Summary
Myoepithelioma is a rare neoplasm of the salivary glands, generally occurring in the parotid gland and less often in the minor accessory salivary gland of the oral cavity. The histological appearance includes solid, myxoid and reticular growth patterns. Vimentin and S-100 protein are very sensitive but non-specific immunohistochemical markers of neoplastic myoepithelium. Conservative surgery is the treatment of choice. A case of myoepithelioma of the minor salivary gland of the cheek with low grade malignancy is described, focusing on clinical behaviour, histopathological and immunohistochemical features and differential diagnosis.
Keywords: Salivary gland tumour, Minor salivary gland, Myoepithelioma, Diagnosis
Riassunto
Il mioepitelioma è un raro tumore delle ghiandole salivari che coinvolge generalmente la ghiandola parotide e meno frequentemente le ghiandole salivari minori della cavità orale. Da un punto di vista istologico si distinguono tre diversi pattern di crescita: solido, mixoide e reticolare. Da un punto di vista immunoistochimico la vimentina e la proteina S-100 rappresentano dei markers molto sensibili ma non specifici di neoplasia mioepiteliale. La chirurgia conservativa radicale è il trattamento di scelta. Viene discusso un caso di mioepitelioma di ghiandola salivare minore della guancia a basso grado di malignità, enfatizzando il comportamento clinico, le caratteristiche istopatologiche e immunoistochimiche e la diagnosi differenziale.
Introduction
Myoepithelioma is defined as a generally benign tumour composed of myoepithelial cells. These cells, which are found in many organs (mainly the salivary glands), are major components of various salivary gland tumours. Morphologic presentation may vary considerably 1 . Myoepithelioma is rare accounting for less than 1% of all salivary gland tumours 2 –6 . The growth patterns may be solid, myxoid or reticular, and the component cells may be spindle-shaped, plasmacytoid, hyaline, clear or epithelioid 7 . When benign, the tumour usually appears as an asymptomatic mass that slowly increases in size over a period of several months or years 8 .
A case of myoepithelioma of a minor salivary gland of the cheek is described, focusing on the clinical and cytohistological findings and emphasizing the problems of the differential diagnosis.
Case report
In January 2004, an 81-year-old female came to our Otorhinolaryngology Unit with a 2-year history of a non-painful submucosa mass in the right cheek that had recently increased in size.
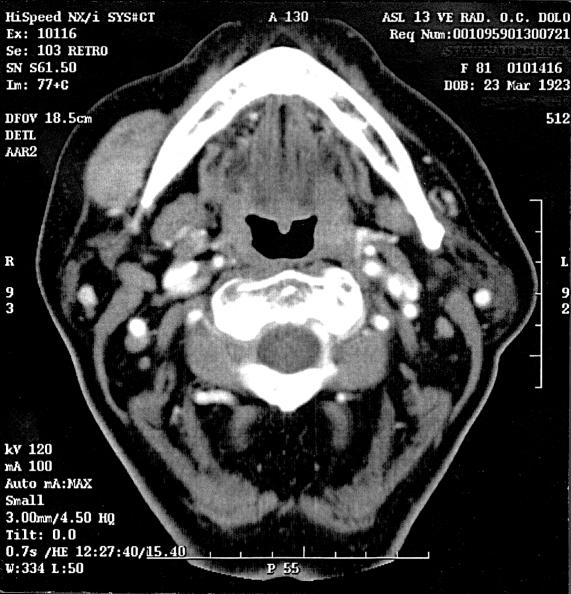
The physical examination of the head and neck showed a mass in the oral portion of the right cheek, laterally to the horizontal tract of the jaw, similar to an apricot, mobile to bimanual palpation, non-painful, covered with normal mucosa. Regional lymphadenopathy was not present and no other lesions of the head and neck were observed. Neurologic evaluation of the sensory and motor function of the oral and maxillofacial region was within normal limits. Axial contrast-enhanced computed tomography (CT) scan demonstrated a solid, well-circumscribed mass (2 x 3.5 cm in size) in the right cheek, laterally to the horizontal tract of the jaw, with homogeneous enhancement; the lesion displaced, but did not infiltrate, the superficial veins and did not appear to involve the bone (Fig. 1).
Fig. 1.
Axial contrast-enhanced CT scan demonstrated a solid, well-circumscribed mass (2x3.5 cm) in right cheek, laterally to horizontal tract of jaw, with homogeneous enhancement; the lesion displaced, but did not infiltrate, the superficial veins and did not appear to involve the bone.
Fine needle aspiration biopsy (FNAB) was performed, but a non-specific diagnosis of tumour of the minor salivary gland was made. The patient underwent a wide local tumour-resection via a transoral approach under general anaesthesia. The final histological diagnosis was myoepithelioma, with low grade malignancy, in an accessory minor salivary gland of the cheek.
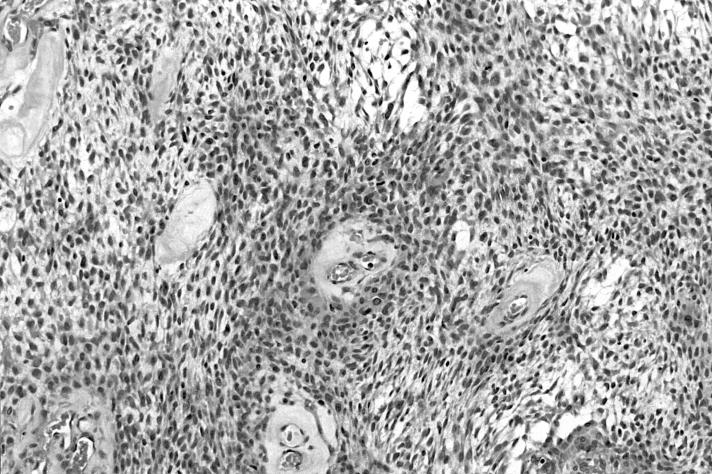
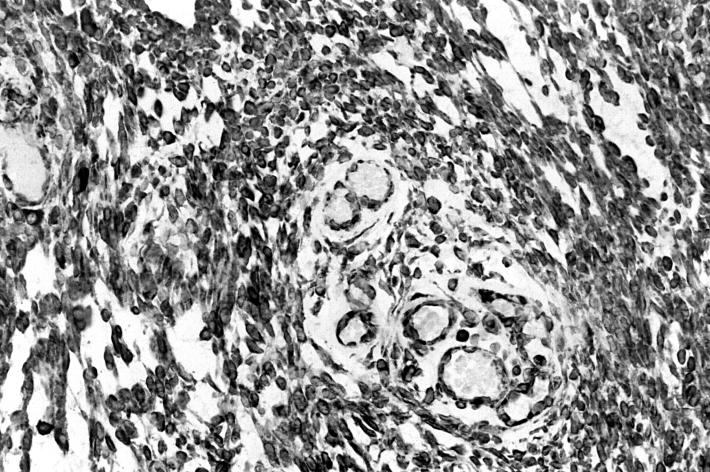
The surgical specimen showed a well-circumscribed, capsulated mass measuring 3.5 x 3 x 2.5 cm; the cut surface appeared solid, homogeneous and white in colour, with occasional pseudocystic areas. Microscopic examination revealed the presence of the small and/or medium spindle-shaped cells, differently interlaced, with eosinophilic cytoplasm, occurring in sheets or swirls; the nuclei were predominantly from round to ovoid in shape, often eccentric, with finely dispersed chromatin and low mitotic activity. The proliferative index (Ki67) was 1%. The stroma was myxomatous with focal pseudocystic areas (Fig. 2). Immunohistochemically, the tumour cells were strongly positive for vimentin and focally for S100 and CD100 protein but were negative for cytokeratins, alpha smooth muscle actin, MNF116, HMB-45 and GFAP (Fig. 3).
Fig. 2.
Myoepithelioma of minor salivary gland of cheek: tumour is composed of small and/or medium spindle-shaped cells, differently interlaced, with eosinophilic cytoplasm, that occur in sheets or swirls and have round to oval nuclei with finely dispersed chromatin. Stroma is myxomatous (haematoxylin and eosin, [H&E], x16).
Fig. 3.
Immunohistochemical study (25x). Myoepithelial cells stain strongly positive for vimentin and focally for S100 and CD100 protein.
At 12-month follow-up, without additional treatment, no evidence of local recurrence or metastases was detected.
Discussion
Myoepithelial cells are ectodermally derived contractile cells, routinely identified in many normal tissues with a secretory function such as major and minor salivary glands, lacrimal glands, sweat glands, breasts and the prostate 8 9 . These cells are one of the most frequent components of salivary gland tumours. Salivary-gland neoplasms that frequently contain myoepithelial cells are pleomorphic adenoma, adenoid-cystic carcinoma and epithelial-myoepithelial carcinoma of intercalated duct origin 10 11 . Neoplasms composed exclusively of myoepithelial cells are uncommon accounting for less than 1% of all salivary gland tumours. Most of these tumours are located in the parotid gland, while others occur in the submandibular gland or in the accessory glands of the oral cavity (hard and soft palate, lip, cheek, tongue, floor of the mouth, gingiva, retromolar area) 2 –6 12 . They sometimes arise from the glands of the respiratory tract (nasal cavity, nasopharynx, larynx, lung). Patients are generally over 50 years of age, with both sexes being equally affected 13 –17 .
The term myoepithelioma was introduced by Sheldon in 1943 18 . However, the precise criteria for inclusion of a tumour in this category still remains controversial. The complex and varied morphologic and immunophenotypic expressions of neoplastic myoepithelium have always attracted numerous investigators, with valuable, but often contradictory, data being presented 19 20 . The traditional definition included only three types of myoepithelioma: plasmacytoid, spindle and mixed cells forms 4 21 . However, with the recent recognition of the different phenotypic and ultrastructural modifications displayed by the neoplastic myoepithelial cells of salivary gland tumours, the morphologic spectrum of myoepithelioma has been expanded 20 22 . By taking into account the variety of cytoarchitectural patterns displayed by the myoepitheliomatous regions of pleomorphic adenoma, Dardick et al. have proposed broader histopathologic guidelines for myoepitheliomas and have included a few previously unrecognized variants 22 23 .
Unlike pleomorphic adenoma, these tumours lack any ductal epithelial differentiation, and present a minor stromal element. Cytogenetic and molecular genetic studies have so far investigated mainly pleomorphic adenomas and recurrent specific chromosomal alterations, at the 8q12 and 12q13-q15 regions, have been reported. The cell origin of these alterations, however, remains speculative. Cytogenetic analysis of the myoepithelioma shows 12q12 involved in a translocation with a previously unreported partner (1q), and nonrandom del (9) (q22.1q22.3) and del (13) (q12q22). The myoepithelial cell is the source of those cells with chromosomal alterations, moreover, the myoepithelioma shares 12q alterations reported in a subset of pleomorphic adenomas 24 .
Vimentin and S-100 protein are not usually present in normal myoepithelial cells and are very sensitive, but non-specific, markers of neoplastic myoepithelium. As with neoplastic transformation, the myoepithelium loses or modifies its smooth-muscle phenotype, immunohistochemical studies to demonstrate smooth-muscle differentiation in these cells have been less fruitful. In most tumours, alpha smooth muscle actin positivity has been observed, at least in a few cells, both of the spindle-shaped and plasmacytoid types. Therefore, myoepithelial carcinomas invariably express keratins and their absence would not be in favour of this diagnosis 25 26 .
The major differential diagnosis of myoepithelioma is from a pleomorphic adenoma. Myoepitheliomas are composed completely, or almost completely, of myoepithelial cells, whereas the amount is variable in the pleomorphic adenoma, but may reach levels comparable to those in myoepithelioma. Pleomorphic adenoma contains abundant ducts, whereas myoepithelioma have few, if any. The range of stromal components is identical, and myxoid and even chondroid areas can be seen in both. The only difference being that a much greater amount is likely to be found in the pleomorphic adenoma. Myoepithelioma probably constitutes one end of a biological spectrum which also includes pleomorphic adenoma and some (non-membranous) basal cell adenomas 7 . Other differential diagnosis includes soft tissues tumours such as leiomyoma, which is S-100 protein negative. Schwannomas are S-100 positive but most display characteristic histological features, although Verocay bodies have been reported in myoepitheliomas 27 . Polymorphous low grade adenocarcinoma may display areas of myoepithelial differentiation, both on Haemoxylin Eosin sections and on immunohistochemical stains. It is infiltrative, unlike the usually well-circumscribed myoepithelioma 28 . Myoepithelial cells may be found in epithelial-myoepithelial carcinoma and may be particularly prominent. However, adequate sampling should reveal the characteristic double cell lining of ducts, as well as evidence of invasive behaviour.
Biologically, myoepitheliomas are benign, in most cases, but occasionally infiltrate locally and metastasize. The malignancy is supported by infiltrative growth, necrotic areas, cytologic atypia, high mitotic rate and cellular pleomorphism 16 26 . It has been suggested, in the literature, that assessment of cell proliferative activity may be helpful in the differential diagnosis between benign and malignant myoepitheliomas, and that a Ki-67 labelling index of more than 10% is diagnostic of myoepithelial carcinoma 29 .
The prognosis of benign myoepithelioma would appear to be good, provided surgical excision is complete. Radiation therapy is used only when surgery is not considered feasible.
References
- 1.Martinez-Madrigal F, Micheau C. Histology of the major salivary glands. Am J Surg Pathol 1989;13:879-99. [DOI] [PubMed] [Google Scholar]
- 2.Sciubba JJ, Brannon RB. Myoepithelioma of salivary glands: report of 23 cases. Cancer 1982;49:52-72. [DOI] [PubMed] [Google Scholar]
- 3.Batsakis JG. Myoepithelioma. Ann Otol Rhinol Laryngol 1985;94:523-4. [DOI] [PubMed] [Google Scholar]
- 4.Seifert G. World Health Organization international histological classification of tumours. Histological typing of salivary gland tumours. 2nd Edn. Berlin, Heidelberg, New York: Springer 1991. [Google Scholar]
- 5.Katsuyama E, Kaneoka A, Higuchi K. Myoepithelioma of the soft palate. Acta Cytol 1997;41:1856-8. [PubMed] [Google Scholar]
- 6.Piattelli A, Fioroni M, Rubini C. Myoepithelioma of gengiva. Report of a case. J Periodontol 1999;6:683-7. [DOI] [PubMed] [Google Scholar]
- 7.Simpson RHW, Jones H, Beasley P. Benign myoepithelioma of the salivary glands: a true entity? Histopathol 1995;27:1-9. [DOI] [PubMed] [Google Scholar]
- 8.Barnes L, Appel BN, Perez H, El-Attar AM. Myoepitheliomas of the Head and Neck: case report and review. J Surg Oncol 1985;28:21-8. [DOI] [PubMed] [Google Scholar]
- 9.Hamperl H. The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumours. Curr Topics Pathol 1970;53:161-220. [PubMed] [Google Scholar]
- 10.Corio RL, Sciubba JJ, Brannon RB. Epithelial-myoepithelial carcinoma of intercalated duct origin. Oral Surg 1982;53:280-7. [DOI] [PubMed] [Google Scholar]
- 11.Thompson SH, Bender S, Richards A. Plasmacytoid myoepithelioma of a minor salivary glands. J Oral Maxillofac Surg 1985;43:285-8. [DOI] [PubMed] [Google Scholar]
- 12.Taylor J, Tighe JV. A minor salivar gland tumour presenting with dysphagia. J Laryngol Otol 1999;113:569-72. [DOI] [PubMed] [Google Scholar]
- 13.Begin LR, Rochon L, Frenkiel S. Spindle cell myoepithelioma of the nasal cavity. Am J Surg Pathol 1991;15:184-90. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim R, Bird DJ, Sieler MW. Malignant myoepithelioma of the larynx with massive metastatic spread to the liver: an ultrastructural and immunocytochemical study. Ultrastruct Pathol 1991;15:69-76. [DOI] [PubMed] [Google Scholar]
- 15.Miura K, Harada H, Aiba S, Tsutsui Y. Myoepithelial carcinoma of the lung arising from bronchial submucosa. Am Surg Pathol 2000;24:1300-4. [DOI] [PubMed] [Google Scholar]
- 16.Suba Z, Nemeth Z, Gyulai-Gaal S, Ujpal M, Szende B, Szabo G. Malignant myoepithelioma. Clinicopathological and immunohistochemical characteristics. Int J Oral Maxillofac 2003;32:339-41. [DOI] [PubMed] [Google Scholar]
- 17.Tuncel U, Ergul G, Ozlugedik S, Unal A. Myoepithelial carcinoma in the nasopharynx: an unusual localization. Yonsei Med J 2004;45:161-5. [DOI] [PubMed] [Google Scholar]
- 18.Sheldon WH. So-called mixed tumours of the salivary glands. Arch Pathol 1943;35:1-20. [Google Scholar]
- 19.Batsakis JG, Kraemer B, Sciubba JJ. The pathology of head and neck tumours: the myoepithelial cell and its participation in salivary gland neoplasia, part 17. Head Neck Surg 1983;5:222-33. [DOI] [PubMed] [Google Scholar]
- 20.Dardick I, Van Nostrand AW. Myoepithelial cells in salivary gland tumour. Head Neck Surg 1985;7:395-408. [DOI] [PubMed] [Google Scholar]
- 21.Ellis GL, Auclair PL. Tumours of the salivary glands. Washington, DC: Armed Forces Institute of Pathology 1996. [Google Scholar]
- 22.Dardick I, Thomas MJ, Van Nostrand AW. Myoepithelioma – new concepts of histology and classification: a light and electron microscopic study. Ultrastruct Pathol 1989;13:187-224. [DOI] [PubMed] [Google Scholar]
- 23.Dardick I. Myoepitheliomas. Definitions and diagnostic criteria. Ultrastruct Pathol 1995;19:359-69. [DOI] [PubMed] [Google Scholar]
- 24.El-Naggar AK, Lovell M, Callender DL, Ordonez NG, Killary AM. Cytogenetic analysis of a primary salivary gland myoepithelioma. Cancer Genet Cytogenet 1999;113:49-53. [DOI] [PubMed] [Google Scholar]
- 25.Alos L, Cardesa A, Bombi JA, Mallofre C, Cuchi A, Traserra J. Myoepithelial tumours of salivary glands: a clinicopathologic, immunohistochemical, ultrastructural and flow cytometric study. Semin Diagn Pathol 1996;13:138-47. [PubMed] [Google Scholar]
- 26.Savera AT, Sloman A, Huvos AG, Klimstra DS. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol 2000;24:761-74. [DOI] [PubMed] [Google Scholar]
- 27.Merino MJ, LiVolsi VA. Pleomorphic adenomas of the parotid gland resembling mesenchymal tumours. Oral Surg 1977;44:405-10. [DOI] [PubMed] [Google Scholar]
- 28.Dardick I, Van Nostrand APW. Polymorphus low-grade adenocarcinoma: a case report with ultrastructural findings. Oral Surg Oral Med Oral Pathol 1988;66:459-65. [DOI] [PubMed] [Google Scholar]
- 29.Nagao T, Sugano I, Ishida Y. Salivary gland malignant myoepithelioma. A clinicopathologic and immunohistochemical study of ten cases. Cancer 1988;83:1292-9. [DOI] [PubMed] [Google Scholar]