of a tumor, as well as to the secondary wave of β-catenin nuclear accumulation which drives the epithelial-mesenchymal transition during the progression to invasivity (Brabletz et al., 2005; Fodde and Brabletz, 2007; Fre et al., 2008). Environmental factors could thus include such mechanical cues in the core of the tumor itself, or in invasive front domains which are a priori particularly exposed to mechanical strains through contact with the surrounding stroma. Furthermore, given our observations, β-catenin signaling may also become activated in the morphologically healthy APC+∕− stroma surrounding a growing APC−∕− tumor in response the internal tumor driven mechanical strain, possibly leading to a loss of adhesion surrounding the tumor, thus facilitating expansion of the tumor. This could also be true for intestinal transit strains. Any of these mechanical events could potentially act as the additional environmental cues necessary for colon cancer initiation and progression.
Indeed, chronic mechanical irritation is known to promote tumorigenesis in many tissues, as shown by the correlations between gallstones and gall bladder carcinoma (Vitetta et al., 2000), urinary calculus and urinary bladder carcinoma (Harzmann et al., 1983), ill-fitting dentures and oral squamous cell carcinoma (Young et al., 1986; Lockhart et al., 1998), and subcutaneously implanted microchips and fibrosarcoma (Tillmann et al., 1997). In addition, it has been shown in vivo that mechanical irritation of the epithelium can lead to an increased mutation rate (Takahashi et al., 2000). This suggests that cells of the APC deficient colon, which can already experience chromosomal missegregation due to dominant negative effects of truncated APC protein or insufficient full length APC during mitosis (Fodde et al., 2001; Tighe et al., 2004; Aoki et al., 2007; Draviam et al., 2006), may be exposed to an alternative route to loss of heterozygosity when subjected to mechanical stress, in addition to the effects demonstrated here on tumor gene expression in the haploinsufficient tissue.
Our results thus show that a tumorlike gene expression pattern can be initiated in preneoplastic tissues of APC deficient mice by mechanical cues, and importantly, that loss of a single APC allele can be sufficient to allow mechanical transcriptional activation of tumor promoting genes. Therefore, APC deficient individuals are potentially constitutively hypersensitive to mechanical perturbations of an endogenous nature (due to peristaltic strains or intestinal transit), which could thereby participate in tumorigenesis by increasing significantly the probability of initiation through β-catenin dependent Myc mechanosensitivity. In addition, sporadic tumors, which necessarily carry Wnt-activating mutations, may also be locally sensitive to pathological mechanical strains arising from tumor growth, which could further contribute to triggering invasion and metastasis through β-catenin dependent Twist mechanosensitivity. We have demonstrated the inherent capacity of APC deficient tissues to respond pathologically to mechanical strain, as well as the potential for pharmacological inhibition of this pathway, thus opening additional possibilities for the management and prevention of colon cancer.
MATERIALS AND METHODS
Inbred and transgenic mice
APC1638N∕+ mice are heterozygous for an APC allele truncated at amino acid 1638, resulting in little or no detectable truncated APC protein, while the wild-type allele is expressed normally. C57Bl/6 inbred mice were used as wild-type controls. Animal experiments were carried out under the authority of the institute veterinarian, under permission granted to the Robine lab N° 75-433 (Prefecture de Police—Direction des Services Veterinaires de Paris).
Mechanical deformation of tissues
The colon was dissected from adult mice, rinsed with PBS and incubated at 37 °C in HEPES-buffered DMEM:F12 medium supplemented with 10% foetal calf serum and 40 μg∕ml gentamicin. Individual tissue segments of approximately 2 cm were opened longitudinally and placed villi up in custom made tissue boxes, consisting of a disk with a central surface recessed a defined distance. The box is submerged in medium, then a 2 cm2 coverslip is placed over the tissue and weighted to compress to the tissue to the thickness of the box depth. Tissues were either fixed immediately, or maintained in culture for a further 4 h. For Src inhibition experiments, PP1 or PP2 (30 μM; BioSource) or SU6656 (20 μm; Sigma-Aldrich), or an equivalent volume of the vehicle DMSO, was added to reduced serum medium 20 min prior to the start of compression.
2PEF microscopy
The morphology of control (1.2 mm) and compressed (0.3 mm) colon tissue was assessed using two-photon excited microscopy of endogenous fluorescence. An excitation wavelength of 740 nm was chosen for efficient excitation of NADH fluorescence, and imaging was performed down to a depth of 100–120 μm. Loss of contrast and signal prevented imaging down to the base of the crypts.
Tissue fixation and immunofluorescence
Tissues were fixed in 3% paraformaldehyde, cryoprotected in 30% sucrose, then transferred to TBS freezing medium (Bios Europe), frozen in an isopentane/dry ice mixture, and cryosectioned. Labeling was carried out by standard protocols using the following antibodies: Total β-catenin (BD mouse monoclonal), pY418 src (Biosource rabbit polyclonal), Myc N262 (Santa Cruz rabbit polyclonal), Twist H81 (Santa Cruz rabbit polyclonal), pY654 β-catenin (Abcam mouse monoclonal), anti-mouse Cy3 (Jackson ImmunoResearch) and anti-rabbit Alexa 488 (Molecular Probes).
Quantification of gene expression
The immunofluorescent signal representing Myc expression was quantified using ImageJ (Rasband WS 1997–2007) by measuring the ratio of the average pixel intensity of crypt epithelial cells to that of the underlying submucosa [Fig. 2B]. For Twist1, the submucosa also showed a slight response to compression, so intensity was quantified simply in relation to the background noise. Data were averaged over three or more replicates and normalized to the basal expression level in wild-type colon.
ACKNOWLEDGMENTS
JW was supported by a French Ministry of Education & Research fellowship and a Marie Curie Intra-European fellowship. This work was supported by grants from ARC (3536), INSERM (ITS2005), HSFP (RGP0014/2006-C) and Equipe Labellisée La Ligue (EL2008.LNCC/SR1). JW carried out the experimental work and imaging, and prepared the manuscript; DJ aided in tissue preparation; CF designed the apparatus; SR provided mice; EB carried out the 2PEF microscopy; EF initiated the project. JW & EF analyzed the data, and all authors contributed to project planning and manuscript preparation. Thanks to François Waharte (Cell and Tissue Imaging Facility, UMR144 CNRS/Institut Curie) for confocal imaging assistance, to Jacques Prost and Padra Ahmadi (UMR168 CNRS/Institut Curie) for experimental advice, and to Olivier Delattre, Xavier Sastre, Daniel Louvard, and members of the Robine lab for helpful discussions.
References
- Anderson, C B, Neufeld, K L, and White, R L (2002). “Subcellular distribution of Wnt pathway proteins in normal and neoplastic colon.” Proc. Natl. Acad. Sci. U.S.A. 99(13), 8683–8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu, P, et al. (2005). “Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine.” Development 132(6), 1443–1451. [DOI] [PubMed] [Google Scholar]
- Aoki, K, et al. (2007). “Chromosomal instability by [beta]-catenin//TCF transcription in APC or [beta]-catenin mutant cells.” Oncogene , 26(24), 3511–3520. [DOI] [PubMed] [Google Scholar]
- Avvisato, C L, Yang, X, Shah, S, Hoxter, B, Li, W, Gaynor, R, Pestell, R, Tozeren, A, and Byers, S W (2007). “Mechanical force modulates global gene expression and beta-catenin signaling in colon cancer cells.” J. Cell. Sci. 10.1242/jcs.03476 120(15), 2672–2682. [DOI] [PubMed] [Google Scholar]
- Bain, J, McLauchlan, H, Elliott, M, and Cohen, P (2003). “The specificities of protein kinase inhibitors: an update.” Biochem. J. 10.1042/BJ20021535371(Pt 1), 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson, M D (2003). “Paradigms for mechanical signal transduction in the intestinal epithelium.” Digestion 68(4), 217–225. [DOI] [PubMed] [Google Scholar]
- Bissell, M J, Kenny, P A, and Radisky, D C (2005). “Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes.” Cold Spring Harbor Symposia on Quantitative Biology 70(1), 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake, R A, Broome, M A, Liu, X, Wu, J, Gishizky, M, Sun, L, and Courtneidge, S A (2000). “SU6656 a selective Src family kinase inhibitor, used to probe growth factor signaling.” Mol. Cell. Biol. 20(23), 9018–9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher, Y, and Jain, R K (1992). “Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: implications for vascular collapse.” Cancer Res. 52(18), 5110–5114. [PubMed] [Google Scholar]
- Brabletz, T, Jung, A, Spaderna, S, Hlubek, F, and Kirchner, T (2005). “Migrating cancer stem cells—an integrated concept of malignant tumour progression.” Nat. Rev. Cancer 5(9), 744–749. [DOI] [PubMed] [Google Scholar]
- Brouzes, E, Supatto, W, and Farge, E (2004). “Is mechano-sensitive expression of twist involved in mesoderm formation?” Biol. Cell 96(7), 471–477. [DOI] [PubMed] [Google Scholar]
- Chen, C S, Alonso, J L, Ostuni, E, Whitesides, G M, and Ingber, D E (2004). “Cell shape provides global control of focal adhesion assembly?” Biochem. Biophys. Res. Commun. 10.1016/S0006-291X(03)01165-3 307(2), 355–361. [DOI] [PubMed] [Google Scholar]
- Draviam, V M, Shapiro, I, Aldridge, B, and Sorger, P K (2006). “Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1-or APC-depleted cells.” EMBO J. 25(12), 2814–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farge, E (2003). “Mechanical induction of twist in the Drosophila foregut/stomodeal primordium.” Curr. Biol. 10.1016/S0960-9822(03)00576-1 13(16), 1365–1377. [DOI] [PubMed] [Google Scholar]
- Fodde, R, and Brabletz, T (2007). “Wnt/beta-catenin signaling in cancer stemness and malignant behavior.” Curr. Opin. Cell Biol. 19(2), 150–158. [DOI] [PubMed] [Google Scholar]
- Fodde, R, et al. (1994). “A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors.” Proc. Natl. Acad. Sci. U.S.A. 91(19), 8969–8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodde, R, et al. (2001). “Mutations in the APC tumour suppressor gene cause chromosomal instability.” Nat. Cell Biol. 3(4), 433–438. [DOI] [PubMed] [Google Scholar]
- Fre, S, Vignjevic, D, Schoumacher, M, Duffy, S L, Janssen, K-P, Robine, S, and Louvard, D (2008). “Epithelial morphogenesis and intestinal cancer: new insights in signaling mechanisms.” Adv. Cancer Res. 100, 85–111. [DOI] [PubMed] [Google Scholar]
- Gregorieff, A, and Clevers, H (2005). “Wnt signaling in the intestinal epithelium: from endoderm to cancer.” Genes Dev. 19(8), 877–890. [DOI] [PubMed] [Google Scholar]
- Hao, X P, Pretlow, T G, Rao, J S, and Pretlow, T P (2001). “Beta-catenin expression is altered in human colonic aberrant crypt foci.” Cancer Res. 61(22), 8085–8088. [PubMed] [Google Scholar]
- Harris, T J C, and Peifer, M (2005). “Decisions, decisions: Beta-catenin chooses between adhesion and transcription.” Trends Cell Biol. 15(5), 234–237. [DOI] [PubMed] [Google Scholar]
- Harzmann, R, Schubert, G E, Gericke, D, Altenahr, E, and Bichler, K H (1983). “Morphology of the urinary bladder following long-term experimental irritation of the urothelium.” Urol. Int. 38(3), 166–172. [DOI] [PubMed] [Google Scholar]
- Hayashi, S, Rubinfeld, B, Souza, B, Polakis, P, Wieschaus, E, and Levine, A J (1997). “A Drosophila homolog of the tumor suppressor gene adenomatous polyposis coli down-regulates beta-catenin but its zygotic expression is not essential for the regulation of armadillo.” Proc. Natl. Acad. Sci. U.S.A. 94(1), 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, T-C, Sparks, A B, Rago, C, Hermeking, H, Zawel, L, da Costa, L T, Morin, P J, Vogelstein, B, and Kinzler, K W (1998). “Identification of c-MYC as a target of the APC pathway.” Science 281(5382), 1509–1512. [DOI] [PubMed] [Google Scholar]
- Hens, J, Wilson, K, Dann, P, Chen, X, Horowitz, M, and Wysolmerski, J (2005). “TOPGAL mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro.” J. Bone Miner. Res. 20(7), 1103–1113. [DOI] [PubMed] [Google Scholar]
- Hirokawa, M, et al. (1997). “Pressure stimulates proliferation and DNA synthesis in rat intestinal epithelial cells.” Life Sci. 61(7), 667–672. [DOI] [PubMed] [Google Scholar]
- Hirokawa, M, et al. (2001). “Loading of mechanical pressure activates mitogen-activated protein kinase and early immediate gene in intestinal epithelial cells.” Dig. Dis. Sci. 46(9), 1993–2003. [DOI] [PubMed] [Google Scholar]
- Howe, L R, Watanabe, O, Leonard, J, and Brown, A M (2003). “Twist is up-regulated in response to Wnt1 and inhibits mouse mammary cell differentiation.” Cancer Res. 63(8), 1906–1913. [PubMed] [Google Scholar]
- Iwamoto, M, Ahnen, D J, Franklin, W A, and Maltzman, T H (2000). “Expression of beta-catenin and full-length APC protein in normal and neoplastic colonic tissues.” Carcinogenesis 21(11), 1935–1940. [DOI] [PubMed] [Google Scholar]
- Le Beyec, J, Xu, R, Lee, S-Y, Nelson, C M, Rizki, A, Alcaraz, J, and Bissell, M J (2007). “Cell shape regulates global histone acetylation in human mammary epithelial cells.” Exp. Cell Res. 313(14), 3066–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart, P B, Norris, C M, and Pulliam, C (1998). “Dental factors in the genesis of squamous cell carcinoma of the oral cavity.” Oral Oncol. 34(2), 133–139. [DOI] [PubMed] [Google Scholar]
- Luebeck, E G, and Moolgavkar, S H (2002). “Multistage carcinogenesis and the incidence of colorectal cancer.” Proc. Natl. Acad. Sci. U.S.A. 99(23), 15095–15100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L, Teruya-Feldstein, J, and Weinberg, R A (2007). “Tumour invasion and metastasis initiated by microRNA-10b in breast cancer.” Nature (London) 449(7163), 682–688. [DOI] [PubMed] [Google Scholar]
- Munne, A, Fabre, M, Marinoso, M L, Gallen, M, Real, F X, and for the Colon Cancer Team at IMAS (1999). “Nuclear beta-catenin in colorectal tumors: to freeze or not to freeze?” J. Histochem. Cytochem. 47(8), 1089–1094. [DOI] [PubMed] [Google Scholar]
- Noria, S, Cowan, D B, Gotlieb, A I, and Langille, B L (1999). “Transient and steady-state effects of shear stress on endothelial cell adherens junctions.” Circ. Res. 85(6), 504–514. [DOI] [PubMed] [Google Scholar]
- Padera, T, Stoll, B, Tooredman, J, Capen, D, Tomaso, Ed, and Jain, R (2004). “Cancer cells compress intratumour vessels.” Nature (London) 10.1038/427695a 427(6976), 695. [DOI] [PubMed] [Google Scholar]
- Paszek, M J, et al. (2005). “Tensional homeostasis and the malignant phenotype.” Cancer Cells 8(3), 241–254. [DOI] [PubMed] [Google Scholar]
- Piedra, J, Martinez, D, Castano, J, Miravet, S, Dunach, M, and de Herreros, A G (2001). “Regulation of beta-catenin structure and activity by tyrosine phosphorylation.” J. Biol. Chem. 276(23), 20436–20443. [DOI] [PubMed] [Google Scholar]
- Radisky, D C, et al. (2005). “Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability.” Nature (London) 436(7047), 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband, W S, (1997–2007). ImageJ http://rsb.info.nih.gov/ij/, U. S. National Institutes of Health, Bethesda, Maryland, USA.
- Robinson, J A, et al. (2006). “Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone.” J. Biol. Chem. 281(42), 31720–31728. [DOI] [PubMed] [Google Scholar]
- Roura, S, Miravet, S, Piedra, J, de Herreros, A G, and Dunach, M (1999). “Regulation of e-cadherin/catenin association by tyrosine phosphorylation.” J. Biol. Chem. 274(51), 36734–36740. [DOI] [PubMed] [Google Scholar]
- Sawada, Y, Tamada, M, Dubin-Thaler, B J, Cherniavskaya, O, Sakai, R, Tanaka, S, and Sheetz, M P (2006). “Force sensing by mechanical extension of the Src family kinase substrate p130Cas.” Cell 127(5), 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena, P, Saviano, M, Monni, S, Losi, L, Roncucci, L, Marzona, L, and Pol, A D (2006). “Subcellular localization of beta-catenin and APC proteins in colorectal preneoplastic and neoplastic lesions.” Cancer Lett. 241(2), 203–212. [DOI] [PubMed] [Google Scholar]
- Smits, R, et al. (1999). “Apc1638T: A mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development.” Genes Dev. 13(10), 1309–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, S, Ikeda, Y, Kimoto, N, Okochi, E, Cui, L, Nagao, M, Ushijima, T, and Shirai, T (2000). “Mutation induction by mechanical irritation caused by uracil-induced urolithiasis in Big Blue® rats.” Mutat Res. 447(2), 275–280. [DOI] [PubMed] [Google Scholar]
- Takahashi, Y, Bontoux, M, and Le Douarin, N M (1991). “Epithelio—mesenchymal interactions are critical for Quox 7 expression and membrane bone differentiation in the neural crest derived mandibular mesenchyme.” EMBO J. 10(9), 2387–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe, A, Johnson, V L, and Taylor, S S (2004). “Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability.” J. Cell. Sci. 117(26), 6339–6353. [DOI] [PubMed] [Google Scholar]
- Tillmann, T, et al. (1997). “Subcutaneous soft tissue tumours at the site of implanted microchips in mice.” Exp. Toxicol. Pathol. 49(3–4), 197–200. [DOI] [PubMed] [Google Scholar]
- Tsukamoto, K, Ariga, H, Mantyh, C, Pappas, T N, Yanagi, H, Yamamura, T, and Takahashi, T (2007). “Luminally released serotonin stimulates colonic motility and accelerates colonic transit in rats.” Am. J. Physiol. 293(1), R64–69. [DOI] [PubMed] [Google Scholar]
- Ukropec, J A, Hollinger, M K, and Woolkalis, M J (2002). “Regulation of VE-cadherin linkage to the cytoskeleton in endothelial cells exposed to fluid shear stress.” Exp. Cell Res. 273(2), 240–247. [DOI] [PubMed] [Google Scholar]
- Vitetta, L, Sali, A, Little, P, and Mrazek, L (2000). “Gallstones and gall bladder carcinoma.” ANZ J. Surg. 70(9), 667–673. [DOI] [PubMed] [Google Scholar]
- Walsh, M F, Woo, R K, Gomez, R, and Basson, M D (2004). “Extracellular pressure stimulates colon cancer cell proliferation via a mechanism requiring PKC and tyrosine kinase signals.” Cell Prolif 37(6), 427–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y, Botvinick, E L, Zhao, Y, Berns, M W, Usami, S, Tsien, R Y, and Chien, S (2005). “Visualizing the mechanical activation of Src.” Nature (London) 10.1038/nature03469 434(7036), 1040–1045. [DOI] [PubMed] [Google Scholar]
- Wheeler, D L, et al. (2003). “Database resources of the National Center for Biotechnology.” Nucleic Acids Res. 31(1), 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z, et al. (2007). “Up-regulation of gastric cancer cell invasion by Twist is accompanied by N-cadherin and fibronectin expression.” Biochem. Biophys. Res. Commun. 358(3), 925–930. [DOI] [PubMed] [Google Scholar]
- Young, T B, Ford, C N, and Brandenburg, J H (1986). “An epidemiologic study of oral cancer in a statewide network.” Am. J. Otolaryngol. 7(3), 200–208. [DOI] [PubMed] [Google Scholar]