Summary
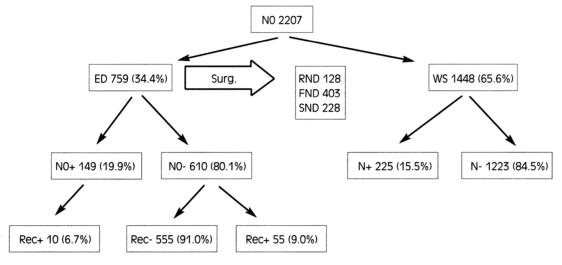
Management of cervical lymph node metastasis is one of the most challenging problems facing clinicians dealing with head and neck cancer. A retrospective evaluation has been made of results in N0 laryngeal cancer patients treated from 1978 to date by comparing historical data reported in related papers previously published by our institution. The medical records of 2207 consecutive patients with cN0 SCC of the larynx were used as the source of data in the present study. Together with primary treatment, 759 (34.4%) received at least unilateral elective neck dissection, while the remaining 1448 (65.6%) were admitted to a wait-and-see protocol. Overall, in the electively dissected patients (ED): 128 (16.9%) cases were submitted to classical radical neck dissection, while 403 (53.1%) cases were submitted to functional neck dissection (FND) and 228 (30.0%) cases to jugular node dissection (JND, removing Level II, III and IV). In 125 of the ED group, a neck procedure on the contralateral N0 neck was associated, of which 15 were RNDs, 35 FNDs and 75 JNDs, respectively.
Based on this large series population, the change in the philosophy was evaluated concerning elective neck treatment in N0 laryngeal cancer, from RND through FND towards JND. As far as concerns the reliability as a staging procedure, no statistically significant difference was found between RND, FND and JND (p = 0.794). The 5-year neck recurrence rate, as estimated by the Kaplan Meier, method, for all ED patients, was 7.7%. No significant difference in the rate of 5-year neck recurrence was detected between RND, FND and JND groups (p = 0.178). In the survival curves, no differences, in terms of actuarial survival by Kaplan Meier analysis, were observed, in our series, as far as concerns type of elective neck dissection performed (p = 0.222). In conclusion, following a critical revision of 25 years’ experience, at our Institution, in the management of cN0 necks in laryngeal cancer patients, definitive changes were observed in the surgical approach to the treatment of occult disease in cN0 cases. JND, compared to more extensive neck dissections, did not show statistically significant differences in terms of neck control (p = 0.233), in terms of impact on survival (p = 0.122) and in terms of accuracy as staging procedure (p = 0.794).
Keywords: Laryngeal cancer, N0 neck, Neck dissection
Riassunto
Il trattamento delle metastasi linfonodali laterocervicali è uno dei problemi più complessi che deve affrontare il chirurgo oncologo del distretto testa-collo. Abbiamo valutato retrospettivamente i pazienti con carcinoma laringeo N0 trattati nel nostro Istituto dal 1978 ad oggi, rivalutando i dati di precedenti pubblicazioni. Sono state considerate le cartelle cliniche di 2.207 pazienti con carcinoma squamocellulare laringeo e collo N0; di questi 759 (34,4%) sono stati sottoposti, contestualmente al trattamento chirurgico del tumoure laringeo, a svuotamento elettivo laterocervicale, mentre i rimanenti 1.448 (65,6%) sono stati sottoposti a protocollo di attesa vigile. Tra i pazienti sottoposti a svuotamento elettivo in 128 casi (16,9%) è stato praticato uno svuotamento radicale (RND), in 403 (53,1%) uno svuotamento funzionale (FND) e in 228 (30,0%) uno svuotamento laterale (JND); in 125 casi è stato associato anche uno svuotamento del collo N0 controlaterale. Basandoci su questi dati abbiamo valutato i cambiamenti della filosofia nel trattamento elettivo del collo N0 da RND attraverso FND fino a giungere alla JND. Per quanto concerne l’affidabilità come procedura di campionamento non abbiamo rilevato differenze statisticamente significative fra i diversi svuotamenti RND, FND e JND (p = 0,794).
Il tasso di recidiva a 5 anni per tutti i colli sottoposti a svuotamento elettivo è stato del 7,7% e non si sono rilevate differenze statisticamente significative tra i gruppi trattati con RND, FND e JND (p = 0,178). Non si sono rilevate differenze significative anche nelle curve di sopravvivenza (p = 0,222). Concludendo, dalla revisione critica dei 25 anni di esperienza, nel trattamento dei colli N0 da carcinoma laringeo primitivo presso il nostro Istituto, abbiamo rilevato un marcato cambiamento nella filosofia di trattamento di questi pazienti. Il JND rispetto agli svuotamenti funzionali e radicali non presenta differenze statisticamente significative per quanto riguarda il controllo regionale di malattia, la sopravvivenza e la capacità di campionamento.
Introduction
Management of cervical lymph node metastasis is one of the most challenging problems facing clinicians dealing with head and neck cancer.
By the end of the 19th Century, the first attempts to remove any cervical lymph nodes involved were described by Kocher, Warren, Butlin and others 1–3.
In 1906, George W. Crile 4 first described a systematic comprehensive approach to cervical lymph node dissection. The operation included the removal of all the cervical node groups from the level of the mandible above, to the clavicle below, together with the sternocleidomastoid muscle, the spinal accessory nerve, the internal jugular vein and the submandibular salivary gland. This operation created the basis for radical neck dissection (RND) as we know it to-day and was standardized and refined by Martin 5 in 1951.
The morbidity and potential complications related to RND led Osvaldo Suarez 6, in 1963, to codify a different approach to the neck where unjustified removal of non-lymphatic structures were avoided, and, at the same time, all the lymphatic tissue within fascial spaces were completely and systematically removed. This philosophically new approach was immediately followed by Bocca who after a preliminary report in 1964 7, presented, in the English literature in 1980 8, the so-called functional neck dissection (FND). FND is a selective neck lymphadenectomy that allows preservation of the spinal accessory nerve, sternomastoid muscle and internal jugular vein with removal of levels II, III, IV and V.
The evidence that, usually, the lymphatic tumour spread to the neck follows predictable pathways 9, recently represented the basis for the development of a neck dissection operation in which a limited number of levels at risk are removed, the so-called selective neck dissection (SND). In jugular node dissection (JND), lymphatic tissue along the internal jugular vein is removed, levels II-III-IV 10 11.
Nodel status has a greater influence on the prognosis of laryngeal cancer, as histologically identified regional metastases are associated with a significantly increased risk of recurrence and reduce survival by about 50% 12.
The overall prevalence of occult neck metastasis in laryngeal carcinomas is about 25%, varying with the sub-site of origin and with the T stage. As reported in the literature 13–15, the incidence of occult metastasis, in electively dissected neck, is about 30% for supraglottic carcinoma and 20% for T3-T4 glottic carcinoma.
If the need for therapeutic neck dissection is not questioned and the clearance of evident metastatic lymph nodes is universally accepted as a major step in the comprehensive surgical management of laryngeal cancer, elective treatment of the clinically uninvolved neck remains more controversial. In most institutions, a frequency rate of occult metastasis exceeding 15% represents the indication for elective neck treatment 16 17.
Elective neck dissection finds its rational in therapeutic proposals related to removal of occult neck disease. Moreover, the pathological assessment of neck nodes is extremely helpful to define the possible need for adjuvant therapy and represents a valuable prognostic indicator providing accurate staging of the disease.
The present report deals with personal experience in elective treatment of the neck, in N0 laryngeal cancer patients, focusing on the evolution of the procedure starting from RND passing through FND to SND. To this end, we retrospectively evaluated the results, in N0 laryngeal cancer patients, treated from 1978 to date, by comparing historical data reported in related papers previously published by our institution 17–20 and dividing this 25-year period into 3 periods: the 80ies, the 90ies and the recent period.
Based on this large population, an attempt is made to evaluate the change in philosophy regarding elective neck treatment in N0 laryngeal cancer over the years, and the survival results of comprehensive, vs. selective, neck lymph-adenectomies in controlling occult neck disease in our Institution.
A critical review of our results was made with the aim to address the following issues:
the usefulness of different neck lymphadenectomies, as staging procedure in N0 laryngeal cancer patients (i.e., the capacity to identify occult neck disease in N0 necks = sensitivity);
neck control in an electively treated N0 population according to neck procedure (i.e., the capacity to control occult disease in N0 necks);
the impact on survival of different surgical procedures in patients who underwent elective treatments.
Materials and methods
The data source, for the present study was obtained from the medical records of 2207 consecutive patients with cN0 SCC of the larynx. All patients underwent surgical treatment at the Department of Oto-Neuro-Ophthalmological Surgical Sciences of the University of Florence, Division of Otolaryngology-Head & Neck Surgery, between January 1978 and December 2003 and were then submitted to a strict follow-up programme.
The population study comprised 1950 males and 257 females, age range 38-82 years (mean 63). No patients who had previously received chemotherapy or radiotherapy for head and neck cancers were included in this study.
Of these 2207 patients, 1118 (51%) presented laryngeal SCC in a supraglottic localization, 1012 (45.4%) presented a glottic development of the cancer lesion, and, finally, 77 (3.5%) had a subglottic involvement (Table I). These tumours were staged according to the UICC-AJCC 2000 classification. Most of the T3-4 cancer patients (852 cases) underwent total laryngectomy, while the remainder underwent a conservative approach with different partial laryngectomies according to tumour site and stage.
Table I. T stage and T localization (ED: elective neck dissection; WS: wait-and-see protocol).
| Localization | ED (%) | WS (%) | Total (%) |
| Supraglottic | 390 (52.0) | 728 (50.4) | 1118 (51.1) |
| Glottic | 311 (40.3) | 701 (48.4) | 1012 (45.4) |
| Subglottic | 58 (7.7) | 19 (1.2) | 77 (3.5) |
| Total | 759 (100) | 1448 (100) | 2207 (100) |
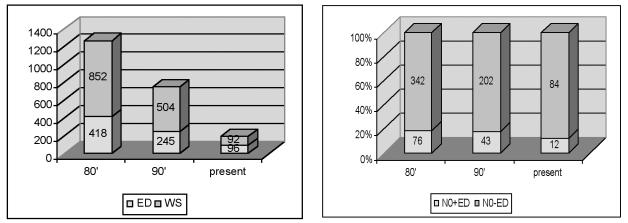
Together with surgical treatment of the laryngeal cancer, 759 (34.4%) received at least an unilateral elective neck dissection, while the remaining 1448 (65.6%) were admitted to a wait-and-see protocol (Fig. 1). Overall, as far as concerns the electively dissected patients (ED, 759 cases): in 128 (16.9%) cases, a classical radical neck dissection (RND) was performed, while 403 (53.1%) cases received a functional neck dissection (FND) and 228 (30.0%) cases a selective dissection or jugular node dissection (JND, removing Levels II, III and IV). In 125 of the ED group, a neck procedure was also associated on the contralateral N0 neck, of which 15 were RNDs, 35 FNDs and 75 JNDs, respectively.
Fig. 1.
Patient distribution.
The criteria for selecting N0 laryngeal cancer patients for elective neck surgery were mostly subjective; however, elective treatment was reserved for patients with: advanced lesions (T3-4), supraglottic lesions, well-lateralized lesions involving “marginal” laryngeal structures (usually at higher risk of occult node metastases), poorly differentiated lesions (G3), short fat neck with clearly difficult clinical examination. Conversely, a wait-and-see policy was often adopted in patients with early stage lesions, mainly glottic, in elderly patients or when the general conditions were poor, implicating high-risk surgical procedures.
In all ED cases, information regarding the pathological involvement of the neck was available from patients’ clinical records; however, only since January 1996, has a well standardized procedure for the analysis of the specimen been used; immediately after lymphadenectomy, the neck specimen was sent for pathological analysis and the node levels were accurately marked on the surgical bed; the neck dissection specimen was sectioned in a routine manner and studied by a head and neck pathologist; finally, the incidence and location of nodal metastases were reported according to the classification suggested by MSKCC for neck levels 21. Thus, a complete pathological assessment of neck specimens was available only for a limited number of patients.
Overall, 453 (20.5%), received post-operative radiotherapy (RT) using a daily fraction of 200 cGy for 5 days a week. The average dose of irradiation was 56.8 Gy (50-65 Gy). Indications for post-operative RT included advanced stage of the primary tumour, positive margins, more than 1 occult positive node, and lymph node metastases with extra-capsular tumour spread. RT was delivered both to the primary lesion and neck, in a few cases treated with partial laryngectomy (tumour-free margin cases), RT was delivered to the neck only.
Follow-up was for a minimum of 5 years or until death in the group treated in the 80ies and the 90ies (mean 69 ± 19 months, minimum 38, maximum 110), a minimum of 3 years for the patients in the more recent group (1998-2003). A comparison was made between cN0pN0- (N0-) and cN0pN+ (N0+) groups and between the RND, FND and JND groups, in terms of treatment failures and actuarial survival according to the Kaplan Meier method by log rank test.
To test the differences in our ED population, Fisher test was used. Considering the day of the initial surgery as the starting day of the observation, the disease-free curve was calculated according to the Kaplan Meier method.
Probability values < 0.05 were considered statistically significant. Statistical analysis was performed by Stata (Stata Corporation, College Station, TX, USA) and StatXact (Cambridge, MA, USA) programmes.
Results
Histo-pathological findings in the neck
Overall, in the ED group (759 cN0 patients who had elective neck treatment), occult lymph node metastases were found in 149 (19.6%) specimens. Of these, 29 received RND (22.6%), 78 FND (19.4%) and, finally, 42 selective JND (18.4%) (Fig. 1).
To test the reliability as a staging procedure for each type of neck dissection, we evaluated, per procedure, the sensitivity (capacity of identifying occult neck disease) measured as a proportion between the number of the identified N0+ patients (true positive) and the addition between these patients plus the patients initially staged as N0- who developed recurrence (false negative). Sensitivity values of 80.5%, 78.8% and 66.6%, respectively, were found for RND, FND and JND. The differences are not statistically significant (Fisher exact test, p = 0.386).
According to tumour site, we documented the higher incidence of occult neck metastases among ED supraglottic cancer patients (98/405, 24.2%) in comparison with advanced glottic cancer (43/317, 13.2%) and sub-glottic lesions (8/37, 21.7%) (p = 0.095).
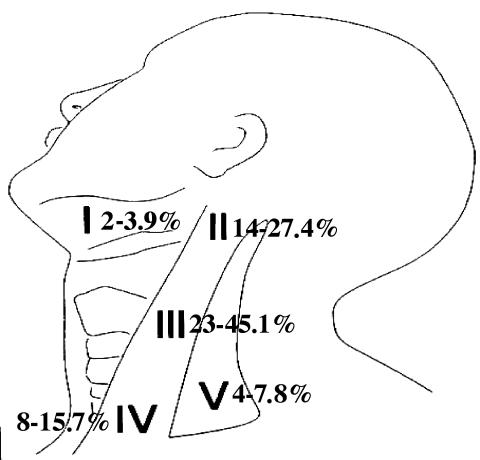
Further detailed data regarding the exact number of occult positive lymph nodes, location and extra-capsular spread were available only for patients treated from 1996 until now (almost 30% of the 149 N0+ ED). The group for whom a complete pathology report was available amounted to 225 neck specimens, the average number of lymph nodes examined by pathologists was 21 per neck (range 15-32). In the ED group, an extracapsular spread of occult metastases was documented in 7/51 (11.8%) of node-positive neck dissections. In these 51 cases, the total number of occult positive nodes was 61, of which 20 (32.8%) were located at Level II, 27 (44.3%) at Level III, 10 (16.4%) at Level IV and only 4 (6.5%) were found at Level V (Fig. 2). These metastases at Level V were found in 3 patients with positive nodes at Level II and III who received a FND and in one patient with positive Level IV nodes who received a classical RND.
Fig. 2.
Localization of detected micrometastases.
Neck relapse
During follow-up, overall 65 neck recurrences were documented in the ED group (8.5%) (ranging from 6 to 21 months). Of these, 9 (7%, 2 in N0+ and 7 in N0) developed in cN0 patients who underwent elective RND; 25 (6.2%, 4 in N0+ and 21 in N0-) in those treated by FND, and, finally, 31 (9.2%, 4 in N0+ and 27 in N0-) following selective JND. Therefore, a higher risk of neck failure was documented in the JND group when compared with those who received a more extended lymph-adenectomy, although the differences were not statistically significant (Fisher exact p = 0.233).
In the wait-and-see group, 225 cN0 laryngeal cancer patients experienced neck relapse in the undissected neck(s) (15.5%), while 84.5% of the remainder were disease-free in the neck.
In the ED group, a lower rate of neck failure was documented in pN0+ necks vs. pN0- patients (Table II). Overall, in the 149 pN0+, 10 recurrences (6.7%) were documented, while in the remaining 610 pN0, 45 (7.4%) experienced a neck failure in the dissected neck during follow-up (p = 0.307), with the highest rate among those who underwent JND (9.2%). In fact, of 45 neck recurrences, 21 affected patients were electively treated by JND (p = 0.178) (Table II).
Table II. Percent recurrences according to surgical procedure.
| N0+ recurrences | No. (%) | Total | N0- recurrences | No. (%) | Total | ||
| RND | 2 (1.5) | 128 | RND | 7 (5.4) | 128 | ||
| FND | 4 (1.7) | 403 | FND | 21 (5.2) | 403 | ||
| JND | 4 (0.9) | 228 | p = 0.434 | JND | 27 (11.8) | 228 | p = 0.178 |
The exact site of recurrence was clearly documented in a limited number of cases. In RND and FND groups, the recurrence was within the field, frequently at Levels II and III. A surgical approach, followed by curative RT was attempted in 18/30 neck recurrences, successfully only in 7 patients (23.3%).
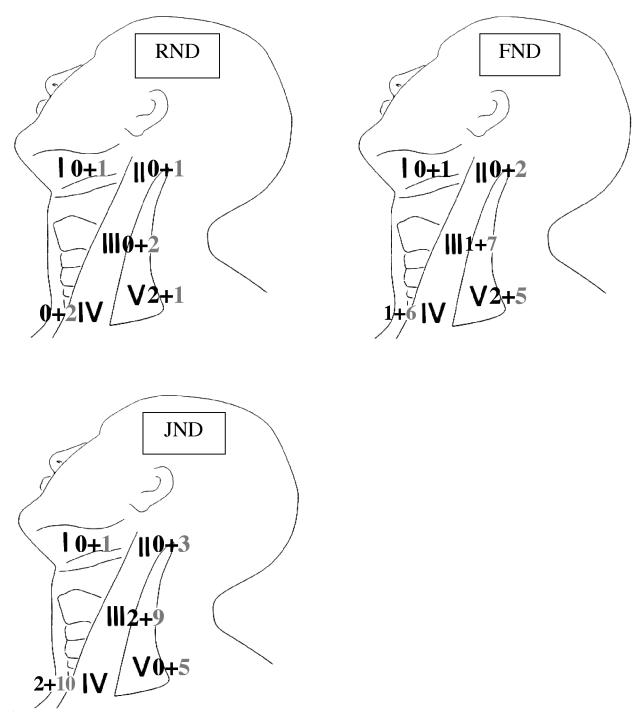
In most JND dissected necks, we found that often, in the pN0 cases, the site of recurrence was clinically documented at the posterior limit of the resection at Levels III and IV, within the dissected JND field (Fig. 2), while the sites of failure in the 4 cases with pN1 disease were, in 2 cases, outside the dissected field (Level I and Level V, respectively) (Fig. 2).
In 14 out of 21 neck failures (66.7%), a classical or modified (Type II or III) RND was performed as salvage procedure, combined with post-operative RT. Among these, 9 were successfully treated (64.3%). Therefore, irrespective of primary tumour control, we found that despite a higher rate of neck relapse in selective JND procedures when compared with more extended lymph-adenectomies (RND and FND), the neck failure following an extended procedure seems to be less salvable by surgery plus RT than a recurrence in a selectively dissected neck (9 out of 21 vs. 7 out of 30, p = 0.154).
The 5-year neck recurrence rate is 7.7%. No significant difference in the rate of 5-year neck recurrence was documented between node positive (pN+) and node negative (pN0) groups (log rank 0.03, p = 0.893) between surgery alone and combined therapy groups (log rank 0.51, p = 0.490), as well as between RND, FND and JND groups (log rank test, p = 0.122); this answers our second issue (Fig. 3).
Fig. 3.
Localization of recurrences (Left number pN0+ necks, Right number pN0- necks).
As far as concerns the third issue, in the survival curves, no differences, in terms of actuarial survival by Kaplan Meier analysis, were documented, in our series, according to type of elective neck dissection performed (p = 0.122) (Figs. 4, 5).
Fig. 4.
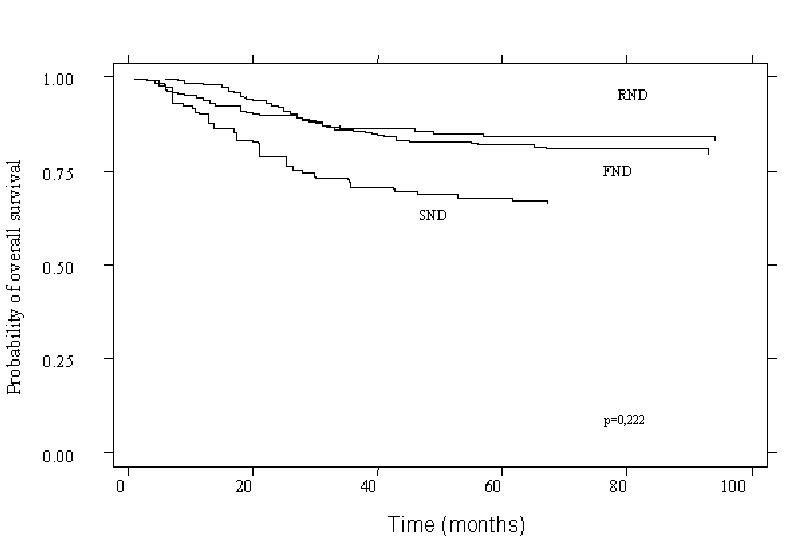
Kaplan Meier survival curve according to surgical procedure (p = 0.222).
Fig. 5.
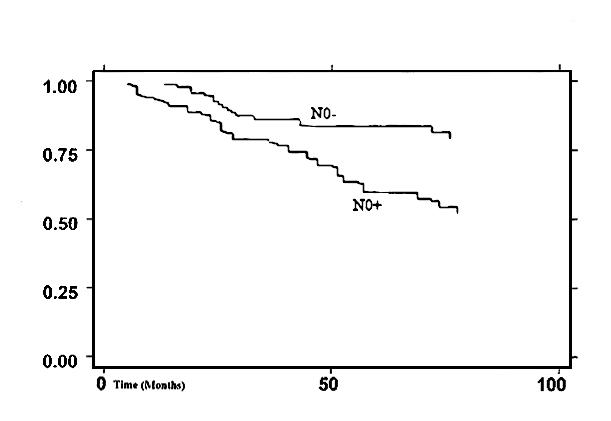
Kaplan Meier survival curve according to N0 status (p = 0.023).
Discussion
RND is a comprehensive procedure involving removal of all five neck levels together with the spinal accessory nerve (SAN) the internal jugular vein (IJV) and the sternocleidomastoid muscle (SCM). Although there is little doubt that RND is oncologically effective, significant functional and cosmetic morbidities induced by this operation give rise to problems. Denervation of the trapezius muscle due to sacrifice of the SAN together with loss of the SCM muscle can result in the well-recognized “shoulder syndrome” involving pain, weakness, and deformity of the shoulder girdle mechanism.
Furthermore, severe facial oedema, a relatively high incidence of fistulas and skin necrosis as well as carotid rupture, have been described as RND-related complications.
This scenario led to a change in thinking towards a more functional type of surgery, supported also by the evidence that, in most cases, classic RND presents no advantage as far as concerns overall survival when compared with modified radical neck dissection (MRND).
According to internationally standardized terminology for neck lymphadenectomies 10, FND might be regarded as a MRND type III sparing Level I. In MRND and FND, nonlymphatic structures are preserved in the attempt to reduce the complication rate and to reach a consistent gain in post-operative shoulder function. Certainly, FND is associated with an important decrease in post-operative complications compared to RND, however the outcomes on shoulder function are not consistent.
Surgical dissection of the SAN, even if extremely carefully carried out, produces direct trauma on the nerve that will result in post-operative trapezius dysfunction of variable degree; furthermore, sacrifice of the cervical plexus nerves interrupts the nerve supply from C2-C3-C4 branches to the SAN. With JND these branches are routinely preserved because they represent the posterior margin of the resection, furthermore the fact that Level V is not dissected prevents injuries to the distal portion of the SAN. Retrospective analysis of 25 years’ experience with cN0 laryngeal cancer patients offers interesting considerations regarding the evolution of treatment philosophy in these patients.
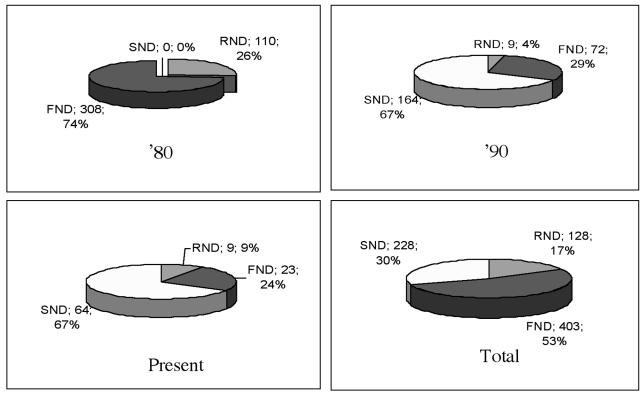
According to an arbitrary division of patients’ data, we compared the type of lymphadenectomies during the Eighties, Nineties and the present. This is clearly outlined in Figure 6. This distribution is influenced by a progressive reduction in treatments for laryngeal cancers, at our Institution, over the past few years. In fact, we treated 1270 cN0 laryngeal cancer patients in the Eighties, this number decreased to 749 in the Nineties, and reaching < 200 procedures for the last 5 years (Fig. 7).
Fig. 6.
Percentage of surgical procedures performed over the years.
Fig. 7.
Number of neck dissections performed over the years and percentage of occult metastases detected.
When we look at the rate of ED patients vs. the WS group, it can be seen that with the introduction of JND in the last few years almost 50% of the cases were submitted to elective neck treatment. Accordingly, we found a marked reduction in the number of more extended neck procedures when SND became more popular in the Department.
Finally, when analysing the rate of occult neck disease detected by ED during this period, the rate of occult disease decreased with time (almost 20% in the past vs. 9.6% more recently), this finding seems to reflect an increased accuracy of pre-operative imaging rather than a less extensive sampling provided by JND (Fig. 6). Our data showed no statistically significant difference between JND, FND and RND as staging procedure (p = 0.672).
Several reports indicate that the routine use of new diagnostic imaging techniques such as ultrasonography, CT, MRI and PET have considerably reduced the number of false negative cN0 patients. Albeit, elective lymph-adenectomy, in the N0 neck, seems to be reasonable with the aim of better staging the tumour and to remove occult micro-metastases in cN0 patients 19 20. With the increased knowledge regarding the routes and site of lymph node metastases in the neck from laryngeal carcinoma, some authors have suggested more limited selective lymph-adenectomies removing only the high risk levels 9 (JND, removing Levels II, III and IV) with less morbidity and lower social costs 11.
Taken together, the critical review of our results suggests some interesting considerations. First of all, the rate and the type of elective neck procedures, in cN0 laryngeal cancer patients at our Institution, reflects the changing philosophy concerning this issue reported in the international literature.
Overall, the rate of ED patients vs. the WS group with the introduction of JND in the last few years has increased, passing from 20% in the Eighties to almost 50% more recently. Accordingly, we found a marked reduction in the number of more extended neck procedures when SND became more popular in the Department.
We report an incidence of occult neck metastases of 18% in the ED group treated during the Nineties, compared to a rate of 9.8% in patients treated more recently.
Therefore, according to the removal of higher risk lymph node levels sparing low risk areas, it seems reasonable to limit neck lymph-adenectomies, in cN0 laryngeal cancer patients, in the attempt to reduce the time of surgery, side-effects and social costs.
Due to the limited information concerning the histological status of neck specimens and site of failure in patients in the Eighties and Nineties, no definite conclusions can be drawn regarding the risk of occult neck disease at Levels I and V, usually spared by a selective procedure. More information is available for the latter patients mainly treated by JND. Analysis of the pattern of neck failure in patients who failed after a following JND that yielded negative nodes (cN0/pN-) was intriguing. In most of these cases, in fact, we found that nearly all recurrences involved the upper posterior or mid-posterior nodes at Levels III-IV. Thus, the site of failure seems to suggest that some of our patients had a JND that was less than complete JND was incomplete in some patients and highlights a technical question regarding the absence of clear surgical boundaries in the posterior limit of the resection. This result together with the analysis of the sites of recurrence in the JND cN0 group indirectly supports the idea that, from a technical point of view, a RND (classic or FND, i.e., mRND type III sparing Level I), is able to completely remove lymph nodes at Levels II to IV en bloc with Level V, while a JND might be inadequate, particularly at the posterior margin of the resection. Interestingly, our data are in agreement with those of an article published in 1993 regarding the role of selective JND in the treatment of cN0 hypo-pharyngeal and laryngeal SCC patients from the MSKCC 11 reporting analogous results in terms of site of relapse after JND. On the contrary, Davidson et al. in a series of patients treated by JND documented 5 out of 6 ipsilateral recurrences within the field 21.
Despite these considerations, we observed a high salvage rate when conventional RND plus RT was performed as a salvage procedure for neck recurrence following JND, no differences in terms of actuarial survival by Kaplan Meier analysis were documented in our series according to type of ED performed (p = 0.122).
Although elective selective dissection of Levels II to IV lymph nodes (JND) seems to remove the more frequently involved lymph nodes in cN0 laryngeal cancer patients 3 4 16, few investigations have analysed the effectiveness of RND vs. FND (mRND type III sparing level I) or mRND type III vs. JND as a staging procedure for cN0 cases and their therapeutic effect in cN0/pN+ cases as well as their impact on patient survival. These topics were satisfactorily addressed by only one prospective study by the Brazilian Head and Neck Cancer Study Group 22, comparing selective JND (Levels II-IV) with type III MRND (Levels I-V) as part of elective treatment for patients with supra-glottic-trans-glottic SCC of the larynx. In this study, JND was converted into a mRND type III if frozen section analysis of a suspicious lymph node, performed during surgery, confirmed the presence of occult metastatic SCC. After adequate follow-up, the Authors reported no difference in the outcome between patients treated by either of these 2 modalities.
Conclusion
In conclusion, following critical revision of 25 years’ experience at our Institution with the management of cN0 necks from laryngeal cancer patients, we documented definite changes in the surgical approach to the treatment of occult disease in cN0 cases.
JND seems to be as reliable as the more extensive neck dissection operations in terms of accuracy as a staging procedure, in terms of neck control as well as in terms of impact on survival.
Finally, it should be pointed out that, the low rate of occult micro-metastases detected in the last few patients in our series, could raise the question of the effective usefulness of prophylactic elective neck lymph-adenectomy in such a minority of cN0 laryngeal cancer patients if we take into account that < 10% of the patients had beneficial neck surgery, in comparison to 90% of patients who could have been over-treated. However, since in this series, the primary tumour was treated surgically, the addition of a JND, in our opinion, is advisable even if the expected occult metastasis rate is only about 10%, because of its very low morbidity. FND and RND in N0 laryngeal carcinomas probably belong to the past.
References
- 1.Kocher H. Über Radicalheilung des Krebses. Deutsche Chir 1980;13:134-66. [Google Scholar]
- 2.Warren JC. Surgical observations on tumours: Cases and operations. Boston: Crocker & Brewster 1838. [Google Scholar]
- 3.Butlin HT, Spencer WG. Diseases of the tongue. 2nd edn. London: Cassell 1900. [Google Scholar]
- 4.Crile GW. Excision of cancer of the head and neck: with special reference to the plan of dissection based on one hundred and thirty-two operations. JAMA 1906;47:1780-6. [DOI] [PubMed] [Google Scholar]
- 5.Martin H, Del Valle B, Ehrlich H, Cahan WB. Neck dissection. Cancer 1951;4:441-99. [DOI] [PubMed] [Google Scholar]
- 6.Suárez O. El problema de las metastasis linfáticas y alejadas del cáncer de laringe e hipofaringe. Rev Otorrinolaringol 1963;23:83-99. [Google Scholar]
- 7.Bocca E. Évidement “fonctionel” du cou dans la térapie de principe des metastases ganglionnaires du cancer du larynx (introduction à la présentation d’un film). J Fr Otorhinolaryngol 1964;13:721-3. [Google Scholar]
- 8.Bocca E, Pignataro O, Sasaki CT. Functional neck dissection. A description of operative technique. Arch Otolaryngol 1980;106:524-7. [DOI] [PubMed] [Google Scholar]
- 9.Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg 1990;160:405-9. [DOI] [PubMed] [Google Scholar]
- 10.Robbins KT, Denys D. The American Head and Neck Society’s revised classification for neck dissection. San Francisco, CA: Proc 5th Int Conf Head Neck Cancer; 2000. p. 365-71. [Google Scholar]
- 11.Spiro RH, Gallo O, Shah JP. Selective jugular node dissection in patients with squamous carcinoma of the larynx or pharynx. Am J Surg 1993;166:399-402. [DOI] [PubMed] [Google Scholar]
- 12.Moe K, Wolf GT, Fisher SG, Hong WK. Regional metastasis in patients with advanced laryngeal cancer. Arch Otolaryngol Head Neck Surg 1996;122:644-8. [DOI] [PubMed] [Google Scholar]
- 13.de Campora E, Radici M, Camaioni A, Pianelli C. Clinical experiences with surgical therapy of cervical metastases from head and neck cancer. Eur Arch Otorhinolaryngol 1994;251:335-41. [DOI] [PubMed] [Google Scholar]
- 14.Alajmo E, Aulisi L, Boccuzzi S. L’envahissement ganglionnaire dans les cancers du larynx: a propos des tumeurs N0. Les Cahiers d’ORL 1989;10:737-47. [Google Scholar]
- 15.Snow GB. The N0 neck in head and neck cancer patients. Eur Arch Otorhinolaryngol 1993;250:423. [PubMed] [Google Scholar]
- 16.Bocca E, Calearo C, de Vincentiis I, Marullo T, Motta G, Ottaviani A. Occult metastases of the larynx and their relationship to clinical and histological aspects of the primary tumour: a four-year multicentric research. Laryngoscope 1984;94:1086-90. [DOI] [PubMed] [Google Scholar]
- 17.Weber PC, Johnson JT, Meyers EN. Impact of bilateral neck dissection on pattern of recurrence and survival in supraglottic carcinoma. Arch Otolaryngol Head Neck Surg 1994;120:703-6. [DOI] [PubMed] [Google Scholar]
- 18.Gallo O, Boddi V, Bottai GV, Franchi A, Storchi OF. Prognostic significance of clinically false positive cervical lymph nodes in patients with laryngeal carcinoma. Cancer 1995;75:1077-83. [DOI] [PubMed] [Google Scholar]
- 19.Gallo O, Boddi V, Bottai GV, Parrella F, Storchi OF. Treatment of the clinically negative neck in laryngeal cancer patients. Head Neck 1996;18:566-72. [DOI] [PubMed] [Google Scholar]
- 20.Sarno A, Bocciolini C, Deganello A, Coscarelli S, Gallo O. Does unnecessary elective neck treatment affect the prognosis on N0 laryngeal cancer patients? Acta Otolaryngol 2004;124:980-5. [DOI] [PubMed] [Google Scholar]
- 21.Gallo O, Fini-Storchi I, Napolitano L. Treatment of the contralateral negative neck in supraglottic cancer patients with unilateral node metastases (N1-3). Head Neck 2000;22:386-92. [DOI] [PubMed] [Google Scholar]
- 22.Spiro RH, Strong EW, Shah JP. Classification of neck dissection: variations on a new theme. Am J Surg 1994;168:415-8. [DOI] [PubMed] [Google Scholar]
- 23.van den Brekel MW, Reitsma LC, Quak JJ, Smeele LE, van der Linden JC, Snow GB, et al. Sonographically guided aspiration cytology of neck nodes for selection of treatment and follow-up in patients with N0 head and neck cancer. Am J Neuroradiol 1999;20:727-31. [PMC free article] [PubMed] [Google Scholar]
- 24.van den Brekel MW, Castelijns JA, Reitsma LC, Leemans CR, van der Waal I, Snow GB. Outcome of observing the N0 neck using ultrasonographic-guided cytology for follow-up. Arch Otolaryngol Head Neck Surg 1999;125:153-6. [DOI] [PubMed] [Google Scholar]
- 25.Tachibana T, Yoshida K. Role of the regional lymph node in cancer metastasis. Cancer Metastasis Rev 1986;5:55-66. [DOI] [PubMed] [Google Scholar]
- 26.Davidson J, Khan Y, Gilbert R, Birt BD, Balogh J, MacKenzie R. Is selective neck dissection sufficient treatment for the N0/Np+ neck? J Otolaryngol 1997;26:229-31. [PubMed] [Google Scholar]
- 27.Brazilian Head and Neck Cancer Study Group. Results of a prospective trial on elective modified radical classical vs. supraomohyoid neck dissection in the management of oral squamous carcinoma. Am J Surg 1998;176:422-7. [DOI] [PubMed] [Google Scholar]
- 28.Andersen PE, Cambronero E, Shaha AR, Shaha JP. The extent of neck disease after regional failure during observation of the neck. Am J Surg 1996;172:689-91. [DOI] [PubMed] [Google Scholar]