Summary
Giant cell reparative granuloma accounts for 1%-7% of all benign lesions of the jaw. Giant cell reparative granuloma often arises in the mandible and in the maxilla and affects children and young adults. It is usually a slow-growing lesion, fast-growing lesions having rarely been reported. The latter, despite the innocent histological appearance, has an aggressive behaviour mimicking a malignant lesion. In the present report the clinical features of an aggressive variety of giant cell reparative granuloma in a 21-year-old female are described focusing on the dental findings at computed tomography and surgical treatment.
Keywords: Mandible, Giant cell granuloma, Diagnosis, Surgical treatment
Riassunto
Il granuloma riparativo giganto-cellulare rappresenta l’1-7% delle lesioni benigne della mandibola con prevalente insorgenza in età pediatrica e nella terza decade di vita. Le sedi più frequentemente interessate sono il mascellare superiore e la mandibola. Presenta, in genere, una lenta evoluzione clinica e raramente sono stati descritti casi a rapida crescita. Questi ultimi sono caratterizzati da un quadro istologico di apparente benignità e da un andamento clinico tipico di una lesione maligna. Nel nostro caso vengono descritte le caratteristiche cliniche di una variante aggressiva di granuloma riparativo giganto-cellulare in una giovane donna di 21 anni con particolare riferimento alle caratteristiche radiologiche e al trattamento chirurgico.
Introduction
Giant cell reparative granuloma (GCRG) is not a true neoplasm but rather a reactive process; its origin could be triggered by trauma or inflammation 1 2. GCRG often arises in the mandible and in the maxilla and affects children and young adults, predominantly females, in the 2nd and 3rd decades of life 3. GCRGs are classified, according to location, as central or peripheral, occurring, respectively, in bone or gingival soft tissues 4. Fast-growing lesions have rarely been reported. In these cases, GCRGs are characterized by an aggressive behaviour against an innocent histological appearance, pain and rapid facial swelling and high recurrence rate (as early as 3, and as late as, 22 years) 5 6. The clinical importance of these benign tumours is that they clinically mimic a malignant lesion. The present report illustrates a rare aggressive variety of GCRG, with an atypical clinical presentation in a 21-year-old female; attention has been focused in particular on dental-computed tomography (CT) findings (pre-operative and long-term follow-up) and surgical treatment.
Case report
A 22-year-old female presented to our department with a 2-month history of an expanding right mandibular swelling with concomitant paresthesias of the third branch of the right trigeminal nerve. There was no history of trauma or dental problems. The results of blood chemistry and routine laboratory tests were normal.
Clinical evaluation revealed a mandibular asymmetry with gross enlargement of the right mandibular body, hard in consistency without overlying redness, and mild paresthesia of the right inferior alveolar nerve. There was no evidence of cervical lymphadenopathy. The intra-oral evaluation revealed a non-ulcerated, firm, elastic vestibular swelling extending from the lower right first molar to the left lateral incisor. There was no loosening or displacement of teeth. The molar and premolars exhibited + 2 mobility and paresthesia.
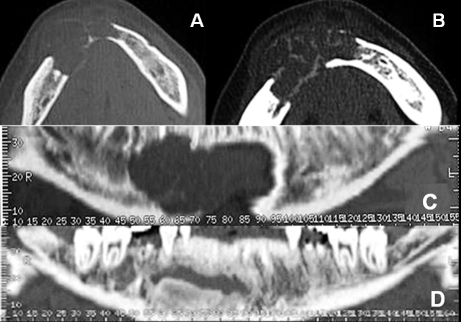
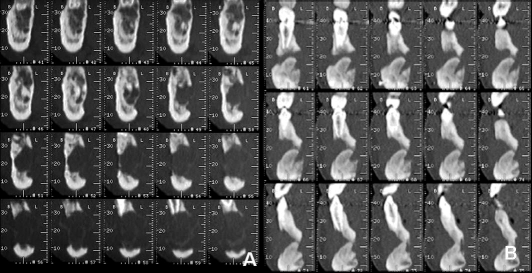
Panoramic radiograph, performed in another hospital, showed a large radiolucent lesion located in the body of the right mandible. The patient underwent computed tomography (CT) with Dental reformatting programme (DentascanTM) that revealed a hypodense, oval-shaped, uni-locular, non mineralized osteolytic lesion, with some hyperdense haemorrhagic regions within (Fig. 1A-B); the lesion was located centrally in the anterior right mandible, with a proximal-distal extension over the midline for 4.7 cm, from 4.5 to 3.3 roots (Fig. 1C), and buccal-lingual diameter of 1.5 cm (Fig. 2A). The lesion showed aggressive imaging features: it thinned, expanded and interrupted cortical bone margins both on the buccal and lingual sides of the mandible, involved the right mandibular canal, the right mental foramen, several teeth roots and surrounding soft tissues (Fig. 1C-2A). 3D-VR (Volume Rendering) reconstructions confirmed cortical bone interruption of buccal and lingual sides of the mandible and the involvement of several teeth roots. A pre-operative intra-oral biopsy of the lesion revealed a morphology consistent with peripheral giant cell granuloma.
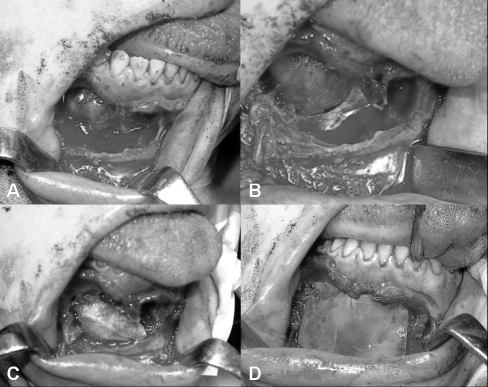
Bearing in mind the radiological and histological features, surgical treatment was planned. Under general anaesthesia, the mandible was approached by an intra-oral access with an endoral vestibular incision deep down to the underlying bone. This approach allowed wide exposure of inferior mandibular border. Thereafter, using appropriate curettes the lesion was excised by curettage. The surgical defect, following excision, showed a well-demarcated area of bone destruction both of the anterior and posterior walls of the jaw, while the lower and upper borders of the mandible were intact (Fig. 4A-B). The inferior alveolar nerve was not identified due to disruption by the lesion. An immediate reconstruction of the mandible was performed with an autologous iliac crest bone graft removing the periosteal surface and a mixture of demineralized bone matrix (Grafton DBM Putty in a Jar – 5 cc.) (Fig. 4C), in order to promote osteogenesis and to repair the large surgical defect of the anterior and posterior walls of the jaw. These implants were then immobilized with two allograft membranes guiding tissue regeneratio (Inion GTR Membrane Products; MBR-2000, 30x40 mm), each fixed by 4 screws and blocking in a “sandwich” fashion the bone implants (Fig. 4D). The wound was closed with interrupted closure to prevent the formation of a haematoma. The post-operative course was uncomplicated and the patient was discharged on post-operative day 6.
The patient was regularly observed at follow-up until healing was complete. The dental vitality was checked every three months. The one-year post-operative clinical follow-up revealed that healing was uneventful and all teeth were vital to electrical and thermal pulp testing.
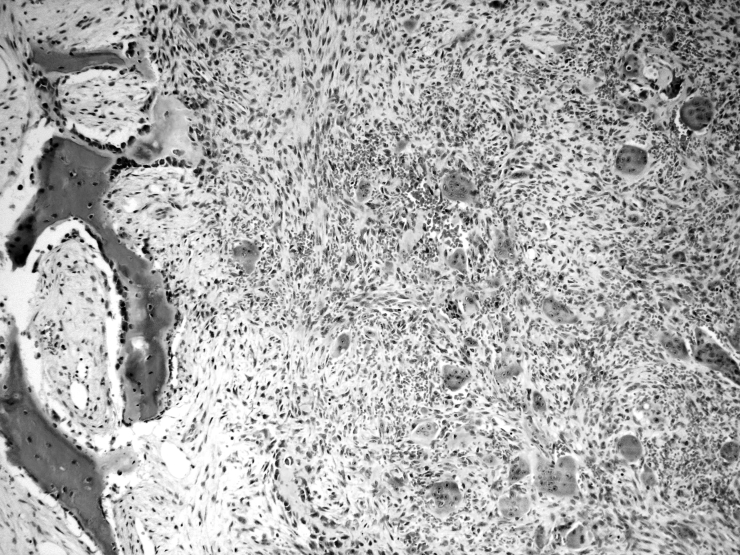
Microscopic examination revealed a mass composed of clumped multinucleated giant cells embedded in a fibrous stroma with focal haemorrhages and new bone formation (Fig. 5). The final diagnosis was GCRG.
Fig. 5.
Sections of tumour: multinucleated giant cells embedded in fibrous stroma with focal haemorrhage; new bone formation with osteoblastic rimming is evident (left) (H&E, original magn. x 100).
The radiological control with dental-CT showed the hyperdense bone graft placed within the surgical cavity with complete ossification around the lower margin and incomplete bone formation around the upper portion; the hypodense lesion showed no modification in size, morphology and its relationship with surrounding structures; there was no evidence of post-operative complications (Fig. 1D-2B-5).
Discussion
The following lesions other besides GCRG have been classified as giant cell lesions of bone: giant cell tumours (GCT), fibrous dysplasia, aneurysmal bone cyst, lesions of primary or secondary hyperparathyroidism (brown tumours) and cherubism. GCT is distinctly unusual in the jaw; moreover, giant cells are regularly and uniformly distributed in GCT, while they are clumped in areas separated by virtually devoid areas in GCRG. Fibrous dysplasia is characterized by the presence of Chinese figure-like trabeculae of woven or immature bone within a proliferating fibroblastic stroma. Aneurysmal bone cysts show large spaces filled by blood. These lesions were thus excluded by histology 7–9. Brown tumours are identical to GCRG, both histologically and radiographically, but they were ruled out on the basis of normal serum levels of calcium, phosphorus, alkaline phosphatase and good renal function. Cherubism (hereditary and intra-osseous fibrous swellings of the jaw) is also microscopically indistinguishable from GCRG, but its usually bilateral presentation, in a young individual with a hereditary autosomal dominant mode, allows its recognition.
Imaging plays an essential role in the detection, characterization, pre-surgical evaluation of focal bone lesions of the mandible as well as in their post-operative follow-up 10. Panoramic radiograph is still the imaging modality of choice, but CT with the Dental reformatting programme allows an optimal view of the bone and provides essential data for differentiating benign from malignant lesions and for planning correct surgical procedure 11. Radiological appearance of GCRG are non-specific, and conflicting descriptions have appeared in various articles. The lesion may appear with a unilocular or multilocular radiolucency, with well-defined or ill-defined margins; varying degrees of expansion and erosion of the cortical plates and resorption of dental root have been described 12. The radiographic appearance is indistinguishable from that of odontogenic cyst, aneurysmal bone cyst (ABC), ameloblastoma, odontogenic myxoma and odontogenic fibroma. Aggressive radiologic features are more likely in GCRG affecting the hands 13 14. In the literature, there is a paucity of Dental-CT pre- and post-surgical evaluation of GCRG characteristics 15 16. In our case, the aggressive imaging features of the lesion were more typical of a malignant tumour and are unusual for a GCRG, which was the final histological diagnosis.
Several morphological parameters, such as mitotic index, stromal characteristics, and values reflecting the average dimension of giant cells and the percent of tumour mass they occupied, have been tested in an effort to reliably divide giant cell lesions of the jaw into indolent and aggressive cases 17. However, none of these have shown a significant association with clinical behaviour. Further studies, on large series of giant cell lesions of the jaw, could possibly produce some useful data on this point.
GCRG might be treated by non-surgical methods such us radiotherapy, daily systemic doses of calcitonin, and intra-lesional injections of corticosteroids. A non-surgical approach is probably a good treatment option for small, slow-growing lesions.
As far as concerns surgery, Becelli et al. 18 described a case of excision of the lesion and reconstruction via an autologous iliac crest bone graft, osseo-integrated implants, and an “overdenture” prosthesis. Other Authors have treated aggressive varieties by combined curettage and cryosurgery 19, or by curettage and peripheral ostectomy 20. Bataineh et al. 21, instead, advocated resection without continuity and peripheral ostectomy and, finally, Choung et al. 17 suggested that aggressive cases should be treated with en bloc resection. However, the traditional treatment of GCRG is represented by surgical removal via an intra-oral approach and the extent of tissue removal ranges from a simple curettage to an en bloc resection. Curettage alone, or in combination with a periostal bone resection is the treatment modality most often used. Curettage has also been supplemented with cryosurgery and peripheral ostectomy. The surgical defect usually heals by secondary intention.
We have successfully treated the present case by excision of the lesion and reconstruction of the jaw with an autologous iliac crest bone graft, allograft bone and membranes fixed on the lateral and medial walls of the mandible.
Fig. 1.
A) Dental-CT scan of mandible: transverse images clearly demonstrate thinning, expansion and interruption of cortical bone margins; soft tissues window. (B) Better depicts haemorrhagic foci within the lesion and its spread on the surrounding soft tissues. C) Preoperative panoramic images: teeth roots, right mandibular canal and right mental foramen involved within the lesion. Left mandibular canal and left mental foramen are spaved. D) One-year post-surgical follow-up: the surgical cavity with hyperdense bone graft within, partially ossified in lower portion.
Fig. 2.
A) Pre-operative cross-sectional views on right anterior mandible: interruption of cortical bone margins both on buccal and lingual sites, involvement of right mandibular canal, right mental foramen and teeth roots. B) Post-operative cross-sectional views on middle portion of mandible: hyperdense bone graft is in inferior third of surgical cavity. Still evident are interruption of cortical bone margins and involvement of roots of teeth.
Fig. 3.
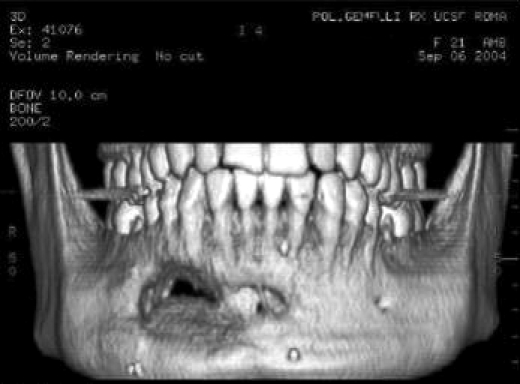
3D volume rendering image shows partial ossification of bone graft.
Fig. 4.
A-B. Surgical defect following excision of lesion showing area of bone destruction of anterior and posterior wall of jaw. C) Reconstruction of mandible with autologous iliac crest bone graft and allograft bone implants. D) Allograft membranes fixed by 4 screws correcting surgical defect of anterior wall of jaw.
References
- 1.Waldron CA, Shafer WG. The central giant cell reparative granuloma of the jaws. Am J Clin Pathol 1966;45:437-47. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe HL. Giant cell reparative granuloma, traumatic bone cyst, and fibrous (fibroosseous) dysplasia of the jaw bones. Oral Surg 1953;6:159-75. [DOI] [PubMed] [Google Scholar]
- 3.Bhaskar SN, Cutright DE, Beasley JD, Perez B. Giant cell reparative granuloma (peripheral): report of 50 cases. J Oral Surg 1971;29:110-5. [PubMed] [Google Scholar]
- 4.Fechner RE, Fitz-Hugh GS, Pope TL. Extraordinary growth of giant cell reparative granuloma during pregnancy. Arch Otolaryngol 1984;110:116-9. [DOI] [PubMed] [Google Scholar]
- 5.Cassatly MG, Greenberg AM, Koop WK. Bilateral giant cell granuloma of the mandible: report of case. JADA 1988;117:731-3. [DOI] [PubMed] [Google Scholar]
- 6.Ficarra G, Kaban LB, Hansen LS. Central giant cell lesions of the mandible and maxilla. A clinicopathologic study. Oral Surg Oral Med Oral Pathol 1987;l64:44-9. [DOI] [PubMed] [Google Scholar]
- 7.Murphey MD, Nomikos GC, Flemming DJ. Imaging of giant cell tumour and giant cell reparative granuloma of bone: radiologic-pathologic correlation. RadioGraphics 2001;21:1283-309. [DOI] [PubMed] [Google Scholar]
- 8.Austin LT, Dahlin DC, Royer RQ. Giant cell reparative granuloma and related conditions affecting the jaws. Oral Surg 1979;12:1285-95. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MA, Hertzanu Y. Radiologic features, including those seen with computed tomography, of central giant cell granuloma of the jaws. Oral Surg Oral Med Oral Pathol 1988;65:255-61. [DOI] [PubMed] [Google Scholar]
- 10.Abrahams JJ. Dental CT imaging. A look at the Jaw. Radiology 2001;219:334-45. [DOI] [PubMed] [Google Scholar]
- 11.Urade M. Mandibular giant cell granuloma. Oral Surg Oral Med Oral Pathol 1988;66:121-2. [DOI] [PubMed] [Google Scholar]
- 12.Kaffe I, Ardekian L, Taicher S, Littner MM, Buchner A. Radiographic features of central giant cell granuloma of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;81:720-6. [DOI] [PubMed] [Google Scholar]
- 13.Bertoni F, Biscaglia R, Bacchini P. Giant cell separative granuloma of the phalanx of the hand with aggressive radiographic features. Skeletal Radiol 1998;27:584-7. [DOI] [PubMed] [Google Scholar]
- 14.Giza E, Stern PJ, Cualing H. Aggressive giant cell reparative granuloma of the metacarpal: a case report. J Hand Surg 1997;22:732-6. [DOI] [PubMed] [Google Scholar]
- 15.Chen SS, Chiang JH, Chang CY, Lao CB, Lirng JF, Teng MM. Giant cell reparative granuloma: a case report. Zhonghua Yi Xue Za Zhi (Taipei) 1999;62:738-42. [PubMed] [Google Scholar]
- 16.Uchino A, Kato A, Yonemitsu N, Hirctsu T, Kudo S. Giant cell reparative granuloma of the cranial vault. Am J Neuroradiol 1996;17:1791-3. [PMC free article] [PubMed] [Google Scholar]
- 17.Chuong R, Kaban LB, Kozakewich H, Perez-Atayde A. Central giant cell lesions of the jaws: a clinicopathologic study. J Oral Maxillofac Surg 1986;44:708-13. [DOI] [PubMed] [Google Scholar]
- 18.Becelli R, Cerulli G, Gasparini G. Surgical and implantation reconstruction in a patient with giant-cell central reparative granuloma. J Craniofac Surg 1998;9:45-7. [DOI] [PubMed] [Google Scholar]
- 19.Webb DJ, Brockbank J. Combined curettage and cryosurgical treatment for the aggressive “giant cell lesion” of the mandible. Int J Oral Maxillofac Surg 1986;15:780-5. [DOI] [PubMed] [Google Scholar]
- 20.Eisenbud L, Stern M, Rothberg M, Sachs SA. Central giant cell granuloma of the jaws: experiences in the management of thirty-seven cases. J Oral Maxillofac Surg 1988;46:376-84. [DOI] [PubMed] [Google Scholar]
- 21.Bataineh AB, Al-Khateeb T, Rawashdeh MA. The surgical treatment of central giant cell granuloma of the mandible. J Oral Maxillofac Surg 2002;60:756-61. [DOI] [PubMed] [Google Scholar]