Summary
Main purpose of this study was to evaluate vestibular function, focusing attention on percentage of peripheral damage in 30 HIV positive patients (23 male, 7 female), age range 26-68 years, belonging to Categories A-C of CDC classification of infection, underwent electronystagmography with bithermic stimulation according to Freyss (125 cc of water at 30 °C and 44 °C in 30 sec). The angular velocity of slow phase was considered as the main value of labirinthine functionality. Peripheral vestibular damage has been found in 35.7% of Class A patients; a similar percentage of peripheral signs was found in Classes B and C, where, on the contrary, increased central vestibular signs were observed. In order to evaluate equilibrium in these patients, a Dynamic Gait Index (DGI) test was performed. Scores were > 21 points in 85.7% of Class A patients and decreased in Classes B and C.
Keywords: Vestibular function, HIV infection, Diagnosis, Caloric tests, D.G.I. test
Riassunto
Scopo del lavoro è valutare in quale percentuale di pazienti HIV positivi esistano segni strumentali di danno vestibolare periferico ed eventuale interessamento delle vie vestibolari centrali; a questo scopo 30 pazienti sieropositivi (23 uomini e 7 donne), di età compresa tra 26 e 68 anni, in Categoria A, B e C di infezione secondo la classificazione CDC sono stati sottoposti ad esame Elettronistagmografico con stimolazione termica secondo Freyss (125 cc. di acqua a 30 °C e 44 °C in 30 secondi). È stata valutata la VAFL (velocità angolare della fase lenta) come parametro di funzionalità labirintica. Il 35,7% dei pazienti in classe A presentava segni di sofferenza vestibolare periferica; nelle classi B e C tale percentuale resta costante ma aumentano i segni clinici di sofferenza vestibolare centrale. L’equilibrio è stato valutato utilizzando il Dynamic gait index (DGI) test. Il punteggio del test è correlabile allo stadio dell’infezione; l’85,7% dei pazienti in classe A hanno ottenuto punteggi superiori a 21, mentre il 40% in classe C inferiori a 19.
Introduction
Compared with the ample literature on cochlear symptoms (sudden and/or fluctuating hearing loss), only a few reports have analyzed vestibular symptoms in HIV+ patients.
Snider et al. 1 did not include dizziness and balance disorders among the neurological symptoms in the study published in 1983 concerning 50 HIV+ patients.
Furthermore, neither Rosemberg et al. 2 nor Marcussen and Soy 3 in their studies in 1985 included cases of vertigo among ORL symptoms in 102 and 165 HIV+ patients, respectively. In 1990, Kohan et al. 4 described vestibular disorders in 15% of HIV+ patients.
Less frequent yet are instrumental studies on labyrinthine function and equilibrium in HIV+ patients. Nonetheless, they emphasize an early alteration of central nervous system (CNS) and peripheral neurological structures, which is also evident in non-symptomatic patients without anamnestic relief for vertigo; these findings appear to be in accordance with liqueural surveys 5.
Chandrasekar et al., in 1992, in an autopsy study on 10 temporal bones from 5 deceased HIV+ patients refer to significant alterations of the neuroepithelium of the posterior labyrinth; less significant alterations are described in cochlea 6.
Cytomegalovirus may damage the vestibular apparatus if localized in the anterior labyrinth, where viral inclusions in Stria Vascularis and Reissner’s Membrane have been found, or in the posterior labyrinth, as can be easily defined by observing the virus in the perilymph, even if there is no definite evidence regarding this latter hypothesis.
Like Cytomegalovirus, several different pathogens can localize in the labyrinth: Treponema, especially in immunosuppressed patients, as stated by Smith et al., in 1988 7, fungi (in particular as a consequence of meningitis) 2, Criptococcus 8, P. Carinii 9. Some autopsy studies revealed virus-like inclusions in the labyrinth (mostly the anterior labyrinth), in the perilymph and structural changes of the utricle and saccular neuroepithelium 10.
Ototoxic drugs (aminoglycosidic, antitubercular, antimycotic) may damage the labyrinth, just as drugs with collateral effects on the Central Nervous System (Sustiva) may cause dizziness.
Studying eye movements in 14 AIDS patients and relating them with data from 8 patients with Alzheimer disease, Tervo et al. 11 found significant ocular motor disturbances (hypermetric saccades, fixation instability, reduced Gain of pursuit), similar to those in Alzheimer patients and strictly correlated to the degree of dementia.
In 1989, Hart et al. published a case report on the outbreak of dizziness in a patient with AIDS 12.
Instrumental findings included normal saccadic movements, saccadization of pursuit, and bilateral areflexia after caloric stimulation. A subsequent autopsy revealed a sub-acute encephalitis with diffuse damage to the cortex, cerebellum and encephalic trunk, basically myelinic degeneration.
In 1991, Koralnik et al., in a study based on 29 HIV+ non-symptomatic patients described alterations in rotational tests in 11% of cases with occurrence of spontaneous nystagmus in 17% of cases and of positional nystagmus in 20.7% of cases 13.
The largest study is that of Hausler et al. (1991). All stage IV HIV+ patients have been found to suffer from some vestibular alteration, in 57% of cases with modification of pursuit, visual vestibular inhibition and evoked bi-thermal and rotational nystagmus. All these parameters were altered in 50% of stage III and 22% of stage II HIV+ patients. Furthermore, it is worthwhile pointing out another interesting result: 45% of non-symptomatic patients presented changes in the OTN test 14.
In 1992, Salami found a reduction of the Gain of VOR in HIV+ patients while this parameter was not remarkably different from normal in patients with AIDS 15.
In our opinion, peripheral vestibular disorders are, in fact, frequent in the HIV population even in early stages of infection. The main purpose of this investigation was to verify this hypothesis.
Materials and methods
Selection of patients
A series of 30 HIV-infected patients seroconverted from 2 to 19 years, were studied at the ENT Unit of IRCCS San Raffaele using electronystagmography and bi-thermal caloric stimulation.
Of these, 23 patients were male (77%), 7 female (23%) age range 26 to 68 years (mean 38.5; 38 years ± for males, 39.7 ± 7.7 for females). Patients belonged to HIV stages A-C: we selected 14 class A, 11 class B and 5 class C. We used the CDC classification (Centres for Disease Control and prevention); defined in 1993, it is now the most widely accepted being based upon general symptoms.
Exclusion criteria:
Evidence of chronic otitis media and/or previous surgery of middle ear;
Drug addiction (during the last 2 years);
Previous therapy with SUSTIVA;
Chemotherapy for tumours;
Previous Central Nervous System disorder;
Use of drugs active on Central Nervous System and for treatment of vertigo (14 days wash-out was applied).
Included in the study were patients with a positive history of balance disorders.
Vestibular tests and evaluation of balance
Vestibular system function was evaluated either by electonystagmography using an Amplaid MK22 and bithermal caloric testing using an Amplaid otocalorimeter as proposed by Freyss (125 cc in 30 sec with water at 30 °C and 44 °C), in a dimly lit room with the patient wearing a pair of Frenzel glasses or looking at a non-patterned background, his/her head anteroflexed 30° from the supine position. The time elapsing between stimuli was 5 min.
We used Angular Velocity of Slow Phase (AVSP), as calculated during 10 seconds of culmination, as a parameter of labyrinthine function; in accordance with data in the international literature, we considered as a normal value an AVSP of 15°/sec +/- 5° sec. Data were interpreted in terms of Directional. Preponderance (DP) and Labyrinthine Preponderance (LP) which were considered relevant when exceeding 25% and 15%, respectively. In a control population of 25 healthy patients, we found values of AVSP comprised between 12° and 25°/sec, with a mean value of 16°/sec; no significant DP or LP were found. These results are in accordance with international reports. Stimulations, when AVSP is < 7°/sec, were considered hyporesponsive, hyperresponsive when > 30°/sec. Caloric stimulation was preceded by a study of spontaneous signs (in particular the presence of spontaneous or positional nystagmus) and oculomotor movements (smooth pursuit and saccadic).
Fig. 1.
CDC classification for HIV stages.
| The CDC Classification Scheme for HIV Disease |
| The CDC classification of HIV disease was first defined as a categorization of HIV-related symptoms into four groups and was explicitly for “ public health purposes” and not “ intended as a staging system” , although it was frequently treated as if it were a staging system in the AIDS literature. The current CDC classification system, from the revision in 1993, combines three categories of the CD4 count with three symptom categories and is closer to a staging system but is still not described as such. The CDC, however, proposed that it be used to “ guide clinical and therapeutic actions in the management of HIV-infected adolescents and adults” . This description of its intended use is close to the use of a staging system. |
| The definitions of the three CD4+ T-lymphocyte categories and the three categories of clinical conditions used in Table I follow: |
|
| These categories correspond to CD4+ T-lymphocyte counts per microlitre of blood. The percentage of CD4+ T cells can be substituted for the count as indicated in parentheses. The lowest accurate, but not necessarily the most recent, CD4+ T-lymphocyte count or percentage should be used for classification purposes. The percentages were derived from correlating counts and percentages from 7 data sources. The correspondence of a 200 count to 14 percent showed little variation (range from the 7 data sources: 13 to 14%), but the correspondence of a 500 count to 29 percent was more variable (range from the 7 data sources: 22.5 to 39%). |
| Category A |
| Category A consists of one or more of the conditions listed below in an adolescent or adult (> 13 years) with documented HIV infection. Conditions listed in Categories B and C must not have occurred. |
|
| Category B |
| Category B consists of symptomatic conditions in an HIV-infected adolescent or adult that are not included among conditions listed in clinical Category C and that meet at least one of the following criteria: (a) the conditions are attributed to HIV infection or are indicative of a defect in cell-mediated immunity; or (b) the conditions are considered by physicians to have a clinical course or to require management that is complicated by HIV infection. Examples of conditions in clinical category B include but are not limited to: |
|
| For classification purposes, Category B conditions take precedence over those in Category A. For example, someone previously treated for oral or persistent vaginal candidiasis (and who has not developed a Category C disease) but who is now asymptomatic should be classified in Category B. |
| Category C |
| Category C includes the clinical conditions listed in the 1993 AIDS surveillance case definition (see subsequent section) For classification purposes, once a Category C condition has occurred, the person will remain in Category C. |
The following day balance was evaluated using the Dynamic Gait Index Test (DGI). To obtain an evaluation as uniform as possible, this latter subjective test has always been performed by the same operator. The purpose of the test is to assess equilibrium in 8 conditions:
walking straight for 50 mt maintaining the same pace;
walking straight, losing or gaining speed after an order;
walking straight, then looking right and left after an order;
walking straight, then looking up or down after an order;
walking straight, then spinning round as fast as possible 180° and stopping;
walking straight, at a normal rate toward a step, then going on;
walking as far as the first obstacle, turning around it on the right, going straight to the second obstacle and turning around it on the left;
climbing the stairs.
Each single test in this group of 8 tests has been assigned a score between 0 and 3 (0 for an awkward realization, 3 for an easy execution).
The DGI test has been introduced, in 1995, by Shumway-Cook and has been adopted mainly in the elderly population to establish the risk of falling (great risk with an overall score less than 19) 16.
Results
Vestibular tests
We divided ENG patterns into normal, with significant LP or DP, significant LP with DP, bilateral hyporesponsiveness or bilateral hyperresponsiveness. We also looked for central signs, in particular alteration of oculomotricity (Gain reduction and saccadization of smooth pursuit, over- and undershooting and velocity reduction of saccades), positional nystagmus (geotropic or apogeotropic nystagmus tested on right and left side and Rose position), rebound nystagmus. Nine of the 14 class A patients had a normal ENG, 4 of them a LP pattern and 1 LP with DP. In this group, 2 patients presented monolateral sensorineural hearing loss (both in LP group) with normal magnetic resonance imaging (MRI) of Central Nervous System. In both cases, patients had a single episode of sudden hearing loss and vertigo with only partial recovery from hearing loss after therapy. No central signs were observed in this group of patients. In class B patients, 5 had a normal ENG, 3 LP with DP, 1 had only DP, 1 showed hyporesponsiveness and 1 hyperresponsiveness. Of 3 patients with LP associated with DP, 2 had significant central signs (1 had pursuit alterations and 1 bidirectional geotropic positional low-frequency nystagmus showing no latency and exhauribility). The remaining 3 patients (pure DP hyper and hyporesponsiveness) showed signs of central nervous system involvement for oculomotricity alterations and positional nystagmus in the patient with a hyperreflexic pattern. In class C patients, 2 had LP with a DP pattern, 1 had hyporesponsiveness and 2 hyperresponsiveness. All had significant signs of central pathways involvement for oculomotricity alterations positional nystagmus while 1 of them showed rebound nystagmus.
Balance evaluation
The purpose of DGI is to evaluate the equilibrium of a subject in eight dynamic conditions 17; each of these is assigned, by the examiner, a score between 0 and 3; the highest possible score is 24 which corresponds to a brilliant function of the vestibular system; a final result < 21 indicates a balance disturbance, while patients with a score < 19 are at high risk of falling down, especially in the elderly population. In our study, 19 seropositive patients reached a score of > 22 (Tab. I), 7 patients between 19 and 22, while for caught up with a DGI outcome inferior to 19DA. As far as concerns the stages of infection, the following results were achieved:
Table I. ENG patterns and distribution in Hiv patients.
| Class | Normal | LP | DP | LP + DP | Hyporesp | Hyperresp |
| A | 9 (64.3%) | 4 (28.5%) | 1 (7.2%) | |||
| B | 5 (45.4%) | 1 (9.1%) | 3 (27.3%) | 1 (9.1%) | 1 (9.1%) | |
| C | 2 (40%) | 1 (20%) | 2 (40%) |
Category A: HIV+ patients: 12 (85.7%) had a DGI score > 21, in 2 of whom (14.3%), the final result was between 19 and 21, and none had a result below 19.
Category B: HIV+ patients: DGI > 21 in 6 patients (54.5%), between 19 and 21 in 3 patients (27.7%) and < 19 in 2 patients (17.8%).
Category C: HIV+ patients: a score > 21 was found only in one patient (20%), while two presented scores between 19 and 21 (40%) and another two reached < 19 (40%).
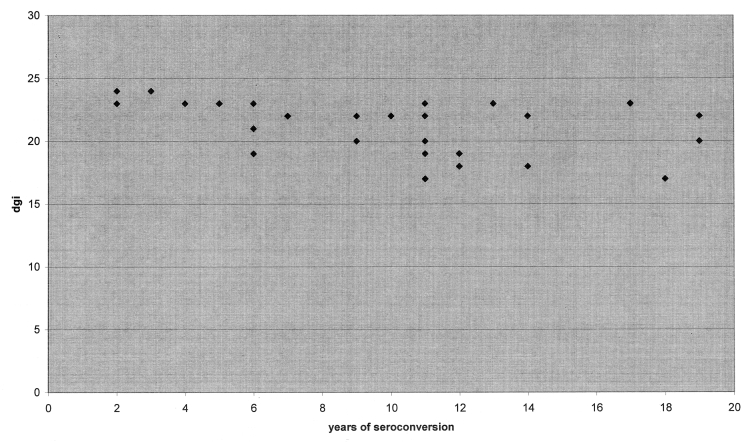
Furthermore, we looked for a possible correlation between DGI test score and years of seroconversion; a linear correlation between these two parameters (p = 0.003 statistical significance r = -0.52). It is likely that, in the evaluation of balance, an important role is played by causes other than labyrinthine damage (e.g. a disorder of the proprioceptive system).
Fig. 2.
Relationship between years of seroconversion and DGI.
Table II. Values of DGI and relationship with stage of infection.
| Category (No. patients) | DGI > 21 | DGI between 19-21 | DGI < 19 |
| A (14) | 12 (85.7%) | 2 (14.3%) | 0 |
| B (11) | 6 (54.5%) | 3 (27.7%) | 2 (17.8%) |
| C (5) | 1 (20%) | 2 (40%) | 2 (40%) |
Discussion
Caloric testing remains the most useful laboratory test to determine the responsiveness of the labyrinth 18. Nonetheless, it has some drawbacks, mainly due to a great inter-individual non-labyrinthine variability (i.e., external canal volume, amount of blood to the temporal bone, temporal bone thickness). Albeit, it is a valid tool as it is simple to perform and allows one labyrinth to be studied independently of the other. We chose not to use stimulation, as developed by Dix-Hallpike, on account of the great risk of intense nausea and vomiting correlated with the severe anxiety of the patients, but, nonetheless, we required sufficient stimulation to ensure a useful result.
It is worthwhile stressing some points.
As far as concerns vestibular function 64.3% of class A patients (9 out of 14) had normal ENG findings, while the remaining 35.7% (5 out of 14) had a pure peripheral pattern (LP without or with DP but no central signs). Only 45.4% of class B patients (5 out of 11) showed normal vestibular reliefs; 27.3% (3 out of 11) had both peripheral and central signs (LP with DP but with oculomotor alterations) while the remaining 27.3% (3 out of 11) had signs of central vestibular pathways damage (DP, hypo- or hyperresponsiveness with oculomotor disorders and/or positional nystagmus).
No C class patients had normal or pure peripheral vestibular findings; 2 out of 5 (40%) had both peripheral and central signs, while the remaining 3 out of 5 (60%) had only central vestibular signs.
The percentage of normal findings decreased from class A (64.3%) to B (45.4%), while we found no C class patients with normal reliefs.
Signs of peripheral vestibular damage are present in 10 patients out of 30 (33%) and are almost equally represented in the 3 classes (35.7% of A, 27.3% of B and 40% of C class); it is likely that peripheral damage is independent from Central Nervous System complications that mostly occur in the advanced phase of infection.
Central signs (with or without peripheral signs), on the contrary, increase considerably from A to C groups (none in A, 54.6% of B and 100% of group C).
Only 2 patients showed a peripheral cochleovestibular syndrome which appeared with sudden hearing loss and vertigo and evolving, with poor recovery from hearing symptoms (both in class A). In our opinion, peripheral cochleovestibular symptoms are provoked by direct viral damage, even in an early phase of infection (the same percentage of peripheral vestibular damage was observed in the three classes) while central vestibular damage may occur later and be related to different causes (sovrainfections, vascular causes).
Moreover, a correlation exists between patient’s balance, as evaluated by DGI test and the years of infection. In our opinion, the balance disorder, in this kind of patient, may depend on damage to the central or peripheral nervous system, not only on a vestibular injury (proprioception due to neuropathy, for example).
Conclusions
Despite the small number of patients studied in this investigation and the fact that the method used in the quantitative evaluation of labyrinthine function is based on many variables, the data obtained seem to demonstrate a correlation between the reduction of labyrinthine function and the increase in the number of years of seroconversion and the stage of disease, even in patients without acute episodes of vertigo. Our data seem to confirm the findings of other Authors. In our opinion, the decreased function of the vestibular apparatus is related to damage to the peripheral receptor, to the nerve and to the vestibular nuclei, possibly in correlation with the length of exposure to the virus (some studies have revealed the presence of the virus in the internal ear and in the perilymph).
Life expectation of HIV+ patients have improved over the last few years as a result of new chemotherapy strategies; life conditions are also improving.
This consideration explains the clinical observation of symptoms such as dizziness that were previously masked, with all probability, by more serious disorders.
From this observation, it emerges that the physician treating HIV+ patients needs to be well aware of medical and rehabilitative therapy of vertigo in order to further improve the quality of life for his/her patients.
In conclusion, we suggest vestibular screening in all HIV+ patients in order to obtain more information on central nervous system function; in our experience, results obtained may also be predictive of an imminent worsening of the clinical condition.
References
- 1.Snider W, Simpson DM, Nielsen S, Gold JW, Metroka CE, Posner JB. Neurological complications on AIDS: analysis of 50 patients. Ann Neurol 1983;14:403-18. [DOI] [PubMed] [Google Scholar]
- 2.McGill T. Micotic infections of the temporal bone. Arch Otolaryngol 1978;104:140-4. [DOI] [PubMed] [Google Scholar]
- 3.Marcussen DC, Sooy CD. Otolaryngologic and head and neck manifestations in AIDS. Laryngoscope 1985;95:401-5. [DOI] [PubMed] [Google Scholar]
- 4.Kohan D, Hammerschlag PE, Holliday RA. Otologic diseases in AIDS patients: CI correlation. Laryngoscope 1990;100:1326-30. [DOI] [PubMed] [Google Scholar]
- 5.Marshall DW, Brey RL, Cahill WT, Houk RW, Zajac RA, Boswell RN. Spectrum of cerebrospinal fluid findings in the various stages of HIV infection. Arch Neurol 1988;45:954-8. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekar SS, Siverls V, Sekhar HK. Histopathologic and ultrastructural changes in the temporal bones of HIV-infected human adults. Am J Otol 1992;13:207-14. [PubMed] [Google Scholar]
- 7.Smith M. Otologic manifestation of AIDS: the otosyphilis connection. Laryngoscope 1988;99:356-72. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi M, Weber SC, Alford BR, Coats AC, Jerger J. Temporal bone findings in Cryptococcal meningitis. Arch Otolaryngol 1975;10:577-83. [DOI] [PubMed] [Google Scholar]
- 9.Sandler ED, Sandler JM, Leboit PE, Wening BM, Mortensen N. P. Carinii in the temporal bone as a primary manifestation of AIDS. Ann Otol Rhinol Laryngol 1988;97:427-31. [DOI] [PubMed] [Google Scholar]
- 10.Pappas DG Jr, Roland JT Jr, Lim J, Lai A, Hillmann DE. Ultrastructural findings in the vestibular-end organs of AIDS case. Am J Otol 1995;16:140-5. [PubMed] [Google Scholar]
- 11.Tervo T, Elovaara I, Karli H, Valle SL, Suni J, Ladehvirta J, et al. Abnormal ocular motility as an early sign of CNS involvement in HIV infection. Lancet 1986;2:512. [DOI] [PubMed] [Google Scholar]
- 12.Hart C, Cokely CG, Schupbach J, Dal Canto MC, Coppleson LW. Neuro-otologic findings of a patient with AIDS. Ear Hearing 1989;10:68-76. [DOI] [PubMed] [Google Scholar]
- 13.Koralnik IJ, Beaumanoir A, Hausler R, Kohler A, Safran AB, Delacoux R. A controlled study of early neurologic abnormalities in men with asymptomatic HIV infection. New Engl J Med 1990;323:864-70. [DOI] [PubMed] [Google Scholar]
- 14.Hausler R, Vibert D, Koralnik IJ, Hirschel B. Neuro-otological manifestations in different stages of HIV infection. Acta Otolaryngol 1991;481(Suppl):515-21. [DOI] [PubMed] [Google Scholar]
- 15.Salami A. Il comportamento del sistema visuo-oculomotore nell’AIDS. Studio preliminare. Rivista Italiana di Otorinolaringologia Audiologia e Foniatria 1992;3. [Google Scholar]
- 16.Shumway-Cook A. Motor control theory and applications. Baltimore: Williams and Wilkins 1995. [Google Scholar]
- 17.Whitney SL, Hudak MT, Marchetti GF. The dynamic gait index relates to self-reported fall history in individuals with vestibular dysfunction. J Vestib Res 2000;10:99-105. [PubMed] [Google Scholar]
- 18.Smith T, Jacobsen J, Gaub J, Helveg-Larsen S, Trojaborg W. Clinical and electro-physiological studies in HIV-seropositive men without AIDS. Ann Neurol 1988;23:295-7. [DOI] [PubMed] [Google Scholar]
- 19.Gray F, Gherardi R, Scaravilli F. The neuropathology of the AIDS. Brain 1988;111:245-66. [DOI] [PubMed] [Google Scholar]
- 20.Elder G, Sever J. AIDS and neurological disorders: an overview. Ann Neurol 1988;23(Suppl):S4-S6. [DOI] [PubMed] [Google Scholar]
- 21.Langford-Kuntz A, Reichart P, Pohle HD. lmpairment of cranio-facial nerves due to AIDS. Report of two cases. Int J Oral Maxillofac Surg 1988;17:227-9. [DOI] [PubMed] [Google Scholar]
- 22.Regesta O, Rizzo F. Alterazioni EEG in corso di AIDS. Riv Neurol 1987;33:283-7. [Google Scholar]
- 23.Rosemberg R, Schneider KL, Cohen NL. Head and Neck presentations of AIDS. Otolaryngol Head Neck Surg 1985;93:700-4. [DOI] [PubMed] [Google Scholar]
- 24.Rosenhall U, Hakansson C, Lowhagen GB, Hanner P, Jonsson-Ehk B. Otoneurological abnormalities in asymptomatic HIV-seropositive patients. Acta Neurol Scand 1989;79:140-5. [DOI] [PubMed] [Google Scholar]
- 25.Scasso F. Manifestazioni otologiche dell’infezione da HIV. Audiol ltal 1989;6:455. [Google Scholar]
- 26.Timon C, Walsh MA. Sudden neurosensorial hearing loss as representation of HIV infection. J Laryngol Otol 1989;103:1071-2. [DOI] [PubMed] [Google Scholar]