Summary
Changes in mitochondrial DNA have been reported in cancer cells. Since little information exists regarding mt DNA mutations in head and neck, the present study focused on ten head and neck cancer cell lines in the attempt to detect alterations in the ND4 gene sequence. DNA was extracted from 10 head and neck squamous cell carcinoma lines from 9 patients. MtDNA sequences were compared in normal and tumour cell line DNA. In ten head and neck squamous cell carcinoma cell lines, 8 somatic mutations and 5 polymorphisms of the mitochondrial gene for ND4 were found. All 5 polymorphisms were silent. Of the 8 somatic mutations, 3 altered the amino acid sequence suggesting a possible effect on enzyme function. The mitochondrial mutations and polymorphisms found demonstrated that these can serve as clonal markers for individual cell lines and demonstrate that the mitochondrial genome remains stable in the cell lines during in vitro culture.
Keywords: Head and neck cancer, Squamous carcinoma, Mitochondrial DNA mutations, ND4 gene, Cancer clonal markers
Riassunto
Negli ultimi anni in alcuni tumouri sono state messe in evidenza mutazioni del DNA mitocondriale, mentre scarse rimangono le informazioni inerenti i tumouri della testa e del collo. Abbiamo ritenuto pertanto interessante studiare su 10 linee cellulari la sequenza del gene mitocondriale ND4. Il DNA mitocondriale di 10 linee cellulari derivate da 9 pazienti affetti da carcinoma della testa e del collo è stato estratto e la sequenza del gene ND4 è stata comparata con quella del DNA mitocondriale normale dello stesso paziente. In 10 linee cellulari sono state trovate 8 mutazioni somatiche e 5 polimorfismi. I 5 polimorfismi erano silenti, 3 delle 8 mutazioni somatiche alteravano la sequenza aminoacidica, suggerendo un possibile effetto sulla funzione enzimatica. Le mutazioni somatiche e i polimorfismi trovati dimostrano che il genoma mitocondriale rimane stabile durante la coltivazione cellulare in vitro e che questi potrebbero essere utilizzati come markers clonali.
Introduction
Mitochondria have many functions. They produce energy by oxidative phosphorylation, support cellular functions in intermediary metabolism, regulate ion homeostasis, support the biosynthesis of lipids, amino acids and nucleotides, have an active transport process, which can affect the movement of cytotoxic drugs, and have roles in cell proliferation and apoptotic processes.
Mitochondria have a characteristic shape and size, carry a genome, and are inherited from the maternal parent in the cytoplasm of the oocyte 1. The human mitochondrial genome consists of circular double stranded DNA of 16 Kb that encodes 13 proteins including 4 enzyme complexes of the respiratory chain (complex I, III, IV, and ATPase synthetase) as well as 2 rRNAs and 22 tRNAs 2 3.
Changes in mitochondrial DNA have been reported in cancer cells 4 5. The frequency of mtDNA mutations and the types of mutations vary in different tumours and in the various studies 6–9. Mutations of mitochondrial DNA have been reported in 16-70% of colon cancers 10–12 and in gastric cancer the percentage with such mutations varies from 5 to 37% 12–14. Alonso et al. 12 advanced the hypothesis that a significant ethnic difference in the pattern of DNA mutations may affect susceptibility to environmental factors. This concept is supported by the observation of numerous polymorphisms, as well as different percentages of mutations, in the various ethnic groups, found in these studies.
In the majority of cases, mitochondrial mutations were multiple, implying possible accumulation of mtDNA damage such as might occur in a clonal expansion model, in which the mutant somatic mitochondrial genome replicates at a higher rate than the wild type 10.
The mutations identified are primarily in the protein coding genes, rRNA genes and in the D-loop region. Most of these mutations are T to C and G to A base transitions, indicating possible exposure to mutagens that generate reactive oxygen species (ROS) 15.
In fact, it is generally accepted that mtDNA mutations are produced during oxidative phosphorylation through mechanisms involving reactive oxygen species (ROS). These mutations may accumulate as the mitochondria lack protective histones and efficient DNA repair mechanisms 4 16.
The respiratory chain protein genes are especially sensitive to oxidative damage, as seen in aging cells 17–19, increased oxidative damage contributes to a decrease of oxidative phosphorylation, and then to a decrease of ATP production as a result of an increase in ROS, which is, in turn, responsible for further mtDNA damage 20 21.
In human epithelia cells, tobacco products increase the production of ROS and induce free radical reactions 22 that may be responsible for single strand breaks in DNA 23–25 especially in the mitochondria where they preferentially accumulate. In fact, it is known that tobacco products are involved in the carcinogenesis process of head and neck cancer 26.
Since little information exists regarding mt-DNA mutations in the head and neck, the present study has been carried out on ten head and neck cancer cell lines and normal cells from the same donors in the attempt to detect alterations in the ND4 gene sequence, an NADHdehydrogenase subunit (nicotinamide adenine dinucleotide hydrogen), that was recently reported to be mutated in these tumours 27.
Materials and methods
DNA samples
DNA was extracted from 10 HNSCC cell lines from 9 patients. Normal DNA was isolated from fibroblasts from the same patients. MtDNA sequences were compared in the normal and tumour cell line DNA to determine which alterations identified in the tumour cell lines were polymorphisms and which were mutations.
Five cell lines (UM-SCC-3, UM-SCC-10B, UM-SCC-11B, UM-SCC-27 and UM-SCC-68) were from recurrent or metastatic sites, and 5 (UM-SCC-11A, UM-SCC-16, UM-SCC-62, UM-SCC-82 and UM-SCC-95) were from primary tumours. UM-SCC-11A was cultured from a biopsy of a primary laryngeal cancer and UM-SCC-11B was derived from the laryngectomy specimen of the same patient after chemotherapy.
The tumour specimen site and the stage of disease, at the time the tissue was taken for culture, are shown in Table I.
Table I. Cell lines.
| Cell line | Specimen Site | Stage |
| UM–SCC–3 | Lymph node | II |
| UM–SCC–10B | Lymph node | III |
| UM–SCC–11A | Larynx | IV |
| UM–SCC–11B | Larynx after cx. | IV |
| UM–SCC–16 | Larynx | II |
| UM–SCC–27 | Lymph node | II |
| UM–SCC–62 | Tonsillar fossa | III |
| UM–SCC–68 | Lymph node | II |
| UM–SCC–82 | Larynx | III |
| UM–SCC–95 | Larynx | IV |
PCR amplification and sequencing
The following oligonucleotide primers were used for ND4 gene PCR amplification and sequencing:
fragment 1 (product size 812bp) from 10688bp to 11500bp,L 5’-TGGGCCAGCCCTACTAGTCT-’3 and R 5’-GTCAGGGGGTTGAGAATGAG-’3;
fragment 2 (product size 712bp) from 11295bp to 12076bp, L 5’-TCACTCTCACTGCCCAAGAA-’3 and R 5’-GGAGAATGGGGGATAGGTGT-’3.
PCR reactions were carried out with 200-500 ng genomic DNA as template, PCR buffer (10x) 10 μl, Mg buffer (25 mM) 6 μl, dNTP (10 mM) 2 μl, primer (10 μM) 2 μl, Taq (5 μl) 0.5 μl (Promega, Wisconsin, Usa) and water for a total volume of 100 μl.
PCR conditions were: 1 cycle at 94 °C for 5 min followed by 30 cycles at 94 °C for 1 min, 60 °C 1 min, 72 °C 1 min, and final extention of 1 cycle at 72 °C for 7 min.
To confirm the correct size of the PCR products, 10 μl of amplified DNA was analyzed in 1.5% agarose gel.
The PCR products were purified using the Qiaquick PCR purification kit (Qiagen, Valencia, CA, Usa). The DNA concentrations were measured with a spectometer and adjusted to 10 ng/μl. The primer concentration used for sequencing was 3.2 pmol/μl.
DNA sequencing was carried out using an Applied Biosystems DNA sequencer model 377.
Results
The mitochondrial DNA sequences obtained with the PCR products were compared to the Cambridge sequences 28 given in the mitochondrial genome databank (htt://www.gen.emory.edu/mitomap.htlm). To distinguish somatic mutations from polymorphisms, we also compare the tumour sequences to the normal DNA for each patient.
ND4 gene sequence mutations were observed in 5 of the 10 (50%) cell lines studied.
The 5 cell lines included UM-SCC-11A and UM-SCC-11B which were from different biopsies in the same patient (Table II). Of the 10 cell lines, 4 contained polymorphisms (Table III).
Table II. Somatic ND4 gene mtDNA mutations.
| Cell lines | Position | DNA sequence change | Protein change |
| U–SCC–10B | 11812 | A → G | L → L |
| UM–SCC–11A–B | 11288 | C → T | L → F |
| 11306 | C → G | A → G | |
| UM–SCC–11B | 11125 | C → G | E → E |
| UM–SCC–27 | 11203 | T → C | F → I |
| 11479 | ins G | frameshift | |
| UM–SCC–95 | 11812 | A → G | L → L |
| 11719 | G → A | G → G |
Table III. ND4 gene polymorphisms.
| Cell lines | Position | DNA sequence change | Protein change |
| UM–SCC–3 | 11298 | T → C | T → T |
| 11467 | A → G | L → L | |
| UM–SCC–11A–B | 10873 | T → C | P → P |
| 11002 | A → G | Q → Q | |
| UM–SCC–27 | 11719 | G → A | G → G |
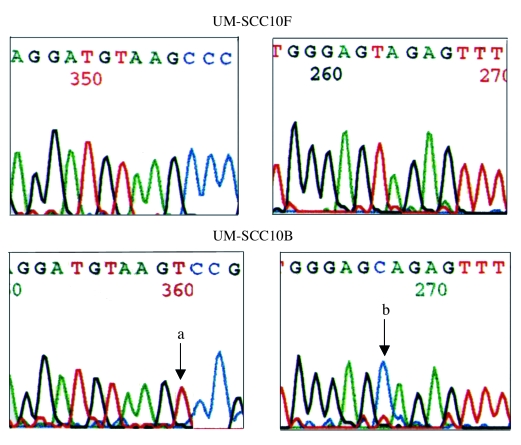
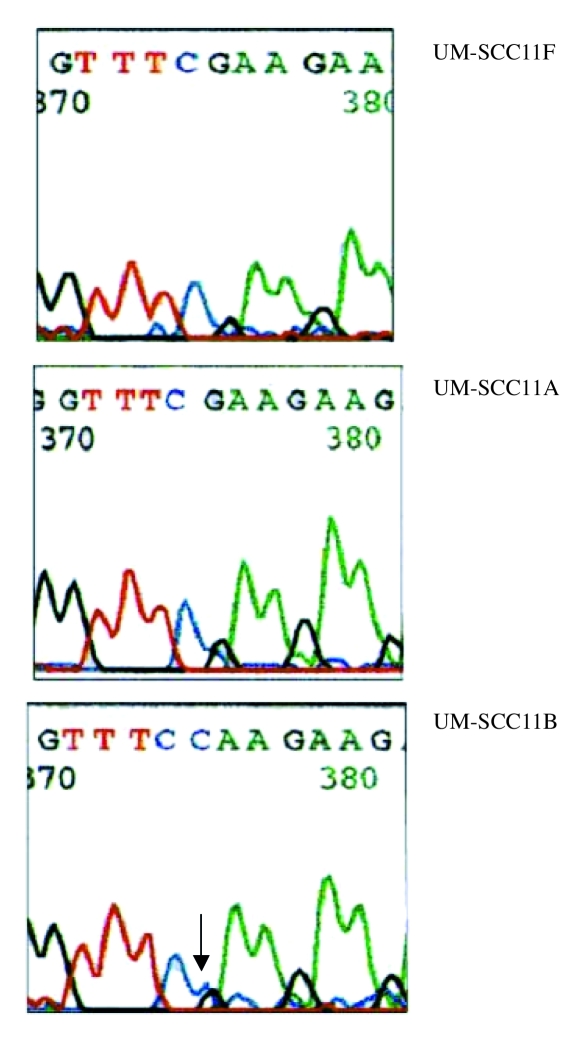
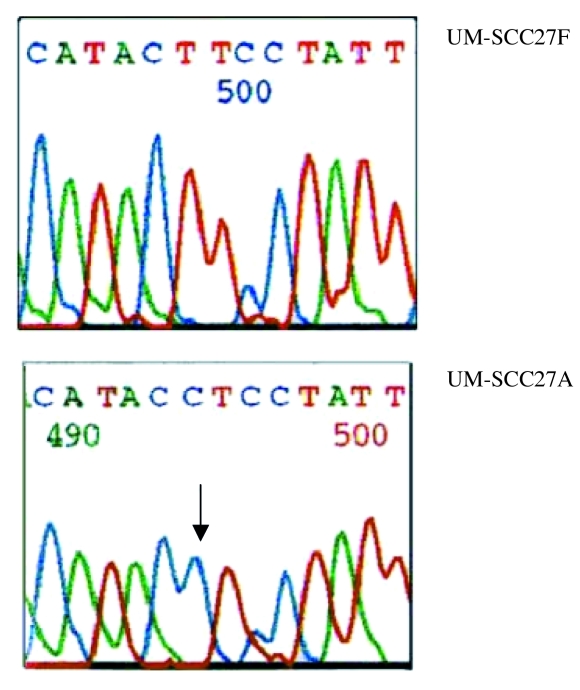
A total of 10 somatic mutations were found in this study. Of these, 9 were nucletide substitutions and one was a single nucleotide insertion. One cell line, UM-SCC-10B, contained a single mutation, 3 cell lines UM-SCC-11A, UM-SCC-27 and UM-SCC-95 had 2 mutations in each and one cell line UM-SCC11B had 3 mtDNA mutations (Table III). Examples of the mutations are shown in Figures 1– 3.
Fig. 1.
ND4 mitochondrial gene mutations present in UM–SCC–10B tumour cell line but not in UM–SCC–10F fibroblast culture positions a) 11719 and b) 11812 (arrows).
Fig. 3.
ND4 mitochondrial gene mutation in position 11125 G>C (arrow).
Overall, 50% of the metastatic lymph nodes and 50% of the primary cell lines showed mutations.
The primary tumour cell lines, with mutations UM-SCC-11A and UM-SCC-95, were from stage IV tumours. The UM-SCC-11B cell line which was derived after chemotherapy treatment contained both of the mutations in the UM-SCC-11A cell line which was from a pre-treatment biopsy. In addition UM-SCC-11B contained a third mutation that was not present at the time of the first biopsy (Table II). The cell lines without mutations (UM-SCC-3, UM-SCC-16, UM-SCC-62 and UM-SCC-68) were from patients with stage II-III tumours (Table I).
Of the 10 mtDNA mutations, 4 were silent, 5 caused amino acid substitutions and one, a nucleotide insertion, caused a frameshift mutation (Table II).
Three patients presented polymorphisms that were present in both the tumour cell lines (UM-SCC-3, UM-SCC-11A, UM-SCC11B, UM-SCC27) and in their respective normal fibroblasts (Table III). All the polymorphisms were silent substitutions and have been previously reported (Mitomap databank). Two of these patients also presented with somatic mutations in their tumour cell lines (UM-SCC-11A, UM-SCC-11B and UM-SCC-27).
Discussion
The upper aerodigestive mucosa is exposed to multiple carcinogenic insults arising from tobacco smoking, as well as from environmental and dietary factors.
If these carcinogens are not fully metabolized to non-hazardous forms, then DNA damage may occur and the multi-step process of carcinogenesis can begin, leading to squamous cell carcinoma.
The ability to metabolize carcinogens and repair DNA defects may vary in different populations due to polymorphisms in the enzyme that carry out these processes and can make some individuals more susceptible to carcinogens.
Mitochondrial DNA is present in multiple copies in each mitochondrion. Damage is thought to occur more frequently to mtDNA than to nDNA, through the production of reactive oxygen species during oxidative phosphorylation, as mitochondria DNA lack protective histones 29–31. Cigarette smoking can cause an increase in reactive oxygen species (ROS) such as H2O2 and O–. In addition, many tobacco smoking metabolic products contain DNA binding agents that can accumulate preferentially in the mitocondria and lead to DNA damage 32–35.
Some polymorphic variations in the mtDNA decrease in oxidative phosphorylation, in synergy with cigarette smoke, could result in subtle changes which generate an increase in ROS levels known to be mitogenic 8 36.
The ND4 gene, encodes one subunit of the NADH dehydrogenase complex. This complex has an important role in the metabolic processing of carcinogen products. Thus alterations of NADH function could contribute to an increased likelihood of mutagen accumulation. In the present study, somatic mutations affecting the ND4 gene in cell lines were found in 44% of the patients. Curiously, 50% of these patients also presented polymorphism of this gene.
The cell lines derived from these patients were mostly from tumours in an advanced stage and, possibly, the high rate of mutation detected is due to the advanced stage of disease in the primary tumours and the fact that some cell lines were from lymph node metastases representing a late stage of progression.
This study was not aimed at determining whether some of these patients were more susceptible to mtDNA mutations on account of the presence of polymorphic changes in the ND4 gene. In fact, with one exception, it is unlikely that the polymorphisms detected had any effect on function since only one resulted in an amino acid change.
However, an interesting finding emerging from this analysis is that mtDNA mutations are fairly common in HNSCC cells and that once developed in the tumour, these mutations are stable clonal markers of the in vitro cell lines. Furthermore, the two cell lines from the same patient reveal that the tumour clone continues to evolve during the time elapsing between the first biopsy and laryngectomy performed six weeks later. Nevertheless, both cell lines maintained the original mutations during in vitro culture.
Interestingly, Fliss et al. 27 detected a mtDNA mutation in the saliva of patients with mtDNA-mutated head and neck cancers, thus suggesting a possible role in early diagnosis.
Ha et al. 37, studying mitochondrial C-tract alterations, have found an increase in mutations in precancerous lesions of the head and neck with increasing severity of dysplasia.
Furthermore, an increase in the quantity of mitochondrial DNA has recently been shown in pre-malignant and malignant head and neck lesions 38 as a result of mtDNA functional alteration, due to mutations 39.
Data emerging from these various studies suggest that it would be interesting to develop research on mtDNA alterations, in pre-malignant and malignant lesions, in the attempt to find a useful biomarker for head and neck cancer.
Fig. 2.
ND4 mitochondrial gene mutation in position 11204 T>C (arrow).
References
- 1.Taanman JW. The mitochondrial genome: structure, transcription, translation and replication. Acta Biochim Biophys 1999;1410:103-23. [DOI] [PubMed] [Google Scholar]
- 2.Anderson S, Bankier T, Barrell BG, de Bruijn MHL, Coulson AR, Drouin J, et al. Sequence and organization of the human mitochondrial genome. Nature 1981;290:457-65. [DOI] [PubMed] [Google Scholar]
- 3.Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet 1999;23:147. [DOI] [PubMed] [Google Scholar]
- 4.Penta JS, Johnson FM, Wachsman JT, Copeland WC. Mitochondrial DNA in human malignancy. Mut Res 2001;488:119-33. [DOI] [PubMed] [Google Scholar]
- 5.Hochhauser D. Relevance of mitochondrial DNA in cancer. Lancet 2000;356:181-2. [DOI] [PubMed] [Google Scholar]
- 6.Ivanova R, Lepage V, Loste MN, Schachter F, Wijnen E, Busson M, et al. Mitochondrial DNA sequence variation in human leukemic cells. Int J Cancer 1998;76:495-8. [DOI] [PubMed] [Google Scholar]
- 7.Horton TM, Petros JA, Heddi A, Shoffner J, Kaufman AE, Graham AD, et al. Novel mitochondrial DNA deletion found in a renal cell carcinoma. Genes, Chromosome Cancer 1996;15:95-101. [DOI] [PubMed] [Google Scholar]
- 8.Lewis PD, Baxter P, Griffiths PA, Parry JM, Skibiski DOF. Detection of damage to the mitochondrial genome in the oncocytic cells of Wartin’s tumour. J Pathol 2000;191:274-81. [DOI] [PubMed] [Google Scholar]
- 9.Yeh JJ, Lunetta KL, van Orsouw JN, Moore FD, Mutter GL, Vijg J, et al. Somatic mitochondrial DNA (mtDNA) mutations in papillary thyroid carcinomas and differential mtDNA sequence variants in cases with thyroid tumours. Oncogene 2000;19:2060-6. [DOI] [PubMed] [Google Scholar]
- 10.Polyak K, Li Y, Zhu H, Lengauer C, Willson JKV, Markowitz SD, et al. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat Genet 1998;20:291-3. [DOI] [PubMed] [Google Scholar]
- 11.Habano W, Sugai T, Yoshida T, Nakamura SI. Mitochondrial gene mutation, but not large-scale deletions, is a feature of colorectal carcinomas with mitochondrial microsatellite instability. Int J Cancer 1999;83:625-9. [DOI] [PubMed] [Google Scholar]
- 12.Alonso A, Martin P, Albarran C, Aquilera B, Garcia O, Guzman A, et al. Detection of somatic mutations in the mitochondrial DNA region of colorectal and gastric tumours by heteroduplex and single-strand conformation analysis. Electrophoresis 1997;18:682-5. [DOI] [PubMed] [Google Scholar]
- 13.Burgart LJ, Zheng J, Shu Q, Strickler JG, Shibata D. Somatic mitochondrial mutations in gastric cancer. Am J Pathol 1995;147:1105-11. [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura G, Nishizuka S, Maesawa C, Suzuki Y, Iwaya Y, Sakata K, et al. Mutations in mitochondrial control region DNA in gastric tumours of Japanese patients. Eur J Cancer 1999;35:316-9. [DOI] [PubMed] [Google Scholar]
- 15.Cadet J, Berger M, Douki T, Ravanat JL. Oxidative damage to DNA: formation, measurement and biologic significance. Rev Physiol Biochem Pharmacol 1997;131:1-87. [DOI] [PubMed] [Google Scholar]
- 16.Sawyer DE, Van Houten B. Repair of DNA damage in mitochondria. Mut Res 1999;434:161-76. [DOI] [PubMed] [Google Scholar]
- 17.Mornstad H, Pfeiffer H, Yoon C, Teivens A. Demonstration and semi-quantification of mt-DNA from human dentine and its relation to age. Int J Legal Med 1999;112:98-100. [DOI] [PubMed] [Google Scholar]
- 18.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA 1994;91:10771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortopassi GA, Shibata D, Soong N-W, Arnheim N. A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proc Natl Acad Sci USA 1992;89:7370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Zhou H, Stansbury K, Trush M. Role of reactive oxigen species in multistage carcinogenesis. In: Thomas C, Kalyanaram B, editors. Oxygen radicals and disease process. Amsterdam: Harwood Academic Publishers; 1997. p. 237-77. [Google Scholar]
- 21.Beckman KB, Ames BN. Oxidative decay of DNA. J Biol Chem 1997;272:19633-6. [DOI] [PubMed] [Google Scholar]
- 22.Kodama M, Kaneko M, Aida M, Inoue F, Nakayama T, Akimoto H. Free radical chemistry of cigarette smoke and its implication in human cancer. Anticancer Res 1997;17:433-7. [PubMed] [Google Scholar]
- 23.Nakayama T, Kaneko M, Kodama M, Nagata C. Cigarette smoke induces DNA single strand breaks in human cells. Nature 1985;314:462-4. [DOI] [PubMed] [Google Scholar]
- 24.Allen JA, Comb MM. Covalent binding of polycyclic aromatic compounds to mitochondrial and nuclear DNA. Nature 1980;287:244-5. [DOI] [PubMed] [Google Scholar]
- 25.Ballinger SW, Bouder TG, Davis GS, Judice JA, Nicklas JA, Albertini RJ. Mitochondrial genome damage associated with cigarette smoking. Cancer Res 1996;56:5692-7. [PubMed] [Google Scholar]
- 26.Schantz SP, Lee NK. Tobacco carcinogenesis and head and neck cancer. In: Werner JA, Lippert BM, Ruppert HH, editors. Head and Neck Cancer – Advanced in Basic Research. Amsterdam: Elsevier; 1996. p. 9-20. [Google Scholar]
- 27.Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, Scott EM, et al. Facile detection of mitochondrial DNA mutations in tumours and bodily fluids. Science 2000;287:2017-9. [DOI] [PubMed] [Google Scholar]
- 28.Mitomap: a human mitochondrial genome database, Center for Molecular Medicine, Emory University, Atlanta, GA, USA. htt://www.gen.emory.edu/mitomap.html, 2000.
- 29.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA 1997;94:514-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zastawny TH, Dabrowska M, Jaskolski T, Klimarczyk M, Kulinski L, Koszela A, et al. Comparison of oxidative base damage in mitochondrial nuclear DNA. Free Rad Biol Med 1998;24:722-5. [DOI] [PubMed] [Google Scholar]
- 31.Hemminki K. DNA adducts, mutations and cancer. Carcinogenesis 1993;14:2007-12. [DOI] [PubMed] [Google Scholar]
- 32.Szyfter K, Szmeja Z, Szyfter W. Analysis of DNA adducts in larynx cancer patients. Otolaryngol Pol 1993;47:496-501. [PubMed] [Google Scholar]
- 33.Szyfter K, Hemminki K, Szyfter W, Szmeja Z, Banaszewski J, Yang K. Aromatic DNA Adducts in laryngeal cancer biopsies and leukocytes. Carcinogenesis 1994;15:2195-9. [DOI] [PubMed] [Google Scholar]
- 34.Szyfter K, Szmeja Z, Szyfter W, Hemminki K, Banaszewski J, Jaskula-Sztul R, et al. Molecular and cellular alterations in tobacco smoke-associated larynx cancer. Mutat Res 1999;445:259-74. [DOI] [PubMed] [Google Scholar]
- 35.Ozawa T, Sahashi K, Nakase Y. Extensive tissue oxygenation associated with mitochondrial DNA mutations. Biochem Biophys Res Commun 1995;213:432-8. [DOI] [PubMed] [Google Scholar]
- 36.Bianchi NO, Bianchi MS, Richard SM. Mitochondrial genome instability in human cancers. Mut Res 2001;488:9-23. [DOI] [PubMed] [Google Scholar]
- 37.Ha PK, Tong BC, Westra WH, Sanchez-Cespedes M, Parrella P, Zahurak M, et al. Mitochondrial C-tract alteration in premalignant lesions of the head and neck. Clin Cancer Res 2002;8:2260-5. [PubMed] [Google Scholar]
- 38.Kim MM, Clinger JD, Masayesva BG, Ha PK, Zahurak ML, Westra WH, et at. Mitochondrial DNA quantity increases with histophatologic grade in premalignant and malignant head and neck lesions. Clin Cancer Res 2004;10:8512-5. [DOI] [PubMed] [Google Scholar]
- 39.Kim MM, Glazer CA, Mambo E, Chatterjee A, Zhao M, Sidransky D, et al. Head and neck cancer cell lines exhibit differential mitochondrial repair deficiency in response to 4NQO. Oral Oncol 2006;42:201-7. [DOI] [PubMed] [Google Scholar]