Summary
Many Authors have discussed the best indication and extension of neck dissection, but few have studied the surgical approach considering the continuity of neck dissection with the primary tumour. This retrospective study refers to patients submitted to major surgery between 1996 and 2001 for floor of mouth and oral tongue squamous cell carcinoma, at the Head and Neck Surgery Department of the Hospital “A.C. Camargo”, São Paulo, Brazil and of the European Institute of Oncology, Milan, Italy. Patients were assigned to one of three groups: group 1 (in-continuity resection); group 2 (discontinuous resection) and group 3 (delayed discontinuous resection). Overall, 193 patients were studied. There were no differences in disease-free survival between the neck dissection groups. Furthermore, no statistical differences were found in disease specific survival between the groups. Discontinuous neck dissection seems not to change the disease-free survival or disease-specific survival when compared to in-continuity neck dissection, in this retrospective study. A prospective randomized trial is necessary to confirm these results.
Keywords: Oral cancer, Surgical treatment, Neck dissection, En-bloc resection, Discontinuous neck dissection
Riassunto
Nei carcinomi della lingua e del pavimento orale l’indicazione ad una dissezione laterocervicale in blocco con la neoplasia primitiva ovvero differita è molto discussa, ed in letteratura esistono pochi contributi su questo argomento. Questo studio retrospettivo analizza i risultati di differenti atteggiamenti terapeutici sulle aree linfatiche laterocervicali in una serie di pazienti con carcinomi della lingua e del pavimento orale trattati dal 1996 al 2001 in due strutture oncologiche: l’Istituto “A. Camargo” di S. Paolo del Brasile e l’Istituto Europeo di Oncologa di Milano. I pazienti sono stati divisi in tre gruppi a seconda della tempistica dello svuotamento laterocervicale rispetto alla rimozione della neoplasia orale: 1) resezione in blocco della neoplasia con i linfonodi del collo; 2) linfoadenectomia laterocervicale sincrona ma non in continuità con la resezione della neoplasia primitiva; 3) linfoadenectomia laterocervicale differita di 30-40 giorni rispetto alla terapia della neoplasia primitiva.
Sono stati studiati globalmente 193 pazienti.
Non è stata evidenziata alcuna differenza statisticamente significativa fra i tre gruppi in termini di sopravvivenza da malattia. Sulla base di questo studio retrospettivo si può ipotizzare che le tre modalità terapeutiche di dissezione in blocco, sincrona discontinua e differita non modifichino la prognosi di pazienti con neoplasie della lingua e del pavimento orale. È necessario uno studio clinico prospettico randomizzato per confermare questa ipotesi.
Introduction
Lymph node metastasis is one of most important prognostic factors in patients with head and neck squamous cell carcinoma (SCC), causing a reduction of 50% in survival expectancy. Many Authors 1–7 have discussed the best indication and extension of neck dissection (ND) but few 8 9 have studied the surgical approach considering the continuity, or not, of ND with the primary tumour and whether discontinuous neck dissection could be delayed or performed at the same time as the primary tumour resection (simultaneous vs. delayed).
One century ago, Crile 10 showed that patients submitted to synchronous resection of primary and ND (en bloc surgery) achieved better results than those who underwent simple primary tumour removal. This en bloc concept has been maintained in time, particularly in the treatment of oral cancer, even if several technical modifications have been made. Today, advanced oral tumours requiring mandibulectomy or a trans-mandibular approach, and often a local reconstruction using a rotation flap or a free flap, also have cervical nodes removed en bloc with the primary tumour, regardless of their clinical status (cN0 or cN1-N3). On the other hand, most small tumours (cT1-cT2) can be removed through a transoral approach, and the treatment of the cN0 neck is debatable: the “wait and see” policy, sentinel lymph node biopsy, en bloc or discontinuous synchronous ND and delayed ND have been proposed and results of these different policies discussed 2 5 8 9 11 12.
In this study, a retrospective analysis has been made of the results of different treatment policies [en bloc, synchronous discontinuous neck dissection (SND) and delayed neck dissection (DND)], performed in a series of patients at the Hospital “A.C. Camargo”, Brazil (HACC) and European Institute of Oncology, Milan, Italy (IEO), for cancer of the oral tongue and floor of the mouth, between 1996 and 2001. Aim of the analysis was to evaluate possible differences in loco-regional recurrences related to the different timing of node removal.
Patients and methods
Records of all patients treated primarily with surgery between 1996 and 2001 for floor of mouth and oral tongue SCC, at the Head and Neck Surgery Departments of the HACC and of the IEO were reviewed.
Following review of patients’ charts, cases were restaged according to the 2002 version of the UICC-AJC 12 classification on the basis of the initial clinical description. Patients pre-treated and those not submitted to a neck ND were excluded from the study.
Overall, 193 patients [141 male (73.4%), 53 female], were studied (56% HACC and 44% IEO). For the purpose of this analysis, cases were divided into early (cT1 or cT2, cN0), or advanced disease (all T3, T4, or any T cN1).
Surgical techniques and timing of ND
Early stage:
-
cT1 of oral tongue and floor of the mouth, and small cT2 (< 3 cm) of the anterior floor of the mouth,
HACC: transoral removal of the primary and SND (supra-omohyoid dissection, levels I-III),
IEO: transoral removal of the primary and DND (levels I-IV and removal of the suprafascial nodes of level V);
-
Large cT2 (> 3 cm) of floor of the mouth,
HACC: en bloc resection of primary and neck (supra-omohyoid dissection, levels I-III),
IEO: en bloc resection of primary and neck (levels I-IV and suprafascial nodes of level V);
-
Advanced stage (cT3, cT4 cN0 or any cT cN1-3),
HACC: en bloc resection of primary and neck (levels I-III in cN0 patients and levels I-V in cN1-3 patients; from the year 2000 surgical protocols changed and cN1 patients underwent removal of I-IV levels),
IEO: en bloc resection of primary and neck (levels I-IV and suprafascial nodes of level V).
Post-operative Radiotherapy
HACC: pT3, pT4; positive or close (< 0.5 cm) resection margins; vascular or peri-neural infiltration; > 1 pN+ or extra-capsular invasion in at least one node;
IEO: pT4; positive resection margins; > 3 pN+ or extra-capsular invasion in at least one node.
Follow-up
In both Institutions, patients were checked clinically every 2 months during the first year after surgery, every 3 months during the second year and thereafter every 6 months. Patients underwent chest X ray yearly, and other imaging assessments [ultrasonography (US), computed tomography (CT) scan, magnetic resonance imaging (MRI)] were planned according to eventual clinical signs or symptoms.
The follow-up period was calculated from the date of the first treatment to the last objective observation of the patient. The disease-free survival time (DFS) was calculated as the period of time between the date of the first treatment to the date of the first recurrence or last follow-up information. Cancer specific survival (CSS) was calculated as the period of time between date of onset of treatment to the date of death with active disease or last follow-up.
Study groups
According to the timing of ND, three groups were identified:
Group 1: en bloc resection;
Group 2: SND;
Group 3: DND.
Statistical analysis:
SPSS 10.0 (Statistical Package for Social Science) for Windows was used for all statistical analyses 13. Descriptive statistics were used to summarize study data. Statistical significance was defined as a two-tailed p value ≤ 0.05. Qualitative and quantitative statistical comparisons were performed using the Chi-Square and Student t tests, respectively. Survival curves were estimated using the Kaplan-Meier method. Survival data were censored for patients alive at the last observation. In addition, the survival data was censored for those dying without evidence of disease in consideration of CSS and those patients without recurrence in consideration of DSF. The Log-Rank test was used to compare survival outcomes.
Results
There were 141 men (73.4%) and 52 women. Tobacco use was reported in 93.8% of patients with initial clinical stage and in 98.2% of patients with advanced clinical stage. Tobacco and alcohol consumption were reported more frequently by the patients treated at HACC. Clinically, 42% of patients had CS I-II and 58% CS III-IV. The stage presentation was cT1, cT2, cN0 in 56% of IEO patients and 30% of HACC patients (Table I). As far as concerns the distribution of CS I-II patients, 50% were included in Group 1, and 50% in Groups 2-3 (Table II), while for CS III-IV patients, 92% were included in Group 1 and 8% in Group 2 (Table III).
Table I. Distribution of cases according to sociodemographic characteristics (stratified according to clinical stage).
| Clinical stages I and II | Clinical stages III and IV | |||||
| IEO | HACC | IEO | HACC | |||
| Mean age (yrs) | 52.5 | 60.7 | p = 0.009 | 50.4 | 54.1 | p = 0.168 |
| Sex (%) | ||||||
| Male | 60.4 | 78.1 | p = 0.098 | 70.3 | 81.6 | p = 0.174 |
| Female | 39.6 | 21.9 | 29.7 | 18.4 | ||
| Use of alcohol n. (%) | 10 (38.2) | 20 (65.3) | p = 0.001 | 13 (23.3) | 47 (64.5) | p = 0.009 |
| Use of tabacco n. (%) | 26 (59.1) | 26 (83.9) | p = 0.022 | 28 (75.7) | 63 (85.1) | p = 0.220 |
| T n. (%) | ||||||
| Tx | 1 (2.1) | 0 | p = 0.305 | 1 (2.7) | 0 | p = 0.001 |
| T1 | 15 (31.3) | 6 (18.75) | 3 (8.1) | 3 (3.9) | ||
| T2 | 32 (66.7) | 26 (81.25) | 23 (62.2) | 14 (18.4) | ||
| T3 | 4 (10.8) | 29 (38.2) | ||||
| T4 | 6 (16.2) | 30 (39.5) | ||||
| cN n. (%) | ||||||
| N0 | 48 (100) | 32 (100) | p = 0.411 | 1 (2.7) | 29 (38.2) | p = 0.002 |
| N1 | 24 (64.9) | 27 (35.5) | ||||
| ≥ N2 | 11 (29.7) | 20 (26.3) | ||||
| pN n. (%) | ||||||
| N0 | 32 (66.7) | 24 (75) | p = 0.426 | 10 (27) | 33 (43.4) | p = 0.092 |
| N+ | 16 (33.3) | 8 (25) | 27 (73) | 43 (56.6) | ||
| N. removed nodes (mean) | 39.4 | 34.0 | p = 0.067 | 37.5 | 50.2 | p = 0.001 |
Table II. Recurrences: distribution of cases according to neck treatment in early stages (stratified according to clinical stage and total population).
| Clinical stages I-II | ||||
| Local | Dissected neck | Untreated neck | Distant metastasis | |
| n. (%) | ||||
| Group 1 (n. = 39) | 5 (12.8) | 2 (5.1) | 2 (5.1) | 2 (5.1) |
| Group 2 (n. = 15) | (0) | (0) | (0) | 1 (6.7) |
| Group 3 (n. = 24) | 3 (12.5) | (0) | (0) | (0) |
Table III. Recurrences: distribution of cases according to neck treatment in advanced stages (stratified according to clinical stage and total population).
| Clinical stages III-IV | ||||||
| Local | Dissected neck | Untreated neck | Distant metastasis | Local + treat. neck | Local + dist. met. | |
| n. (%) | ||||||
| Group 1 (n = 105) | 21 (20.8) | 8 (7.9) | 3 (3.0) | 9 (8.9) | 5 (5.0) | 2 (2.0) |
| Group 2 (n = 9) | 2 (22.2) | 1 (11.1) | (0) | (0) | 1 (11.0) | (0) |
DND was performed on average 40 days (range 26-72 days) after removal of the primary tumour.
In CS I-II, 24 patients (30%) had pN+ and 25 (31%) underwent post-operative radiotherapy. Overall, 70 patients (61.9%) with advanced disease had pN+ and 75 CS III-IV patients underwent post-operative radiotherapy. As far as loco-regional recurrences are concerned, 17 cases (9.7%) had a relapse in the dissected neck and 6 of them also developed local recurrence. Two of them were CSI-II patients and both belonged to Group 1; 15 were CS III-IV and 13 underwent en bloc resection (Group 1) while 2 underwent SND (Group 2).
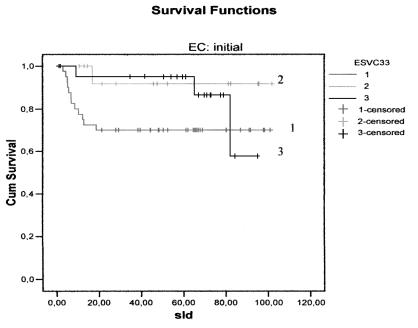
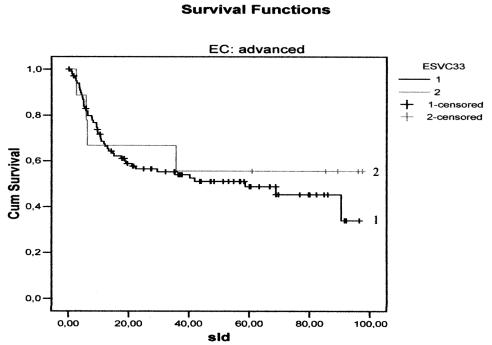
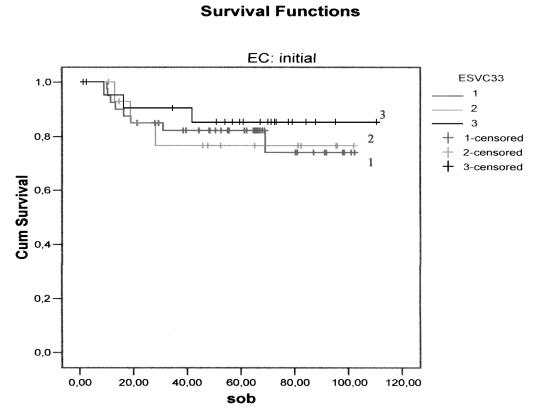
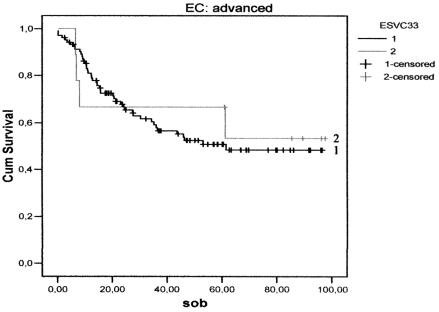
There were no differences, either in DFS (Figs. 1, 2) between the three groups, or in relation to the CS: CS I-II p = 0.10 and CS III-IV p = 0.59. Furthermore, no difference was found between the groups with regard to CSS (Figs. 3, 4) and stage: CS I-II (p = 0.78), CS III-IV (p = 0.80).
Fig. 1.
Disease-free survival curves for the three treatment groups in early stages: continuous (1); discontinuous simultaneous (2); discontinuous delayed (3).
Fig. 2.
Disease-free survival curves for the two treatment groups in advanced stages: continuous (1); discontinuous simultaneous (2).
Fig. 3.
Cancer-specific survival curves for the three treatment groups in early stages: continuous (1); discontinuous simultaneous (2); discontinuous delayed (3).
Fig. 4.
Cancer-specific survival curves for the two treatment groups in advanced stages: continuous (1); discontinuous simultaneous (2).
Discussion
Advanced oral cancers have a high risk of developing nodal metastases and there is general consensus in performing ND in all cases (cN0-3). Moreover, removal of these large tumours often requires a cervical approach allowing en bloc (in continuity) removal of the primary tumour and neck lymph nodes. On the other hand, small tumours (T1-T2) can be safely and reliably removed through a transoral approach. In these cases, treatment of the cN0 neck is discussed on account of the risk of occult metastatic nodes, which should be removed. Up to 30% of early oral SCCs have occult node metastases. Several Authors have studied risk factors for occult metastases and often deep infiltration is reported as the most significant 2 4 11 14–17. Spiro et al. 14, showed that deep infiltration > 2 mm of the tumour is highly predictive of the development of nodal metastases. O’Brien et al. 15 also evaluated tumour infiltration as a risk factor, but according to their experience the cut-off depth was 4 mm. Several other authors identified infiltration and muscle involvement predictive of nodal metastases although each of them evaluated a different cut-off (≥ 5 mm) 16 17. In the present study, both Institutions performed ND in cN0 patients, based on a tumour infiltration > 3 mm.
In small oral cancers, modalities and timing of treatment of cN0 nodes are debatable. The controversies include the approach to the primary tumour and the timing of ND (“wait and see”, sentinel lymph node biopsy, en bloc or discontinuous simultaneous, delayed). SND allows the oral and neck operative fields to be kept separate, with a faster recovery, fewer local complications and better functional results than the en bloc operation. However, the lymphatic network between the primary tumour and the neck is not completely removed and the risk of local recurrence is theoretically higher, moreover several authors stress the prognostic relevance of sublingual nodes, which could not be removed with the discontinuous approach 18–20. Spiro and Strong 9 in 1973 suggested that in oral tumours, operable through a trans-oral approach, en-bloc operation might not be performed. They studied 229 patients treated for tongue SCC and found the same oncological outcome in the three treatment schedules: 1) trans-oral glossectomy without ND (n = 185); 2) en block operation (n = 103), and trans-oral glossectomy + discontinuous simultaneous ND (n = 41). This study presented a bias due to different staging in the groups: T2/3 cases in group 2 = 65.8% and 6.38%, respectively, and in group 3 = 59.2% and 37.8%.
In 1991, Leemans et al. 8 published results of a clinical trial on 61 patients with cT2 oral tongue and floor of the mouth SCC. Patients were randomized into two arms: a) trans-oral resection and SND (n = 27), b) continuous simultaneous removal of the primary and ND (n = 34). OS was 80.2% in group b vs. 67.1% in group a (p < 0.0236).
In 1995, Eckel et al. 21 treated oral and oropharyngeal SCCs with laser trans-oral resection and discontinuous delayed ND (1-3 weeks after removal of the primary lesion). Five-year specific survival was 81% in Stages I-II patients with oral cancer and 86% in those with oropharyngeal cancer, respectively; 73% and 65% in stage III oral and oropharyngeal cancers, respectively, and 21% in all Stage IV patients regardless of the site. This study, however, did not evaluate the possible differences related to different treatment of neck nodes.
In the present study, the population of the two Institutions differed regarding sex (males 64.7% at IEO vs. 80.55% at HACC) and habits: 29.67% of Italian patients drank alcohol vs. 65% of Brazilian patients while 66.6% of IEO patients smoke tobacco vs. 84.79% of Brazilian patients. Regarding the clinical stage, HACC had a larger number of patients with advanced (CS III-IV) disease (67%). This difference can be explained by the socio-economic differences between the two countries, already discussed by the Authors 22. Because of this difference, we grouped all patients into two categories, initial (CS I-II) and advanced (CS III-IV), in order to compare the three different treatment policies.
As far as concerns treatment of the primary tumour, there were no significant differences between the two Institutions. Regarding neck dissection, in small tumours, HACC used SND vs. DND used at IEO. The latter was justified, based on the concept that cells take approximately 30-40 days to go from the primary to the neck lymph nodes; if this hypothesis is correct, discontinuous dissection performed more than one month after removal of the primary tumour fails to remove in transit cells, thus allowing development of local recurrence within the undissected tract between the primary tumour and the neck lymph nodes (T-N tract).
Indications for post-operative RT were similar in both Institutes: advanced cases (pT3-pT4 HACC, pT4 IEO), positive margins and extra-capsular spread.
No differences were found between the groups as far as concerns DFS, either in the early stage (p = 0.10), or in the patients with advanced stages (p = 0.59) who underwent discontinuous dissection (only 9 patients). Also no difference was found regarding the surgical technique, in the early (p = 0.78) and the advanced stages (p = 0.80) or in the number and site of local and regional recurrences when patients were stratified according to clinical stage.
Based on this retrospective study type and timing of neck dissection (en-bloc, SND or DND) seem not to affect oncological results; these data should be confirmed by a prospective randomized trial.
Presented at 11th International Congress on Oral Cancer, 2006. Grado, Italy. Study funded by a Grant from the Umberto Veronesi Foundation
References
- 1.Shah J, Patel SG. Head and Neck Surgery & Oncology. 3rd Edition. Edinburgh: Mosby 2003. [Google Scholar]
- 2.Zbaren P, Nuyens M, Caversaccio M, Stauffer E. Elective neck dissection for carcinomas of the oral cavity: occult metastases, neck recurrences, and adjuvant treatment of pathologically positive necks. Am J Surg 2006;191:756-60. [DOI] [PubMed] [Google Scholar]
- 3.Kaya S, Yilmaz T, Gursel B, Sarac S, Sennaroglu L. The value of elective neck dissection in treatment of cancer of the tongue. Am J Otolaryngol 2001;22:59-64. [DOI] [PubMed] [Google Scholar]
- 4.De Zinis LO, Bolzoni A, Piazza C, Nicolai P. Prevalence and localization of nodal metastases in squamous cell carcinoma of the oral cavity: role and extension of neck dissection. Eur Arch Otorhinolarygol 2006;263:1131-5. [DOI] [PubMed] [Google Scholar]
- 5.Amar A, Curioni OA, Franzi SA, Ortelado DK, Rapaport A. Neck dissection in squamous cell carcinoma of the tongue. Rev Bras Otorrinolaringol (Engl Ed) 2005;71:29-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crean SJ, Hoffman A, Potts J, Fardy MJ. Reduction of occult metastatic disease by extension of the supraomohyoid neck dissection to include level IV. Head Neck 2003;25:758-62. [DOI] [PubMed] [Google Scholar]
- 7.Vartanian JG, Pontes E, Agra IM, Campos OD, Goncalves-Filho J, Carvalho AL, et al. Distribution of metastatic lymph nodes in oropharyngeal carcinoma and its implications for elective treatment of the neck. Arch Otolaryngol Head Neck Surg 2003;129:729-32. [DOI] [PubMed] [Google Scholar]
- 8.Leemans CR, Rammoban T, Nauta JJP, Snow GB. Discontinuous vs. in-continuity neck dissection in carcinoma of the oral cavity. Arch Otolaryngol Head Neck Surg 1991;117:1003-6. [DOI] [PubMed] [Google Scholar]
- 9.Spiro RH, Strong EW. Discontinuous partial glossectomy and radical neck dissection in selected patients with epidermoid carcinoma of the mobile tongue. Am J Surg 1973;126:544-6. [DOI] [PubMed] [Google Scholar]
- 10.Crile G. Excision of cancer of the head and neck, with special reference to the plan of dissection based on one hundred and thirty-two operations. JAMA 1906;47:1780-6. [DOI] [PubMed] [Google Scholar]
- 11.Dunne AA, Folz BJ, Kuropkat C, Werner JA. Extent of surgical intervention in case of N0 neck in head and neck cancer patients: an analysis of data collection of 39 hospitals. Eur Arch Otorhinolaryngol 2004;261:295-303. [DOI] [PubMed] [Google Scholar]
- 12.American Joint Committee on Cancer. Cancer Staging Handbook. TNM classification of malignant tumors. 6th edn. New York: Springer; 2002. [Google Scholar]
- 13.SPSS. Statistical package for social science, statistical data analysis for Windows. Chicago: SPSS Inc.; 2000. [Google Scholar]
- 14.Spiro RH, Huvos AG, Wong GY, Spiro JD, Gnecco CA, Strong EW. Predictive value of tumor thickness in squamous carcinoma confined to the tongue and floor of the mouth. Am J Surg 1986;155:345-50. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien CJ, Lauer CS, Fredricks S, Clifford AR, McNeil EB, Bagia JS, et al. Tumor thickness influences prognosis of T1 and T2 oral cavity cancer – but what thickness? Head Neck 2003;25:937-45. [DOI] [PubMed] [Google Scholar]
- 16.Fukano H, Matsuura H, Hasegawa Y, Nakamura S. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck 1997;19:205-10. [DOI] [PubMed] [Google Scholar]
- 17.Pimenta Amaral TM, Da Silva Freire AR, Carvalho AL, Pinto CA, Kowalski LP. Predictive factors of occult metastasis and prognosis of clinical stages I and II squamous cell carcinoma of the tongue and floor of the mouth. Oral Oncol 2004;40:780-6. [DOI] [PubMed] [Google Scholar]
- 18.Calabrese L, Renne G, De Cicco C, Chiesa F. Metastatic cancer to the floor of mouth: the lingual lymph nodes (Letter to the Editor). Head Neck 2003;25:341-3. [DOI] [PubMed] [Google Scholar]
- 19.Dutton JM, Graham SM, Hoffman HT. Metastatic cancer to the floor of mouth: the lingual lymph nodes. Head Neck 2002;24:401-5. [DOI] [PubMed] [Google Scholar]
- 20.Ozeki S, Tashiro H, Okamoto M, Matsushima T. Metastasis to the lingual lymph node in carcinoma of the tongue. J Maxillofac Surg 1985;13:277-81. [DOI] [PubMed] [Google Scholar]
- 21.Eckel HE, Volling P, Pototschnig C, Zorowka P, Thumfart W. Transoral laser resection with staged discontinuous neck dissection for oral cavity and oropharynx squamous cell carcinoma. Laryngoscope 1995;105:53-60. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho AL, Singh B, Spiro RH, Kowalski LP, Shah JP. Cancer of the oral cavity: a comparison between institutions in a developing and a developed nation. Head Neck 2004;26:31-8. [DOI] [PubMed] [Google Scholar]