Summary
Aim of the study was to evaluate the role of Echo Color/Power Doppler and magnetic resonance imaging in the diagnosis of space occupying parotid lesions, in the attempt to establish criteria for differential diagnosis between benign and malignant types. The study population comprised 49 patients (23 male, 26 female), age range 30-85 years, with a space occupying lesion of the parotid gland. Each lesion was carefully studied with ultrasound integrated with colour/power doppler and magnetic resonance imaging; patients were then submitted to echo-guided needle-biopsy and surgical excision. A preliminary evaluation by means of ultrasound revealed the site, size of lesion, echostructure and borders. Echo colour/power doppler and magnetic resonance imaging can play a very important role both in diagnosis and pre-operative surgical planning of parotid lesions, although cost and availability, the former to be recommended vs. magnetic resonance imaging, which is poorly accessible, expensive, and not always accepted by the patients.
Keywords: Parotid gland, Malignant tumours, Diagnosis, Magnetic resonance imaging, Colour/Power doppler
Riassunto
Lo scopo del presente studio è valutare il ruolo dell’eco-color power doppler e della risonanza magnetica nella diagnosi delle lesioni occupanti spazio della ghiandola parotide, nel tentativo di stabilire i criteri per una diagnosi differenziale tra formazioni benigne e maligne.
Lo studio ha preso in considerazione 49 pazienti (23 maschi e 26 femmine), di età compresa tra 30 e 85 anni, che presentavano lesioni tumorali della parotide. Tali formazioni sono state valutate con eco-color power doppler e risonanza magnetica, diretta e dopo somministrazione endovenosa di mezzo di contrasto paramagnetico quindi sottoposte a biopsia eco-guidata e successivamente ad escissione chirurgica. La valutazione preliminare condotta con ecografia ha considerato la posizione della lesione, le dimensioni, l’ecostruttura ed i margini. L’eco-color power doppler e la risonanza magnetica giocano entrambe un ruolo importante nella diagnsoi e nella pianificazione preoperatoria delle lesioni tumorali della parotide. La risonanza magnetica, a differenza dell’eco-color power doppler, presenta costi più elevati, un più difficile accesso e minore tollerabilità da parte di alcuni pazienti.
Introduction
Diseases of major salivary glands are common and can be classified into: lithiasis, inflammation, and spreading disorders.
In contrast, salivary gland tumours are rare and account for < 3% of all neoplasms 1. In 80% of cases, these tumours arise from the parotid gland, while, in most of the remaining cases, the submandibular gland is affected.
Neoplasms of the parotid gland are peculiar on account of remarkable histological variability. Benign tumours represent 80% of all cases; of these, 80% include pleomorphic adenoma, 10% Warthin’s tumour, and 10% monomorphic adenoma. Malignant tumours account for 20% of cases, and include mucoepidermoid carcinoma (35%), pleomorphic adenoma with malignant degeneration (20%) and acinous cells carcinoma (10-25%) 2 3.
Tumours of the salivary glands usually appear as a painless lump, and both palsy of facial nerve and infiltration of overlying skin should be considered clinical malignant features, while local lymphadenopathy is relatively rare.
Ultrasound-scan (US) is the first diagnostic procedure to evaluate both morphological and structural changes of major salivary glands, and, coupled with cytology, can yield a conclusive diagnosis for superficial and small lesions (< 3 cm) 4.
Unlike the surrounding glandular parenchyma, neoplasms usually show hypoechoic features. US can also reveal the edges 5 and possible invasion of the surrounding parenchyma.
Echo color doppler (CD) and Power doppler (PD) differentiate the solid nature of a hypoechoic lesion 4, therefore leading to diagnosis of malignancy.
The main limitations of US consist in difficulty in evaluating deep parotid lesions, parapharyngeal extension, retropharyngeal and deep cervical lymphadenopathies, as well as spreading to the base of the skull and to the ascendant mandibular branch.
Computed tomography (CT) and magnetic resonance imaging (MRI) are considered the most reliable techniques for optimal assessment of salivary gland lesions, thereby allowing accurate analysis of location, size, and margins of the lesion, extra-capsular extension, involvement of adjacent structures, presence of cystic, necrotic, haemorrhagic, or calcific elements.
Aim of the present study was to evaluate the role of CD/PD and of MRI in the diagnosis of tumoural lesions, in the attempt to establish criteria for differential diagnosis between benign and malignant forms. In fact, while several studies have attempted to differentiate parotid tumours, no clearly predictive diagnostic and differential criteria attention 7-10 have been achieved so far.
Materials and Methods
Patients
The present study population comprised 49 patients (23 male, 26 female), age range 30-85 years, all presenting a space-occupying lesion of the parotid gland.
Each lesion was carefully assessed by US, integrated with CD/PD and MRI, the examiners being unaware of the histology and/or cytology of the lesion(s). Patients were submitted to US-guided needle-biopsy and surgical excision only after diagnostic imaging.
Diagnostic methods
For US and CD/PD, an AU 5 device (Esaote, Genova, Italy) was used with a 7.5-10 MHz linear probe and Diasonics Spectra with a 10 MHz linear probe. A Pulse-Repetition Frequency (PRF) of 600-750 Hz was also used. MRI was performed using a Siemens (Erlangen, Germany) device with a superconductive magnet 1.5 T with a coil for the skull. The study was performed with Spin Echo (SE) sequences T1 weighted, and fast SE T2 weighted, with and without the suppression of the adipose signal with axial, coronal and, in some cases, sagittal plans, in basal conditions and after administration of paramagnetic Gd-DTPA (Magnevist, Schering, Berlin, Germany) at a dose of 0.2 ml/kg iv stat. Fifteen layers each 3-5 mm thick and at 0-0.5 mm interval were scanned, matrix 256 x 192, 1 or 2 excitation. A preliminary evaluation, by means of US, selected the following parameters: site, size of the lesion, echostructure, and borders.
In order to study blood supply to the lesion with CD/PD, the sample was divided into four groups according to the following criteria:
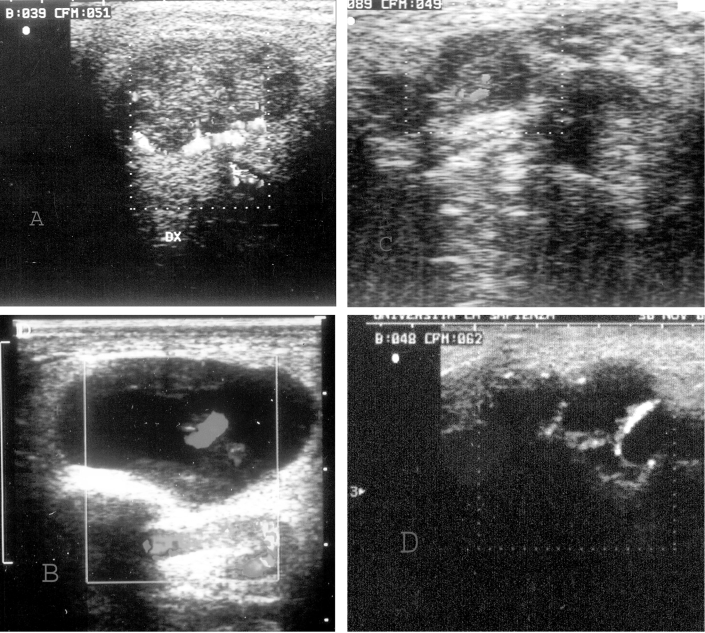
Degree 0: absence of CD/PD signal; First degree: perilesional flow or at least one vessel detectable inside the lesion (Fig. 1 a); Second degree: presence of vessel penetrating into the lesion (Fig. 1 b, c); Third degree: presence of a rich intra- and peri-lesional blood supply (Fig. 1 d); Fourth degree: random distribution of blood supply (Fig. 1 d).
Fig. 1.
A) Echo Color Doppler: Longitudinal scan. Pleomorphic adenoma with prevalent periphery vascularization with few central spots. TYPE 1.
B) Cystic lesion with hypervascularized septum (Color Doppler). TYPE 2. This finding is indicative of Warthin’s cystoadenolymphoma.
C) Hypoechogen neoplasm with intralesion vascularization (Echo Doppler). TYPE 2. Non Hodgkin Lymphoma.
D) Hypoechogen lesion with total parotid alteration (Echo Color Doppler): spread vascularization. TYPE 3-4. Unidifferentiated carcinoma.
Our MRI diagnostic criteria of parotid expansive lesions were site, size, intensity of signal, behaviour of the lesion following contrast, connections with facial nerve and retromandibular vein, recognition of borders, and proximity to adjacent structures.
Results
The histological test showed that 35 out of 49 lesions were benign (18 pleomorphic adenomas, 15 Warthin’s tumours, one neurofibroma, one lipoma) and 14 malignant (12 carcinoma and 2 non-Hodgkin lymphoma).
Color and Power Doppler ultrasound
The CD/PD showed poor blood supply of benign lesions with a mainly peri-lesional blood supply, with a vascular pattern of first degree (Fig. 1 a) and second degree. Mixed tumours showed prevalence of peri-lesional blood supply (first degree in 15/18 patients, second degree in the remaining 3 cases). In the solid version of Warthin’s tumour (6 cases), first degree; in cystic version (9 cases), a marked blood supply of septum with second degree (Fig. 1 b). In only one case of neurofibroma were features of second degree found, while in the only lipoma, total absence of flow was found.
The malignant variants arising from the epithelium, in 5 cases, showed a dishomogeneous pattern with a totally disorganised blood supply and distribution of vessels (fourth degree) (Fig. 1 d).
The other 7 cases were classified as third degree. As already stated, 2 cases of lymphoma showed second degree vascularization (Fig. 1 c).
MRI
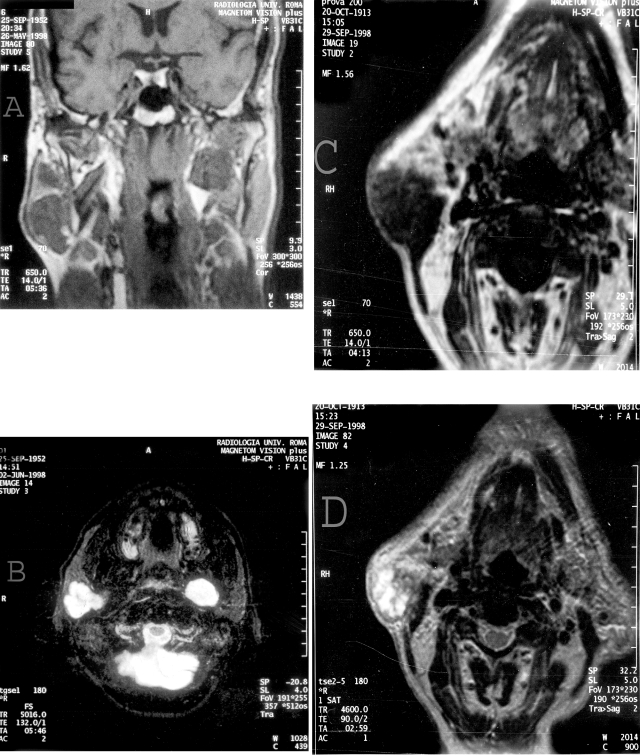
Of the 49 cases studied by MRI, SE T1 weighted sequences showed 47 hypointense and 2 hyperintense lesions (Fig. 2 a, c).
Fig. 2.
A) SE T1 sequence weighted. Coronal scan. Bilateral hypointense lesion localized in the right superficial parotid. Pleomorphic adenoma in the left deep parotid.
B) FSE T2 sequence weighted. Axial scan. Evident hyperintensity of neoplasm in the right superficial parotid. Pleomorphic adenoma in the left deep part.
C) SE T2 sequence weighted. Axial scans. Hypointense lesion weight irregular margins infiltrating adipose subcutaneous tissue. Masseter muscle relationship is poorly defined. Undifferentiated carcinoma.
D) FSE T2 sequence weighted. Hyperintense lesion with non-homogenous features due to the presence of small necrotic areas. The muscle appears to be hyperintense with a suspicious infiltration. Undifferentiated carcinoma.
In FSE T2 weighted sequences, we identified 26 highly visible and homogeneously hyperintense formations, 3 isointense, 14 with a dishomogeneous intensity signal. As far as concerns MRI findings and pathological findings results were as follows:
of the 18 pleomorphic adenomas, in T1 weighted sequences, 17 were hypointense and 1 hyperintense due to haematoma following recent needle-aspiration; in T2 weighted FSE sequences, 18 were strongly and homogeneously hyperintense; after the introduction of iv contrast, 17 appeared with a considerable and homogeneous increase in signal intensity, while one could not be evaluated due to the presence of blood (Fig. 2 a, b);
in T1 weighted sequences, Warthin’s tumours, both in the cystic 9 and solid 6 variants, appeared hypointense; in T2 weighted FSE sequences, of those presenting as a cystic lesion, 7 resulted markedly and homogeneously hyperintense and 2 had non-homogeneous signals on account of thickened septums; among the solid versions, 4 were hypointense and 2 isointense; after the introduction of contrast iv, 6 cases of the solid type showed no considerable enhancement, whereas 9 cases of the cystic version presented marked enhancement;
at T1 weighted sequences, lipoma was hyperintense; while at T2 weighted FSE sequences, it was homogeneously hyperintense; after the introduction of contrast iv, there was no relevant enhancement;
at T1 weighted sequences, the neurofibroma was hypointense, whereas at T2 weighted FSE, it was isointense; after the introduction of contrast iv, a moderate enhancement appeared, with a small hypointense central area;
lymphoma (2) appeared hypointense, both with T1 weighted and T2 weighted FSE sequences; after contrast iv, these showed poor enhancement;
each neoplasm (12), at T1 weighted sequences, appeared hypointense (Fig. 2 c), while at T2 weighted FSE sequences, each had a dishomogenous intensity of signal after intravenous administration of contrast media, 10 had a marked and homogeneous enhancement, 2 were homogeneously enhanced (Fig. 2 d).
Lesion borders
Margins of the benign lesions appeared well defined in most cases;
of the malignant lesions, 9/12 showed irregularities and a poorly-defined shape suggesting infiltration of adjacent structures.
Sequences
Sequences with adipose signal suppression were not useful to characterise lesions, except for lipomas;
T1 sequences, particularly after GA-DTPA administration, allowed, however, a better definition to be achieved of the relationship with the adjacent structures.
Displacement of the retromandibular vein
Outwards in 7 cases;
inwards in 16 cases.
Dimension of the lesion was found to be:
< 2 cm in 21 cases;
between 2-3 cm in 13 cases;
> 3 cm in 15 cases.
Discussion
Over the last few years, the progressive development of non-invasive imaging techniques has led to the detection of early lesions arising from the salivary glands.
The role of diagnostic imaging is to recognise and confirm lesions of glandular origin, define diagnosis (benign vs. malignant), thus allowing tumour staging for optimal therapeutic management 9.
The use of CD and/or PD should be considered part of the US procedure 10.
Both CD and PD can recognise whether or not a highly hypoechoic lesion is solid: pleomorphic adenoma and particularly Warthin’s tumour can show a hypo-echoic structure, and, sometimes, re-enforcement of the posterior wall 4 (Fig. 1 a, b).
Both CD and PD can reliably diagnose malignancy when a high blood supply presents with a high index of resistance and high level of systolic speed 11-13 (Fig. 1 d).
Benign tumours are thought to present lower vascularization than malignancies, and a peri-lesional blood supply, in the first instance, is indicative of pleomorphic adenoma (Fig. 1 a).
On the basis of our experience, on 35 benign lesions, a dyshomogeneous echographic pattern was detected in 6 cases. In three malignancies, US showed 3 hypoechoic structures with regular edges; two of these structures were diagnosed as lymphoma (14-16) (Fig. 1 c).
In our series, while using CD/PD, grade 0 and grade 1 were found only in benign tumours (Fig. 1 a, b). In malignancies, we noted mainly grade 3 and 4 depending on the presence of arteriovenous shunt and neovascularity 11 16; only two malignancies (non-Hodgkin’s lymphoma) have been classified as grade 2 (Fig. 1 c, d). MRI resulted critical for tissue characterization and, also, for optimal pre-operative anatomical assessment and its relationship with adjacent and locoregional structures (such as masseter muscle and the ascending branch of the mandible). Furthermore, MRI was extremely accurate in deep parotid space evaluation, in contrast with US 17. The course of facial nerve is similar to that of the retromandibular vein; nevertheless, visualization of the facial nerve, within the parotid gland, is rare on account of alterations in the glandular structure occurring in the presence of wide lesions, and, also, due to the presence of structures presenting a similar MRI aspect, such as ducts and vessels 17.
In our study, the facial nerve was not detected; therefore, the possibility of proper identification by means of MRI still remains debatable 1. Moreover, involvement of the facial nerve by the lesion was suspected in the presence of displacement of the retromandibular vein 2 7 12 14. Scans performed with T2 FSE weighted, increased contrast resolution between parotid and malignancy 9 18.
FSE sequences with adipose signal removal was poorly important.
Administration of intravenous contrast media allows reliable evaluation of the margins of the edges of the lesion and of peri-glandular invasion, and, also, allows good support to distinguish between solid and cystic lesions 2 8 9 18.
In most cases, in the present series, the use of paramagnetic contrast allowed accurate detection of the lesions by means of an increase in the signal differences between lesion and parenchima 19.
Our results show that both pleomorphic adenomas and most Warthin’s tumours (7/9), of the cystic histotype, were hypointense in T1 weighted and hyperintense in T2 20. Only contrast media administration could improve the differentiation as pleomorphic adenomas appeared with a homogenous and good signal, whereas Warthin’s tumour, of the cystic histotype, showed marked enhancement.
The T1 signal always appeared hypointense, therefore not specific, while features of the lesions in T2 were more significant, presenting as dyshomogeneous and with marked enhancement in most patients (10/12), after contrast administration 20. A hypotense T2 could suggest high cellularity and negligible presence of water, predictive aspects of a malignancy, except for the adenocystic carcinoma which can present with a hyperintense signal (Fig. 2 c, d) 19.
Comparing results obtained with US and MRI, the following conclusions can be made:
when dealing with a poorly vascularised mass at Color/Power Doppler and signal hyperintensity in T2 with marked or partial enhancement after contrast, lesions can be diagnosed as pleomorphic adenoma or Warthin’s tumour, in its cystic variant; whereas, in the case of a reduced signal in T2 and without significant enhancement, lesions can be considered as Warthin’s tumour (solid form);
in the presence of a mass showing rich intra- and peri-lesional blood supply, with dyshomogeneous hyperintensity in T2 and marked and not homogeneous enhancement, the lesion can be considered a true neoplasm.
Conclusions
Echo Color/Power Doppler evaluation and MRI play an important role both in the diagnosis and pre-operative surgical planning of parotid lesions, although on account of cost and availability, the former are to be recommended vs. MRI, which is rarely available, expensive, and not always accepted by the patients. Proper use of one, or, when available, both the above-mentioned methods, contributes to achievement of correct diagnosis 21. Echo Color/Power Doppler evaluation should always be performed to achieve a conclusive diagnosis. In fact, this procedure, provides an exact distinction between benign and malignant lesions, and, in our study, we could classify benign tumours as grade 0, 1 and 2 and malignant lesion as grade 3 and 4.
An accurate analysis, by means of MRI, when this is feasible, provides a more precise locoregional staging, thus being critical both for optimal therapeutic choice, and for good distinction between the different types of lesions. In fact, during pre-operative work-up, the anatomic relationship of the tumour with intraglandular structures and extraparotid structures, can be conclusively achieved only by MRI.
In conclusion, satisfactory differentiation between a number of benign lesions and malignancies can be achieved by combining the diagnostic possibilities offered by these two procedures, or, at least, by careful assessment of Echo Color/Power Doppler which, as such, provides adequate pre-operative information.
References
- 1.Yousem DM, Kraut MA, Chalian AA. Major salivary gland imaging. Radiology 2000;216:19-29. [DOI] [PubMed] [Google Scholar]
- 2.Joe VQ, Westesson PL. Tumours of the parotid gland: MR imaging characteristics of various histologic types. Am J Roentgenol 1994;163:433-8. [DOI] [PubMed] [Google Scholar]
- 3.Wolf JS, Goldberg AN, Bigelow DC. Pleomorphic adenoma of the parotid. Am Fam Physician 1997;56:185-92. [PubMed] [Google Scholar]
- 4.Grazioli L, Olivetti L, Matricardi L, Zanetti U, Burlini D, Negrini S, et al. Comparison of ultrasonography, computerized tomography, and magnetic resonance in the study of parotid. Radiol Med 1993;86:268-80. [PubMed] [Google Scholar]
- 5.Gritzmann N. Sonography of the salivary glands. Am J Roentgenol 1989;153:161-6. [DOI] [PubMed] [Google Scholar]
- 6.Keyes JW Jr, Harkness BA, Greven KM, Williams DW 3rd, Watson NE Jr, McGuirt WF. Salivary gland tumour: pretherapy evaluation with PET. Radiology 1994;192:99-102. [DOI] [PubMed] [Google Scholar]
- 7.Takashima S, Noguchi Y, Okumura T, Aruga H, Kobayashi T. Dynamic MR imaging in the head and neck. Radiology 1993;189:813-21. [DOI] [PubMed] [Google Scholar]
- 8.Goto TK, Yoshiura K, Nakayama E, Yuasa K, Tabata O, Nakano T, et al. The combined use of US and MR imaging for the diagnosis of the masses in the parotid region. Acta Radiol 2001;42:88-95. [DOI] [PubMed] [Google Scholar]
- 9.Shah GV. MR imaging of salivary glands. Magn Reson Imaging Clin N Am 2002;10:631-62. [DOI] [PubMed] [Google Scholar]
- 10.Howlett DC. High resolution ultrasound assessment of the parotid gland. Br J Radiol 2003;76:271-7. [DOI] [PubMed] [Google Scholar]
- 11.Schick S, Steiner E, Gahleitner A, Bohm P, Helbich T, Ba-Ssalamah A, et al. Differentiation of benign and malignant tumours of the parotid gland: value of pulsed Doppler and Color Doppler sonography. Eur Radiol 1998;8:1462-7. [DOI] [PubMed] [Google Scholar]
- 12.Gritzmann N. Sonography of the salivary glands. Am J Roentgenol 1989;153:161-6. [DOI] [PubMed] [Google Scholar]
- 13.Freling NJ, Molenaar WM, Vermey A, Mooyaart EL, Panders AK, Annyas AA, et al. Malignant parotid tumours: clinical use of MR imaging and histologic correlation. Radiology 1992;185:691-6. [DOI] [PubMed] [Google Scholar]
- 14.Soler R, Bargiela A, Requejo I, Rodriguez E, Rey JL, Sancristan F. Pictorial review: MR imaging of parotid tumours. Clin Radiol 1997;52:269-75. [DOI] [PubMed] [Google Scholar]
- 15.Martinoli C, Derchi LE, Solbiati L, Rizzatto G, Silvestri E, Giannoni M. Color Doppler sonography of salivary glands. Am J Roentgenol 1994;163:933-41. [DOI] [PubMed] [Google Scholar]
- 16.Takashima S, Sone S, Takayama F, Maruyama Y, Masegawa M, Morii A, et al. Assessment of parotid masses: which MR pulse sequences are optimal? Eur J Radiol 1997;24:206-15. [DOI] [PubMed] [Google Scholar]
- 17.Thibault F, Halimi P, Bely N, Chevallier JM, Bonfils P, Lellouch-Tubiana A, et al. Internal architecture of the parotid gland at MR imaging: facial nerve or ductal system? Radiology 1993;188:701-4. [DOI] [PubMed] [Google Scholar]
- 18.Sumi M, Takagi Y, Uetani M, Morikawa M, Hayashi K, Kabasawa H, et al. Diffusion-weighted echoplanar MR imaging of the salivary glands. Am J Roentgenol 2002;178:959-65. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto M, Sasano T, Higano S, Takahashi S, Iikubo M, Kakehata S. Usefulness of heavily T(2) weighted magnetic resonance images for the differential diagnosis of parotid tumours. Dentomaxillofac Radiol 2003;32:295-9. [DOI] [PubMed] [Google Scholar]
- 20.Okahara M, Kiyosue H, Hori Y, Matsumoto A, Mori H, Yokoyama S. Parotid tumors: MR imaging with pathological correlation. Eur Radiol 2003;13(Suppl 4):L25-33. [DOI] [PubMed] [Google Scholar]
- 21.Koyuncu M, Sesen T, Akan H, Ismailoglu AA, Tanyeri Y, Tekat A, et al. Comparison of computed tomography and magnetic resonance imaging in the diagnosis of parotid tumours. Otolaryngol Head Neck Surg 2003;129:726-32. [DOI] [PubMed] [Google Scholar]