Summary
Our group has 25 years’ experience in the use of molecular predictive markers in head and neck cancer, on a large patient population, enrolled from a single institution, with a long follow-up, and, most of all, homogeneous regarding histology (squamous cell carcinoma) and site (larynx). Among the most frequent malignancies in the US, cancers of the larynx and uterine corpus are the only types not showing an increase in 5-year Survival Rates over the last 30 years. As far as concerns laryngeal squamous cell carcinoma, we can identify several potential reasons for this failure, the most relevant probably lies in the neck. For this reason, a key issue in laryngeal oncology is to assess metastatic potential of squamous cell carcinoma at diagnosis. Nevertheless, the combination of clinical and histological parameters is not sufficiently reliable in the prediction of lymph node metastases. Molecular characterization, by the study of molecular predictive factors, is a clinical approach aimed to define homogeneous subgroups for clinical metastatic behaviour. Defining invasiveness by means of studies on selected molecular markers (among which the most reliable is probably Epidermal Growth Factor Receptor (EGFR)) may be useful in the choice of the most appropriate treatment on both T and on N.
Keywords: Laryngeal carcinoma, Neck metastases, Molecular markers
Riassunto
Viene discussa l’esperienza monocentrica dell’uso di fattori predittivi molecolari su una casistica numerosa ed omogenea di pazienti con carcinomi laringei. Questi carcinomi non hanno avuto un miglioramento prognostico significativo negli ultimi trent’anni. La causa è verosimilmente riferibile alle ricadute sul collo. Per questo risulta indispensabile caratterizzare la aggressività biologica della neoplasia integrando informazioni desunte dallo studio di marcatori predittivi clinici e molecolari. Fra questi il più affidabile è probabilmente il fattore EGFR.
25 years’ experience
Our group has 25 years’ experience in the use of molecular predictive markers in head and neck cancer, on a large patient population, enrolled from a single institution, with a long follow-up, and, most of all, homogeneous regarding histology (squamous cell carcinoma – SCC) and site (larynx) 1–11.
In our opinion, this may be a definite advantage, avoiding the bias derived from collecting together all head and neck SCCs. In fact, although the larynx is considered a site of the head and neck, several peculiarities, of both a clinical and a molecular nature, can be highlighted. The statistics of the American Cancer Society classify larynx as part of the respiratory system, separately from the oral cavity and pharynx 12. As for the incidence, the male/female ratio is markedly higher than in the other sites of the head and neck 12. Differences in chromosomal pattern and carcinogenic progression between laryngeal squamous cell carcinoma (LSCC) and the other head and neck squamous cell carcinomas (HNSCCs) have been detected by comparative genomic studies 13. In particular, p53 is normally expressed in LSCC more frequently and p53 gene has a mutation pattern more closely resembling lung SCCs than other HNSCCs 14.
Improving survival in LSCC: a missed target
LSCCs represent the vast majority (approximately 96%) of laryngeal malignancies 15. Other histologic types have not been taken into consideration in the present report.
Of the most frequent malignancies in the US, cancers of the larynx and the uterine corpus are the only types not presenting an increase in the Five-year Survival Rates during the last 30 years 12. As for LSCC, we can identify several potential reasons for this failure, probably the most relevant lying in the neck:
TNM classification appears, in some cases, inadequate. For example, it has been observed that regrouping cases in stages III and IV into locally advanced disease vs. regional metastasis appears to predict survival better 15. In fact, neck metastasis remains the first cause of treatment failure and death in LSCC 16. Thus, in our opinion, the treatment of the neck should be the primary concern of every head and neck oncologist;
despite the multiplicity of clinical prognostic factors, the only consistent clinical predictors for disease control and disease-specific survival in LSCC are T and, to a greater extent, N 17–19. The prognostic stratification of LSCC patients is inadequate since similar patients, affected by tumours with similar clinico-pathological parameters, and undergoing the same treatment, may differ considerably in prognosis. This is probably due to the extreme biological heterogeneity of LSCCs and contributes to a lack of consistency in treatment planning 20;
an example of this lack of consistency is the management of cervical lymph nodes, which is the most important component of the overall treatment strategy, especially for supraglottic tumours. Surgery remains the mainstay of neck treatment since it provides comprehensive clearance of all grossly enlarged lymph nodes and allows accurate histological information to be obtained also concerning micrometastases in the clinically negative neck. Nevertheless, while the indications for comprehensive surgical clearance of the neck, for clinically palpable metastatic lymph nodes (cN+), are obvious, the indications for elective selective treatment of cN0 neck appear less clear 19 21–23.
Clinical predictive markers of neck node metastases
For the above-mentioned reasons, a key issue in head and neck, and laryngeal oncology in particular, is to assess the metastatic potential of SCCs at diagnosis.
Differences in the natural histories of the various SCCs of the larynx, as for neck metastasis, are related, according to our present knowledge, to the anatomy and to the lymphatic drainage patterns of the respective subsite(s).
The paucity of lymphatic drainage of the true vocal cords, in all areas other than the posterior commissure, makes metastasis of early lesions extremely unlikely. As for the rare primitive subglottic cancers, the incidence of cervical metastasis, in this group of cancers, is reported to be 20-30%, but that figure is somewhat obscured by the fact that the primary drainage pattern of these lesions is to the less detectable pre-tracheal and para-tracheal nodes. The incidence of metastasis may, therefore, be significantly higher 24 25.
Albeit, neck node metastasis is mainly a ‘supraglottic issue’. In fact, because of the profuse lymphatic network of the supraglottic larynx, carcinomas of this area metastasize frequently to the cervical lymph nodes, and failure of treatment is usually a result of metastasis rather than local disease 26–28. The incidence of patients with clinically positive lymph nodes at the time of diagnosis is 23-50% for all supraglottic sites and stages combined 29–32. A substantial number of those patients with clinically negative necks are found to have histologic disease, as demonstrated when neck dissection is performed, or, if left untreated, they convert to clinically positive necks 33 34. In supra-glottic cancers, the probability of cervical metastasis and the probability of delayed contralateral metastasis increase in direct proportion to the size of the primary lesion (i.e., the T stage) 35–37. Lindberg 26 reported impressive overall metastatic rates with various supraglottic carcinomas: 63% of T1, 70% of T2, 79% of T3, and 73% of T4 cases metastasized.
In patients with supraglottic lesions presenting with a clinically positive cervical node 2 cm in diameter or more, the possibility for contralateral neck metastasis is 40% or higher 24. The epiglottis is particularly prone to bilateral metastasis, and even in smaller lesions of that site, the incidence of contralateral metastasis is > 20% 38.
Nevertheless, it has been observed that the localization of SCCs does not totally account for their clinical behaviour. In fact, glottic tumours extending or recurring in the supraglottic region have a markedly lower proclivity to neck metastasis than primitive supraglottic SCCs.
Other “spatial” factors influencing metastatic tendency have been hypothesized to be location (central vs. marginal), volume, T-stage, growth modalities (exophytic vs. endophytic).
Nevertheless, the combination of these parameters is not adequately reliable in the prediction of lymph node metastases. In fact, clinically homogeneous LSCCs can be characterized by a different behaviour.
Histological predictive markers of neck node metastases
Histopathological features of tumours have been evaluated with the aim to assess correlations with metastatic potential.
It has been observed that primitive supraglottic lesions are more likely to be non-keratinizing and poorly differentiated, and they have more aggressive local behaviour in general 35. Those lesions of the vocal cords, on the other hand, are more often well differentiated and tend to be less aggressive locally. Although the degree of cellular differentiation is not thought to be the most significant fact in tumour grading, it has been reported to correlate with the probability of cervical metastasis 39–42, which, in turn, has a strong impact on survival 43. On the other hand, several molecular markers of differentiation have so far been studied in relation with propensity to neck metastasis and relapse-free survival, with often very promising results 44–48 (see below). Other local characteristics, such as tumour-host interface 35 40, peritumour inflammatory response 49, and vascular and perineural invasion 50, also seem important in determining performance. Finally, the actual tumour thickness and depth of invasion almost certainly have an influence on metastasis and, ultimately, on survival. A variety of studies have attempted to standardize the predictive value of thickness in SCCs of the upper aerodigestive tract with the probability of cervical metastasis and, therefore, prognosis 49 51–54. Although head and neck oncologists, for some time, intuitively favoured a direct correlation between the two, a number of studies have failed to demonstrate a statistically significant association between tumour thickness and nodal metastasis 38 50 55. Furthermore, it should be pointed out that those studies demonstrating a correlation between thickness and metastasis generally focused on sites other than the larynx, and because of the anatomic complexity and embryologic uniqueness of the larynx, one cannot necessarily transpose such data from other head and neck organs.
In conclusion, histopathologic parameters are not employed, at present, in treatment planning and prognostic assessment in laryngeal cancer, also because of the difficulties in the standardization of methods and, therefore, the low reproducibility of results. Furthermore, in our opinion, a notable biological heterogeneity can hide behind up histologically homogeneous SCCs of the larynx and after all accounts for the different clinical behaviours.
Molecular characterization
Molecular, by the study of molecular predictive factors, is a clinical approach aimed to define more homogeneous groups of patients for treatment selection; it represents an attempt to overcome the well-known lack of consistency in the choice of treatment and to eventually improve overall survival. Even if a plethora of reports have attempted to evaluate their potential clinical role, no molecular marker contributes, at present, to the clinical decision-making process.
The perfect marker for molecular characterization of LSCC may not always be present in malignant cells, but invariably associated with precise biological features and a predictable clinical behaviour, and easily detectable by a standardized, reliable and simple assay on a small sample, such as a biopsy specimen. So far, no such marker has been described.
In previous reports 10 56, we hypothesized that the search for three or four well-defined characterizing biological markers might allow us to classify tumours as positive (Mc+) or negative (Mc-), at molecular characterization.
TNM staging would thus become TNM-Mc staging. This would result in a better prognostic stratification of patients and the most suitable individualized (“a la carte”) treatment could be selected. It would prevent overtreatment of Mc- patients and, most importantly, the undertreatment of Mc+ patients, which has probably contributed to the above-mentioned failure in improving LSCC prognosis in the last 30 years.
Furthermore, by means of selected and specific molecular markers, we can try to separately assess some specific biological/clinical features of tumours, such as aggressiveness, invasiveness, radio- and chemo-sensitivity. Here, we define tumour aggressiveness as the tendency to local disease progression. Invasiveness is the intrinsic tendency of tumours to metastasise. Our group evaluated several potential markers of invasiveness (Table I) (see below). These obviously have a great impact on prognosis and would lead to modifications in therapeutic decisions, offering information to define the most suitable management of the neck, both in N0 and N+.
Table I. Molecular markers of invasiveness evaluated on patients from a single institution.
| Molecular marker | Function in the normal cells | Alterations in tumour cells |
| Epidermal Growth Factor Receptor | Receptor for growth factors (TGF and EGF) with tyrosine kinase activity. Upstream activator of MAPkinase pathway and of other pathways involved in cell growth, cell migration, block of apoptosis (Fig. 1). | Frequently and early overexpressed in LSCC, mainly by post– translational mechanisms. At present, the most reliable biological marker for molecular characterization. Marker of aggressiveness4,7 and of invasiveness 1. |
| Overexpression and amplification of cyclin D1 gene (CCND1) | Cyclin D1 gene transcriptional activity normally strictly depends on mitogen stimulation, and leads to cell commitment to mitosis through START checkpoint. | Early CCND1 overexpression is often detectable without evidence of gene amplification, it can be used for molecular epidemiology but it seems to retain a lower prognostic value, if compared with CCND1 amplification, marker of aggressiveness in LSCC 5. Its role as marker of invasiveness is still unproven. |
| Cathepsin D | Lytic enzyme active in extracellular matrix rearrangement | Overexpression is often detectable in tumour cells, where it seems to contribute to invasiveness 8. |
| S100–A2 Ca2+ binding protein | Increasing levels of expression during differentiation of squamous epithelial cells; absent in basal layers. | Underexpression in cancer cells, inversely proportional to tumour differentiation. Starting from data concerning NSCLC, a role as a real oncosuppressor has been hypothesized 44. It may act as a marker both of aggressiveness and of invasiveness. |
| Methyl–p– hydroxyphenyllactate esterase (MEPHLase) activity | Enzyme involved in the metabolism of methyl–p–hydroxyphenyllactate, ligand of type II EBS, with a role in growth and differentiation of several tissues (breast, uterus), normally expressed in larynx. | In LSCC, a low activity is associated with poor differentiation, and shorter overall survival and metastasis–free survival 85. |
| Type 2 cyclo– oxigenase 2 (Cox–2) | Enzyme involved in arachidonic acid metabolism and autacoid synthesis, induced by various stimuli in several cell types. Inhibited by FANS. | Cox–2 activity seems to promote tumour neoangiogenesis. Nevertheless, evidence exists showing that low Cox–2 expression indicates poor differentiation and higher |
In fact, management of cervical lymph nodes represents a vitally important component of the overall approach to patients with LSCC. Surgery remains the mainstay of treatment for cervical lymph nodes since it provides comprehensive clearance of all grossly enlarged lymph nodes and allows accurate histological information to be obtained concerning micrometastases in the clinically negative neck. Nevertheless, while the indication for comprehensive surgical clearance of the neck with clinically palpable metastatic lymph nodes (cN+) is obvious, the indication for elective selective treatment of clinically negative neck (cN0) is less clear 21–23 57.
In Mc-, “a wait and see” (observation) approach to cN0 tumours and even selective, rather than comprehensive, ipsilateral neck dissection, without elective contralateral neck dissection, in cN1 tumours, could be justified.
In Mc+, the clinically negative neck would be managed by a more aggressive approach involving elective bilateral neck dissection or elective irradiation, also considering that invasive tumours may be prone to N2 neck recurrences, with a markedly lower salvageability than N1 16. In cN1 patients, comprehensive ipsilateral neck dissection, with elective selective contralateral neck dissection might be hypothesized. Adjuvant radiotherapy could be recommended also in tumours with histologically negative resection margins, and/or in stage pN1 without extracapsular spread. On the other hand, in N1 patients a planned neck dissection treated by chemoradiation may be recommended. Extracapsular spread and N2-3 spread have been shown to be important prognostic indicators for distant metastasis (DM), in large retrospective reviews 58 59. Although induction chemotherapy did not improve loco-regional recurrence, a trend suggesting decreased rates of distant metastases was seen for patients treated with some chemotherapy protocols 60–63. Positive markers of invasiveness might further suggest the indication for induction or adjuvant chemotherapy in order to decrease DMs, which, in some subsets of advanced disease, are also a relevant issue.
Several features of SCCs have been evaluated, at molecular level, in order to assess their correlation with clinical behaviour in terms of aggressiveness, chemo-radiosensitivity and, in particular, invasiveness.
Among the markers evaluated so far, some appear to be potentially reliable and suitable, from a clinical viewpoint.
Characterizing molecular markers of invasiveness
In the last few years, cDNA microarrays, which can be a powerful tool from which large amounts of genetic information can be obtained, have already been used for an initial attempt at molecular classification based on patterns of global gene expression in HNSCC 64 65. However, thus far, this technology can only be applied to frozen tissues, because RNA is destroyed during the formalin-fixation process. Therefore, one would need a frozen tumour bank in conjunction with a powerful clinical database and complex statistical analytical ability to make use of this expensive technology.
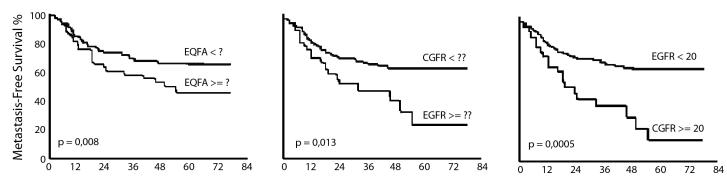
The Epidermal Growth Factor Receptor (EGFR) belongs to the Type I receptor tyrosine kinase family. This family includes four members: EGFR (also known as erbB1/HER1), erbB-2/Neu/HER2, erbB-3/HER3 and erbB-4/HER4. EGFR is a 170 kDa transmembrane glycoprotein with great homology with the avian erythroblastosis virus-transforming protein v-erbB. The effects of EGFR activation lead to generation of intracellular second messengers and different biological responses, such as cell proliferation, gene transcription and other cellular activities through several downstream signalling pathways 66 67. There is provocative evidence supporting a strong role for EGFR expression (and, to a lower extent, for its ligand, tumour growth factor alpha TGFα) in predicting prognosis of LSCC, as it adversely influences both overall, relapse-free and, in particular, regional metastasis-free, survival in LSCC 1 (Fig. 1). Such a strong predictive value is retained by EGFR independently of treatment (surgery, chemotherapy and radiation) 1 4 7 68–71 and, in our opinion, EGFR the most reliable prognostic molecular marker, at present.
Fig. 1.
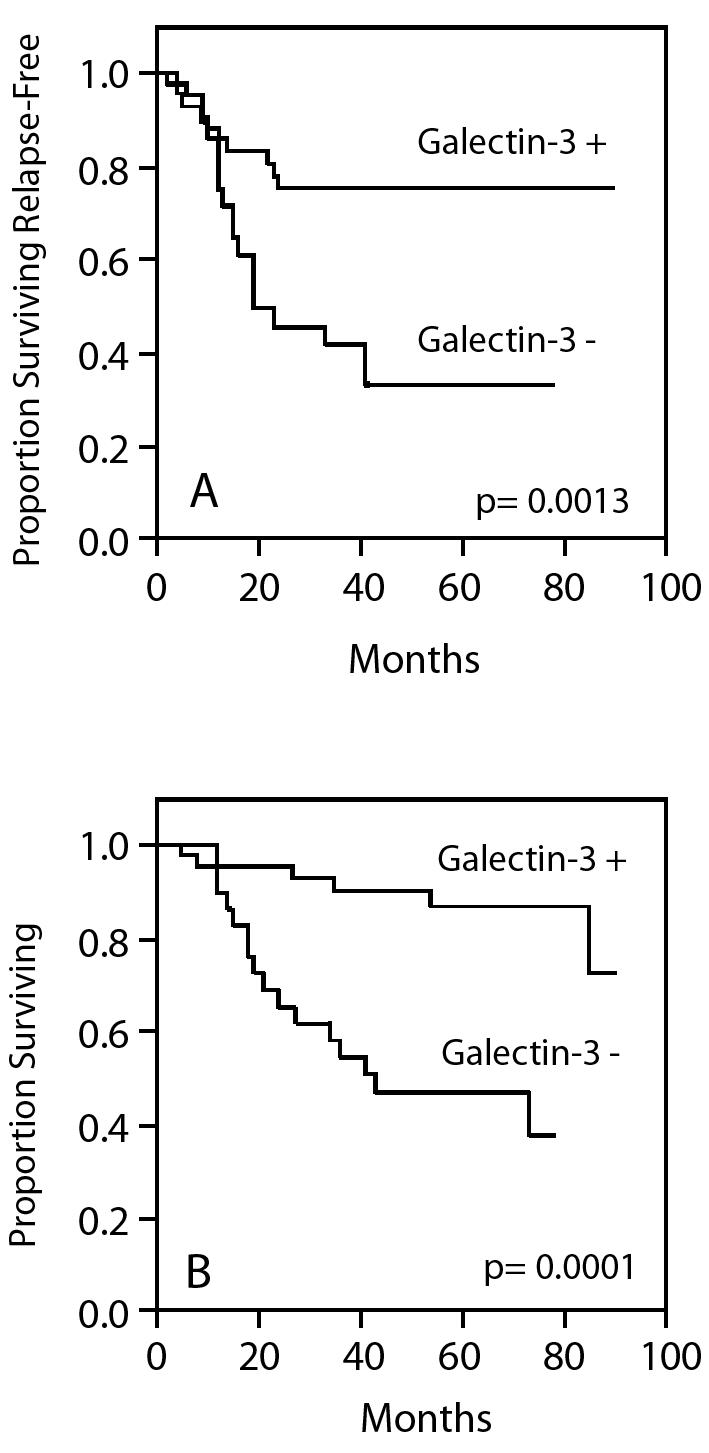
Regional metastasis–free and overall survival in N0 LSCC according to galectin–3 tumour immunostaining.
Moreover an overexpression of EGFR is associated with an increased degradation of the extracellular matrix by metalloproteases and cathepsin D, which plays an important role in tumour growth, invasion and metastasis, as well as in tumour-induced angiogenesis 8 11 72, and is potentially correlated with invasiveness.
Markers of epithelial differentiation, such as laminin-5, galectin-3, Cox-2, have been evaluated in order to integrate the classical histological evaluation and have shown a predictive value of neck node relapse (Fig. 2) 46–48. S100 A2, which also seems to have a role as predictor of neck node relapse in N0 necks, has been recently hypothesized not merely as a differentiation marker, but as a real oncosuppressor with a prognostic significance stronger than the simple histopathological grading 44 45.
Fig. 2.
Regional metastasis–free survival in LSCC according to EGFR status (predictive value increases with higher cut–off).
Alterations of p53 protein expression and mutations of p53 gene have been extensively studied for the evaluation of their predictive role. P53 alterations have been proposed as independent predictors of recurrence in LSCC 73 74, but this prognostic value seems controversial 14, especially in surgically treated patients 75. P53 overexpression, detected by immunohistochemistry (IHC) in a high percentage of LSCCs 76, was hypothesized to correlate well with p53 mutation 77, but a recent study documented significant disagreement between p53 IHC and genotyping data 78. P53 gene mutation has been hypothesized to be more reliable than IHC overexpression for characterization and it has been reported to predict the reponse to radiotherapy in LSCC patients 78. This is consistent with the biological role of p53, which mediates apoptosis associated with DNA damage. Nevertheless, evidence of a correlation with overall, and most of all, regional metastasis-free survival is still lacking 14 75.
Other promising markers of invasiveness in LSCC may be nm23-H1 protein 79 80, PCNA 81 82, p27 83, CD44H 84.
However, various problems have prevented the clinical application of molecular markers for tumour characterization. First of all, the perfect marker for molecular characterization, as described above, remains to be demonstrated. In particular, detection assays must be practical and reliable and should be readily available. The inconsistency of assay methods for the most studied factors, as well as patient and treatment heterogeneity, all contribute to the impossibility to draw definitive conclusions. It is necessary to further evaluate the most promising molecular marker proposed for clinical practice both by a metanalysis of data present in the literature and by multidisciplinary and multicentric clinical trials.
Conclusion
At present, we are probably at a crossroad: the time for the integration of molecular markers in prognostic assessment and treatment selection, as already observed, for example, in breast cancer, is probably very close. Moreover, this perspective appears very intriguing inasmuch as it may be the key to overcoming the above-mentioned lack of consistency in treatment selection, in particular for neck management 21 23, which remains the most controversial issue both in N0 (in particular if the primary tumour is supraglottic) and in N+ LSCCs.
References
- 1.Almadori G, Cadoni G, Galli J, Ferrandina G, Scambia G, Exarchakos G, et al. Epidermal growth factor receptor expression in primary laryngeal cancer: an independent prognostic factor of neck node relapse. Int J Cancer 1999;84:188-91. [DOI] [PubMed] [Google Scholar]
- 2.Scambia G, Panici PB, Battaglia F, Ferrandina G, Almadori G, Paludetti G, et al. Receptors for epidermal growth factor and steroid hormones in primary laryngeal tumours. Cancer 1991;67:1347-51. [DOI] [PubMed] [Google Scholar]
- 3.Scambia G, Catozzi L, Benedetti PP, Ferrandina G, Almadori G, Paludetti G, et al. Expression of ras oncogene p21 protein in normal and neoplastic laryngeal tissues: correlation with histopathological features and epidermal growth factor receptors. Br J Cancer 1994;69:995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurizi M, Scambia G, Benedetti PP, Ferrandina G, Almadori G, Paludetti G, et al. EGF receptor expression in primary laryngeal cancer: correlation with clinico-pathological features and prognostic significance. Int J Cancer 1992;52:862-6. [DOI] [PubMed] [Google Scholar]
- 5.Bellacosa A, Almadori G, Cavallo S, Cadoni G, Galli J, Ferrandina G, et al. Cyclin D1 gene amplification in human laryngeal squamous cell carcinomas: prognostic significance and clinical implications. Clin Cancer Res 1996;2:175-80. [PubMed] [Google Scholar]
- 6.Hohaus S, Cavallo S, Bellacosa A, Genuardi M, Galli J, Cadoni G, et al. Telomerase activity in human laryngeal squamous cell carcinomas. Clin Cancer Res 1996;2:1895-900. [PubMed] [Google Scholar]
- 7.Maurizi M, Almadori G, Ferrandina G, Distefano M, Romanini ME, Cadoni G, et al. Prognostic significance of epidermal growth factor receptor in laryngeal squamous cell carcinoma. Br J Cancer 1996;74:1253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurizi M, Almadori G, Cadoni G, Scambia G, Ottaviani F, Ferrandina G, et al. Cathepsin D concentration in primary laryngeal cancer: correlation with clinico-pathological parameters, EGFR status and prognosis. Int J Cancer 1996;69:105-9. [DOI] [PubMed] [Google Scholar]
- 9.Almadori G, Cadoni G, Cattani P, Posteraro P, Scarano E, Ottaviani F, et al. Detection of human papilloma virus DNA in laryngeal squamous cell carcinoma by polymerase chain reaction. Eur J Cancer 1996;32A:783-8. [DOI] [PubMed] [Google Scholar]
- 10.Almadori G, Bussu F, Cadoni G, Galli J, Paludetti G, Maurizi M. Molecular markers in laryngeal squamous cell carcinoma: towards an integrated clinicobiological approach. Eur J Cancer 2005;41:683-93. [DOI] [PubMed] [Google Scholar]
- 11.Ferrandina G, Scambia G, Benedetti PP, Almadori G, Paludetti G, Cadoni G, et al. Cathepsin D in primary squamous laryngeal tumours: correlation with clinico-pathological parameters and receptor status. Cancer Lett 1992;67:133-8. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin 2006;56:106-30. [DOI] [PubMed] [Google Scholar]
- 13.Huang Q, Yu GP, McCormick SA, Mo J, Datta B, Mahimkar M, et al. Genetic differences detected by comparative genomic hybridization in head and neck squamous cell carcinomas from different tumour sites: construction of oncogenetic trees for tumour progression. Genes Chromosomes Cancer 2002;34:224-33. [DOI] [PubMed] [Google Scholar]
- 14.Bosch FX, Ritter D, Enders C, Flechtenmacher C, Abel U, Dietz A, et al. Head and neck tumour sites differ in prevalence and spectrum of p53 alterations but these have limited prognostic value. Int J Cancer 2004;111:530-8. [DOI] [PubMed] [Google Scholar]
- 15.Shah JP, Karnell LH, Hoffman HT, Ariyan S, Brown GS, Fee WE, et al. Patterns of care for cancer of the larynx in the United States. Arch Otolaryngol Head Neck Surg 1997;123:475-83. [DOI] [PubMed] [Google Scholar]
- 16.Shah JP, Patel KJ. Head and Neck Surgery and Oncology. 3rd Ed., Edinburgh: Mosby 2003. [Google Scholar]
- 17.Licitra L, Bernier J, Grandi C, Locati L, Merlano M, Gatta G, et al. Cancer of the larynx. Crit Rev Oncol Hematol 2003;47:65-80. [DOI] [PubMed] [Google Scholar]
- 18.Franchin G, Minatel E, Gobitti C, Talamini R, Vaccher E, Sartor G, et al. Radiotherapy for patients with early-stage glottic carcinoma: univariate and multivariate analyses in a group of consecutive, unselected patients. Cancer 2003;98:765-72. [DOI] [PubMed] [Google Scholar]
- 19.Spector JG, Sessions DG, Haughey BH, Chao KS, Simpson J, El Mofty S, et al. Delayed regional metastases, distant metastases, and second primary malignancies in squamous cell carcinomas of the larynx and hypopharynx. Laryngoscope 2001;111:1079-87. [DOI] [PubMed] [Google Scholar]
- 20.Ferlito A, Bradley PJ, Rinaldo A. What is the treatment of choice for Tl squamous cell carcinoma of the larynx? J Laryngol Otol 2004;118:747-9. [DOI] [PubMed] [Google Scholar]
- 21.Ferlito A, Rinaldo A, Silver CE, Gourin CG, Shah JP, Clayman GL, et al. Elective and therapeutic selective neck dissection. Oral Oncol 2006;42:14-25. [DOI] [PubMed] [Google Scholar]
- 22.Ferlito A, Rinaldo A, Silver CE, Shah JP, Suarez C, Medina JE, et al. Neck dissection: then and now. Auris Nasus Larynx 2006;33:365-74. [DOI] [PubMed] [Google Scholar]
- 23.Wei WI, Ferlito A, Rinaldo A, Gourin CG, Lowry J, Ho WK, et al. Management of the N0 neck – reference or preference. Oral Oncol 2006;42:115-22. [DOI] [PubMed] [Google Scholar]
- 24.Som ML. Conservation surgery for carcinoma of the supraglottis. J Laryngol Otol 1970;84:655-78. [DOI] [PubMed] [Google Scholar]
- 25.Harrison DF. The pathology and management of subglottic cancer. Ann Otol Rhinol Laryngol 1971;80:6-12. [DOI] [PubMed] [Google Scholar]
- 26.Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer 1972;29:1446-9. [DOI] [PubMed] [Google Scholar]
- 27.Crissman JD. Laryngeal keratosis and subsequent carcinoma. Head Neck Surg 1979;1:386-91. [DOI] [PubMed] [Google Scholar]
- 28.Levendag P, Vikram B. The problem of neck relapse in early stage supraglottic cancer – results of different treatment modalities for the clinically negative neck. Int J Radiat Oncol Biol Phys 1987;13:1621-4. [DOI] [PubMed] [Google Scholar]
- 29.Ogura JH, Sessions DG, Spector GJ. Conservation surgery for epidermoid carcinoma of the supraglottic larynx. Laryngoscope 1975;85:1808-15. [DOI] [PubMed] [Google Scholar]
- 30.Kirchner JA, Owen JR. Five hundred cancers of the larynx and pyriform sinus. Results of treatment by radiation and surgery. Laryngoscope 1977;87:1288-303. [DOI] [PubMed] [Google Scholar]
- 31.Hansen HS. Supraglottic carcinoma of the aryepiglottic fold. Laryngoscope 1975;85:1667-81. [DOI] [PubMed] [Google Scholar]
- 32.Shah JP, Tollefsen HR. Epidermoid carcinoma of the supraglottic larynx. Role of neck dissection in initial surgical treatment. Am J Surg 1974;128:494-9. [DOI] [PubMed] [Google Scholar]
- 33.Ogura JH, Biller HF, Wette R. Elective neck dissection for pharyngeal and laryngeal cancers. An evaluation. Ann Otol Rhinol Laryngol 1971;80:646-50. [DOI] [PubMed] [Google Scholar]
- 34.Putney FJ. Elective vs. delayed neck dissection in cancer of the larynx. Surg Gynecol Obstet 1961;112:736-42. [PubMed] [Google Scholar]
- 35.McGavran MH, Bauer WC, Ogura JH. The incidence of cervical lymph node metastases from epidermoid carcinoma of the larynx and their relationship to certain characteristics of the primary tumour. A study based on the clinical and pathological findings for 96 patients treated by primary en bloc laryngectomy and radical neck dissection. Cancer 1961;14:55-66. [DOI] [PubMed] [Google Scholar]
- 36.Biller HF, Davis WH, Ogura JH. Delayed contralateral cervical metastases with laryngeal and laryngopharyngeal cancers. Laryngoscope 1971;81:1499-502. [DOI] [PubMed] [Google Scholar]
- 37.Ogura JH, Spector GJ, Sessions DG. Conservation surgery for epidermoid carcinoma of the marginal area (aryepiglottic fold extension). Laryngoscope 1975;85:1801-7. [DOI] [PubMed] [Google Scholar]
- 38.Morton RP, Ferguson CM, Lambie NK, Whitlock RM. Tumour thickness in early tongue cancer. Arch Otolaryngol Head Neck Surg 1994;120:717-20. [DOI] [PubMed] [Google Scholar]
- 39.Lauerma S. Treatment of laryngeal cancer. A study of 638 cases. Acta Otolaryngol Ital 1967;(Suppl 225):1. [PubMed] [Google Scholar]
- 40.Kashima HK. The characteristics of laryngeal cancer correlating with cervical lymph node metastasis. Can J Otolaryngol 1975;4:893-902. [PubMed] [Google Scholar]
- 41.Reid AP, Robin PE, Powell J, McConkey CC, Rockley T. Staging carcinoma: its value in cancer of the larynx. J Laryngol Otol 1991;105:456-8. [DOI] [PubMed] [Google Scholar]
- 42.Hirabayashi H, Koshii K, Uno K, Ohgaki H, Nakasone Y, Fujisawa T, et al. Extracapsular spread of squamous cell carcinoma in neck lymph nodes: prognostic factor of laryngeal cancer. Laryngoscope 1991;101:502-6. [DOI] [PubMed] [Google Scholar]
- 43.Spiro RH, Alfonso AE, Farr HW, Strong EW. Cervical node metastasis from epidermoid carcinoma of the oral cavity and oropharynx. A critical assessment of current staging. Am J Surg 1974;128:562-7. [DOI] [PubMed] [Google Scholar]
- 44.Lauriola L, Michetti F, Maggiano N, Galli J, Cadoni G, Schafer BW, et al. Prognostic significance of the Ca(2+) binding protein S100A2 in laryngeal squamous-cell carcinoma. Int J Cancer 2000;89:345-9. [DOI] [PubMed] [Google Scholar]
- 45.Lee SW, Tomasetto C, Swisshelm K, Keyomarsi K, Sager R. Down-regulation of a member of the S100 gene family in mammary carcinoma cells and reexpression by azadeoxycytidine treatment. Proc Natl Acad Sci USA 1992;89:2504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nordemar S, Kronenwett U, Auer G, Hogmo A, Lindholm J, Edstrom S, et al. Laminin-5 as a predictor of invasiveness in cancer in situ lesions of the larynx. Anticancer Res 2001;21:509-12. [PubMed] [Google Scholar]
- 47.Piantelli M, Iacobelli S, Almadori G, Iezzi M, Tinari N, Natoli C, et al. Lack of expression of galectin-3 is associated with a poor outcome in node-negative patients with laryngeal squamous-cell carcinoma. J Clin Oncol 2002;20:3850-6. [DOI] [PubMed] [Google Scholar]
- 48.Ranelletti FO, Almadori G, Rocca B, Ferrandina G, Ciabattoni G, Habib A, et al. Prognostic significance of cyclooxygenase-2 in laryngeal squamous cell carcinoma. Int J Cancer 2001;95:343-9. [DOI] [PubMed] [Google Scholar]
- 49.Mohit-Tabatabai MA, Sobel HJ, Rush BF, Mashberg A. Relation of thickness of floor of mouth stage I and II cancers to regional metastasis. Am J Surg 1986;152:351-3. [DOI] [PubMed] [Google Scholar]
- 50.Close LG, Brown PM, Vuitch MF, Reisch J, Schaefer SD. Microvascular invasion and survival in cancer of the oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg 1989;115:1304-9. [DOI] [PubMed] [Google Scholar]
- 51.Spiro RH, Huvos AG, Wong GY, Spiro JD, Gnecco CA, Strong EW. Predictive value of tumour thickness in squamous carcinoma confined to the tongue and floor of the mouth. Am J Surg 1986;152:345-50. [DOI] [PubMed] [Google Scholar]
- 52.Rasgon BM, Cruz RM, Hilsinger RL Jr, Sawicki JE. Relation of lymph-node metastasis to histopathologic appearance in oral cavity and oropharyngeal carcinoma: a case series and literature review. Laryngoscope 1989;99:1103-10. [DOI] [PubMed] [Google Scholar]
- 53.Platz H, Fries R, Hudec M, Min TA, Wagner RR. The prognostic relevance of various factors at the time of the first admission of the patient. Retrospective DOSAK study on carcinoma of the oral cavity. J Maxillofac Surg 1983;11:3-12. [DOI] [PubMed] [Google Scholar]
- 54.Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G-T and A-C substitutions. J Biol Chem 1992;267:166-72. [PubMed] [Google Scholar]
- 55.Ravasz LA, Hordijk GJ, Slootweg PJ, Smit F, Tweel IV. Uni- and multivariate analysis of eight indications for post-operative radiotherapy and their significance for local-regional cure in advanced head and neck cancer. J Laryngol Otol 1993;107:437-40. [DOI] [PubMed] [Google Scholar]
- 56.Almadori G, Galli J, Cadoni G, Bussu F, Scarano E, Maurizi M. Prospects and therapeutic decisions in the light of biological findings in laryngeal cancer. Acta Otorhinolaryngol Ital 2000;20:407-12. [PubMed] [Google Scholar]
- 57.Ferlito A, Rinaldo A, Robbins KT, Silver CE. Neck dissection: past, present and future? J Laryngol Otol 2006;120:87-92. [DOI] [PubMed] [Google Scholar]
- 58.Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer 1993;71:452-6. [DOI] [PubMed] [Google Scholar]
- 59.Myers JN, Greenberg JS, Mo V, Roberts D. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer 2001;92:3030-6. [DOI] [PubMed] [Google Scholar]
- 60.Brockstein BE. Reduction of distant metastases in head and neck cancer with concomitant chemotherapy. J Clin Oncol 2000;18:3320-1. [DOI] [PubMed] [Google Scholar]
- 61.No Authors listed. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med 1991;324:1685-90. [DOI] [PubMed] [Google Scholar]
- 62.Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst 1996;88:890-9. [DOI] [PubMed] [Google Scholar]
- 63.Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy vs. radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310-7. [DOI] [PubMed] [Google Scholar]
- 64.Belbin TJ, Singh B, Barber I, Socci N, Wenig B, Smith R, et al. Molecular classification of head and neck squamous cell carcinoma using cDNA microarrays. Cancer Res 2002;62:1184-90. [PubMed] [Google Scholar]
- 65.Leethanakul C, Patel V, Gillespie J, Shillitoe E, Kellman RM, Ensley JF, et al. Gene expression profiles in squamous cell carcinomas of the oral cavity: use of laser capture microdissection for the construction and analysis of stage-specific cDNA libraries. Oral Oncol 2000;36:474-83. [DOI] [PubMed] [Google Scholar]
- 66.Song JI, Grandis JR. STAT signaling in head and neck cancer. Oncogene 2000;19:2489-95. [DOI] [PubMed] [Google Scholar]
- 67.Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer 2001;8:11-31. [DOI] [PubMed] [Google Scholar]
- 68.Wen QH, Miwa T, Yoshizaki T, Nagayama I, Furukawa M. Nishijima H. Prognostic value of EGFR and TGF-alpha in early laryngeal cancer treated with radiotherapy. Laryngoscope 1996;106:884-8. [DOI] [PubMed] [Google Scholar]
- 69.Gupta AK, McKenna WG, Weber CN, Feldman MD, Goldsmith JD, Mick R, et al. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res 2002;8:885-92. [PubMed] [Google Scholar]
- 70.Rubin GJ, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst 1998;90:824-32. [DOI] [PubMed] [Google Scholar]
- 71.Dassonville O, Formento JL, Francoual M, Ramaioli A, Santini J, Schneider M, et al. Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer. J Clin Oncol 1993;11:1873-8. [DOI] [PubMed] [Google Scholar]
- 72.Johansson N, Kahari VM. Matrix metalloproteinases in squamous cell carcinoma. Histol Histopathol 2000;15:225-37. [DOI] [PubMed] [Google Scholar]
- 73.Narayana A, Vaughan AT, Gunaratne S, Kathuria S, Walter SA, Reddy SP. Is p53 an independent prognostic factor in patients with laryngeal carcinoma? Cancer 1998;82:286-91. [DOI] [PubMed] [Google Scholar]
- 74.Osman I, Sherman E, Singh B, Venkatraman E, Zelefsky M, Bosl G, et al. Alteration of p53 pathway in squamous cell carcinoma of the head and neck: impact on treatment outcome in patients treated with larynx preservation intent. J Clin Oncol 2002;20:2980-7. [DOI] [PubMed] [Google Scholar]
- 75.Alsner J, Sorensen SB. Overgaard J. TP53 mutation is related to poor prognosis after radiotherapy, but not surgery, in squamous cell carcinoma of the head and neck. Radiother Oncol 2001;59:179-85. [DOI] [PubMed] [Google Scholar]
- 76.Anwar K, Nakakuki K, Imai H, Naiki H, Inuzuka M. Over-expression of p53 protein in human laryngeal carcinoma. Int J Cancer 1993;53:952-6. [DOI] [PubMed] [Google Scholar]
- 77.Maestro R, Dolcetti R, Gasparotto D, Doglioni C, Pelucchi S, Barzan L, et al. High frequency of p53 gene alterations associated with protein overexpression in human squamous cell carcinoma of the larynx. Oncogene 1992;7:1159-66. [PubMed] [Google Scholar]
- 78.Taylor D, Koch WM, Zahurak M, Shah K, Sidransky D, Westra WH, et al. Immunohistochemical detection of p53 protein accumulation in head and neck cancer: correlation with p53 gene alterations. Hum Pathol 1999;30:1221-5. [DOI] [PubMed] [Google Scholar]
- 79.Lee CS, Redshaw A, Boag G. nm23-H1 protein immunoreactivity in laryngeal carcinoma. Cancer 1996;77:2246-50. [DOI] [PubMed] [Google Scholar]
- 80.Gunduz M, Ayhan A, Gullu I, Onerci M, Hosal AS, Gursel B, et al. nm23 protein expression in larynx cancer and the relationship with metastasis. Eur J Cancer 1997;33:2338-41. [DOI] [PubMed] [Google Scholar]
- 81.Liu M, Lawson G, Delos M, Jamart J, Ide C, Coche E, et al. Predictive value of the fraction of cancer cells immunolabeled for proliferating cell nuclear antigen or Ki67 in biopsies of head and neck carcinomas to identify lymph node metastasis: comparison with clinical and radiologic examinations. Head Neck 2003;25:280-8. [DOI] [PubMed] [Google Scholar]
- 82.Franchi A, Gallo O, Boddi V, Santucci M. Prediction of occult neck metastases in laryngeal carcinoma: role of proliferating cell nuclear antigen, MIB-1, and E-cadherin immunohistochemical determination. Clin Cancer Res 1996;2:1801-8. [PubMed] [Google Scholar]
- 83.Pruneri G, Pignataro L, Carboni N, Buffa R, Di Finizio D, Cesana BM, et al. Clinical relevance of expression of the CIP/KIP cell-cycle inhibitors p21 and p27 in laryngeal cancer. J Clin Oncol 1999;17:3150-9. [DOI] [PubMed] [Google Scholar]
- 84.Spafford MF, Koeppe J, Pan Z, Archer PG, Meyers AD, Franklin WA. Correlation of tumour markers p53, bcl-2, CD34, CD44H, CD44v6, and Ki-67 with survival and metastasis in laryngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 1996;122:627-32. [DOI] [PubMed] [Google Scholar]
- 85.Maurizi M, Ferrandina G, Almadori G, Scambia G, Cadoni G, D’Agostino G, et al. Prognostic significance of methyl-p-hydroxy-phenyllactate-esterase activity in laryngeal squamous cell carcinoma. Br J Cancer 1998;77:1253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]