Summary
Vertigo is a very frequent disorder, associated with highly disabling symptomatology. Since the aetiology cannot always be easily identified, treatment is often addressed to the symptoms. Betahistine, a drug characterized by a multi-factorial mode of action of the modulatory type, has been widely employed in the management of various vertiginous syndromes. Its use in Italy is, currently, authorized to treat the vertiginous symptoms related to Ménière’s disease. A meta-analysis has, therefore, been carried out to assess, the efficacy of betahistine in the treatment of other vertiginous syndromes, such as positional paroxysmal vertigo (cupulo-canalolithiasis) and vertigo secondary to arterial deficiency of the vertebrobasilar area, regardless of the specific cause. A review has been made of the literature concerning clinical trials performed with betahistine versus placebo in a randomised double-blind, parallel-group or cross-over design. Only studies evaluating betahistine in patients with vertiginous symptomatology not related to Ménière’s disease were selected. Of the 104 publications, obtained from an analysis of “Medline”, “EMBASE” and “CINAHL” databases, 7 clinical studies, which met the selection criteria, for a total of 367 patients, were extrapolated and analysed. The meta-analysis was conducted using the “Cochrane Collaboration’s Review Manager” software in all the case series and in the sub-groups identified by the experimental design (parallel or crossover design), range of dosages (32-48 mg/day) and range of treatment duration (from 3 weeks to 4 months). The various parameters used to evaluate efficacy, adopted in the trials, and taken into account in the meta-analysis, as overall judgement of the patient or physician, number of vertiginous episodes and their duration, were classified according to the binary classification of “improved” and “not improved”.
The results of the meta-analysis confirm the therapeutic benefit of betahistine versus placebo. In particular, the investigation carried out on the overall sample shows an odds ratio of 3.52 (95% confidence interval 2.40-5.18) and a relative risk of 1.78 (95% confidence interval 1.48-2.13), while the analysis of the sub-groups denotes a maximum efficacy after doses of 32 to 36 mg and with a period of treatment of 3-8 weeks. The present meta-analysis confirms the benefit of drug treatment with betahistine for the vertiginous symptomatology related to cupulo-canalolithiasis and vertebro-basilar arterial insufficiency.
Keywords: Vertigo, Cupulo-canalolithiasis, Vertebrobasilar insufficiency, Medical treatment, Betahistine
Riassunto
La vertigine è un disturbo molto frequente, associato ad una sintomatologia fortemente invalidante. Poiché la sua eziologia non risulta sempre di facile identificazione, le terapie impiegate sono spesso indirizzate al trattamento dei sintomi. La betaistina, molecola caratterizzata da un meccanismo d’azione multifattoriale di tipo modulatorio, ha trovato un vasto impiego nel trattamento delle sindromi vertiginose di varia natura. Attualmente in Italia il suo impiego è autorizzato per il trattamento della sintomatologia vertiginosa correlata alla malattia di Ménière. Si è voluto quindi valutare, attraverso una meta-analisi, l’efficacia di betaistina nel trattamento di altre sindromi vertiginose, quali la vertigine parossistica di posizione (cupolo–canalolitiasi) e la vertigine secondaria a deficit arterioso, qualunque ne sia la causa specifica, del distretto vertebro-basilare. È stata considerata la letteratura riguardante studi clinici controllati, randomizzati a gruppi paralleli o crossover, condotti in doppio cieco, betaistina vs. placebo. Sono stati selezionati i soli studi volti alla valutazione di betaistina in pazienti con sintomatologia vertiginosa non riferibile a malattia di Ménière. Dalle 104 pubblicazioni estrapolate mediante analisi dei database Medline, EMBASE e CINAHL, sono stati estratti ed analizzati 7 studi clinici che rispondevano ai criteri scelti, per un totale di 367 pazienti. La meta-analisi è stata condotta mediante l’utilizzo del Cochrane Collaboration’s Review Manager software sia sull’intera casistica, sia su sottogruppi identificati dal disegno sperimentale (parallelo o crossover), dai range di dosaggi (da 32 a 48 mg/die) e dai range di durata del trattamento (da 1 a 3 mesi). I diversi parametri di valutazione dell’efficacia usati nei trial e presi in considerazione nella meta-analisi, quali il giudizio complessivo del paziente o del medico, il numero di episodi vertiginosi e la loro durata, sono stati uniformati secondo la classificazione binaria di “Migliorati” e “Non migliorati”. I risultati della meta-analisi confermano il beneficio terapeutico di betaistina vs. placebo. In particolare, l’indagine condotta sul campione totale evidenzia un Odds Ratio (OR) di 3,52 (IC 95% 2,40-5,18) ed un Relative Risk (RR) di 1,78 (IC 95% 1,48-2,13), mentre l’analisi dei sottogruppi suggerisce un’efficacia massima ottenuta a dosaggi compresi tra 32 e 36 mg e con periodo di trattamento di 3-8 settimane. La presente meta-analisi conferma l’utilità del trattamento farmacologico con betaistina della sintomatologia vertiginosa correlata alla cupolo-canalolitiasi ed alla insufficienza arteriosa vertebro-basilare.
Introduction
Vertigo is a wrong sensation of movement of the surrounding environment towards our own body (objective vertigo) or of our body towards the environment (subjective vertigo), caused by a dysfunction of the labyrinth, vestibular nerve, brainstem structures, cerebellum, or, more seldom, of other areas of the Central Nervous System (CNS). In the vestibular forms, it is often accompanied by an auditory dysfunction (such as hypoacusis, tinnitus, and auricular fullness) and, especially in critical periods, by neuro-vegetative symptoms.
Generally, the adaptation mechanisms of the CNS assure a functional recovery after a first acute episode. However, various vertiginous syndromes have a recurring evolution, either sub-acute or chronic, and, sometimes, evolve into a status of instability and static and/or dynamic insecurity.
Since this symptom is common to various disorders presenting with different aetio-pathogenic mechanisms, vertigo is so frequent that it is reported in about 5% of the in-patient services of General Medicine and in 15-20% of the specialistic visits of otorhinolaryngology.
In all cases, the vertiginous symptomatology is highly disabling, severely restricting the patient’s social life and often leading to impairment of the patient’s psychological status.
The aetiology or pathogenesis of the vertiginous syndrome cannot always be identified with certainty and, therefore, the main objective of therapy is to reduce the number of crises or the entity of symptoms without changing the physiological mechanisms of adaptation to pathology. In this way, it is easier to prevent the attitude to avoid potentially disabling conditions and thus to make the patient resume his/her usual lifestyle, which is an indispensable condition for functional recovery.
There are numerous forms of treatment with potential symptomatic activity, but, in general, these are characterized by a considerable inhibitory effect on vestibular function and a sedative effect on the CNS, in general.
For this reason, as well as for the potential undesirable effects, their use should be restricted to the first few days after an acute event and not prolonged for weeks or months. Therefore, a more functional symptomatic therapy should modulate less abruptly the impaired function and better safeguard the central mechanisms of adaptation and compensation of vestibular pathology 1 2.
The experimental and clinical data, currently available, suggest that betahistine possesses these features. Betahistine is a histamine analogue which improves the circulation of the inner ear and with a partial agonist action on the post-synaptic H1-receptors and antagonist of the presynaptic H3-receptors present in different types of neurons, above all, but not limited to, histaminergic neurons.
Normally, histamine inhibits its own release by means of these autoreceptors. Therefore, betahistine, an antagonist of H3-receptors, enhances the release of histamine in the CNS and the labyrinthine sensors 2 3. This activity is not limited to the vestibular system: histaminergic neurons, are, actually, located also in the mammary nuclei and in the posterior hypothalamus and their endings have wide projections in the CNS 4. For this reason, betahistine has a more complex and polymorphous potential modulatory activity. On account of its neurochemical and microcirculatory activity, betahistine is widely employed in the treatment of various types of vertiginous syndromes 5 13. Its use has been approved by the Italian Regulatory Authorities for the treatment of vertiginous symptoms related to Ménière’s disease.
In controlled clinical trials, betahistine administered orally was found to be more effective than placebo or other drugs in improving the symptoms related to Ménière’s disease, such as vertigo sensation. In clinical practice, the dose range adopted is overall 24 to 48 mg/day administered two or three times daily 2.
As the efficacy of betahistine, in the treatment of Ménière’s disease, has been demonstrated and its clinical use accepted by the Regulatory Authorities, we aimed, with the present meta-analysis, to analyse the potential evidence of the clinical efficacy of the drug in the treatment of various types of vertigo, such as cupulo-canalolithiasis and vertigo, secondary to arterial insufficiency in the vertebrobasilar system due to any specific cause.
Methods
The meta-analysis reviewed clinical trials published in English and other languages from an analysis of “Medline”, “EMBASE” and “CINAHL” databases using the key-words “betahistine” and “peripheral vertigo” or “betahistine” and “vertigo”. Furthermore, articles published and mentioned in the bibliography of the literature consulted were also considered, while data related to non-published studies and repeated publications were excluded. Inclusion criteria of the meta-analysis were established prior to the bibliographic research. Only clinical studies where treatment with betahistine (drops and tablets) was compared with the use of a placebo substance were included and moreover, in which, the patients were assigned by a double-blind randomized design. Randomized clinical trials, both cross-over and parallel groups, were included in the meta-analysis. The clinical criterion of inclusion comprised only patients with cupulo-canalolithiasis or vertigo secondary to arterial insufficiency of the posterior circle. Thus, patients with Ménierè’s disease, diagnosed on the basis of the labyrinthine syndrome, which occurs with a typical ictal, recurrent and unpredictable course of symptomatologic cluster (hypoacusis, tinnitus, numbness and vertigo), sometimes associated with panic disorders, were excluded. In the case of studies of mixed aetiology, only patients with paroxysmal vertigo or vertebrobasilar insufficiency were considered, while patients with Ménière’s disease were excluded. For the meta-analysis assessment, as parameters for the evaluation of efficacy, we considered the subjective evaluation of the patient or physician, the number of vertiginous episodes within the period of time considered and their duration. The extracted meta-analytical sample was stratified on the basis of two dose ranges of betahistine and two dose ranges of treatment. The meta-analytical assessment of the efficacy of betahistine versus placebo was conducted in all cases, regardless of the parallel-group or cross-over design, and on the sub-groups identified by the experimental design and by the above-mentioned stratifications based on dose ranges and on ranges of treatment duration. The meta-analysis was carried out by means of the Cochrane Collaboration’s Review Manager software (RevMan Rev. 4.2 - 2003). Using the key-words established, 104 publications were extracted from the data base, 29 of these had a design meeting the selection criteria, i.e., double-blind, placebo-controlled randomised studies, to evaluate the efficacy of betahistine versus placebo. Of these 29 studies, 7 were selected for our analysis, while 22 studies were excluded because they referred only to patients with Ménière’s disease or the end-points of efficacy did not meet the criteria selected for the meta-analysis. Therefore, the meta-analysis was carried out on 7 double-blind, placebo-controlled randomised clinical studies versus placebo (Table I).
Table I. Double-blind, randomized clinical studies included in the meta-analysis.
| Ref. | Year | No. patients | Type of study | Dose | Period of treatment | Therapeutic indication | Clinical endpoints |
| 6 | 1979 | 14 | Double-blind | 8 mg x 4/day | 20 days | Vertiginous syndromes | Therapeutic efficacy |
| 7 | 1981 | 32 | Double-blind; | 16 mg + | 20 weeks | Vertiginous syndromes | Therapeutic efficacy, vestibular examination, audiometry, tolerability |
| Cross-over | 8 mg + | ||||||
| 8 mg/day | |||||||
| 8 | 1984 | 24 | Double-blind; | 12 mg x 3/day | 6 weeks | Vertiginous syndromes | Therapeutic efficacy, audiometry, vestibular examination, tolerability |
| Cross-over | |||||||
| 9 | 1988 | 81 | Double-blind | 16 mg x 3/day | 12 weeks | Vertiginous syndromes | Therapeutic efficacy, |
| tolerability | |||||||
| 10 | 1989 | 82 | Double-blind; | 16 mg x 3/day | 5 weeks | Benign paroxysmal vertigo | Therapeutic efficacy, tolerability |
| Cross-over | |||||||
| 11 | 1985 | 73 | Double-blind | 16 mg x 3/day | 16 weeks | Benign paroxysmal vertigo | Therapeutic efficacy |
| 12 | 2003 | 63 | Double-blind | 16 mg x 2 | 3 months | Vertiginous syndromes | Therapeutic efficacy, tolerability |
As the experimental designs, doses, period of treatment and methods used to assess the clinical results are not reported homogeneously in the 7 articles considered, the characteristics of each study included in the meta-analysis should be fully analysed. Furthermore, the criteria and considerations adopted for the selection of patients and their reclassification on the basis of the outcome of treatment by adapting the variety of the trial end-points according to the binary classification of “improved” or “not improved”, need to be cleared.
“Studio in doppio cieco sull’efficacia del cloroidrato di betaistina nel trattamento ambulatoriale di un gruppo di pazienti affetti da vertigine di posizione e acufeni” 6. Of the 20 patients, aged between 29 and 67 years included in the study, only 14 returned for assessment of the therapeutic efficacy after approximately 4 weeks and were thus eligible for an overall evaluation (unchanged or improved symptomatology). For the test treatment, 8 mg betahistine tablets, at a daily dose of 32 mg, were used. As far as concerns the clinical end-points, in Table III the Author reported the results of the treatment, dividing the subjects into two groups according to improved or unchanged symptomatology.
Betahistine in peripheral vertigo. A double-blind, placebo-controlled, cross-over study of Serc versus placebo 7. Due to drop-outs during the study, of the 32 subjects enrolled in the treatment group, 29 were available for the meta-analysis and 30 in the control group. All patients in the two groups were aged ≤ 70 years. In accordance with the study design, the two groups of patients received, in sequence, the test treatment (2 tablets of betahistine twice daily – daily dose 32 mg) for 8 weeks, followed or preceded by a period of the same duration with placebo. From this study, we used Table I where the Authors reported the sum of the scores of the vertiginous symptoms, recorded by the single patients in their own diaries during the weeks of treatment.
Betahistine dihydrochloride in the treatment of vertigo of peripheral vestibular origin. A double-blind placebo-controlled study 8. The 24 eligible patients, divided into two groups, received for 6 weeks, in different sequences, a placebo and the test treatment (1 betahistine tablet (12 mg), 3 times daily – daily dose 36 mg). In this study 8, both the assessments expressed by the patients concerning improvement of their symptoms following treatment with betahistine or placebo (Table V), and the score assigned by the investigators to the interference of the vertiginous symptomatology with the daily activities, are in agreement.
Vertiges paroxystiques itératifs et Serc. Étude clinique contrôlée 9. A series of 39 patients treated for 90 days with a daily dose of 48 mg of betahistine were compared with 42 patients treated with placebo. From the study of Legent, we considered data reported in Figure 3 and then the number of patients reporting good overall results after 90 days of treatment. Therefore, this finding completes the data concerning the number of episodes, intensity and duration of the vertiginous episodes.
Betahistine versus placebo in paroxysmal vertigo; a double-blind trial 10. The study analysed 82 out of 114 patients enrolled (age ≤ 65 years) treated for a period of 5 weeks with placebo and for the same period with betahistine tablets (16 mg) three times a day (daily dose 48 mg) with different sequences. In this study of Oosterveld W.J., the data used to express assessment of the number of patients showing a therapeutic improvement, in the two groups, are reported in Table VI (changed rate of vertiginous attacks) and in Table XI (opinion of treatment).
Betahistine bij de behandeling van aanyalsgewijs optredende duizeligheid. Een Dubbelblind onderzoek 11. Overall 36 patients were treated with betahistine tablets (16 mg) three times a day (daily dose 48 mg), while 37 patients received placebo. The period of observation lasted 4 months. Judgement concerning improvement of symptoms corresponded to that expressed by the investigators (Table II.2a) and to that expressed by the patients (Table II.2b).
Betahistine dihydrochloride in the treatment of peripheral vestibular vertigo 12. In the meta-analysis, patients who had been diagnosed with Ménière’s disease, were excluded and, therefore, 29 patients treated with placebo and 34 patients treated with betahistine (16 mg twice daily for 3 months – daily dose 32 mg) were included. The number of patients whose symptomatology was improved, at the end of the study, was obtained from Figure 2, which outlined the improvement in the score of the intensity of the vertiginous symptomatology.
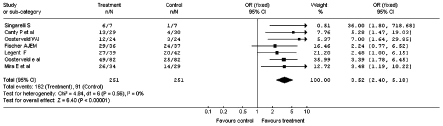
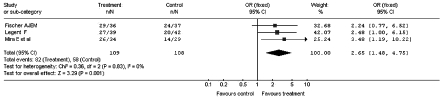
Fig. 3.
Improvement of the vertiginous symptomatology after administration of betahistine or placebo: sub-analysis of cross-over studies (Odds Ratio).
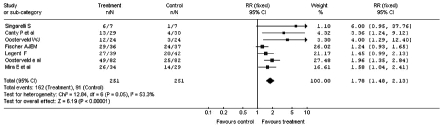
Fig. 2.
Improvement in vertiginous symptomatology after administration of betahistine or placebo: global case study (Relative Risk).
Results
A preliminary analysis of the studies taken into account for the meta-analysis reveals the variability of the daily doses (from 32 to 48 mg) and of the periods of treatment (from 1 to 3 months).
The overall case series obtained from the 7 studies selected comprises 367 patients. As the subjects included in the double-blind cross-over studies were assessed after both treatments (with betahistine and placebo), 251 subjects treated with betahistine and 251 with placebo were available.
The period of time, when the studies were carried out (from 1979 to 2003) allows an estimation to be made, as mentioned above, of the variability in the clinical end-points considered in the various studies. In order to make the end-ponts of the different studies as uniform as possibile, we re-classified the subjective evaluations of the physicians and/or patients in the binary variables “Improved” and “Not improved”. Overall, 175 patients showed an “Improved” outcome in the group treated with betahistine and 92 in the control study, treated with placebo.
Meta-analysis of the overall sample
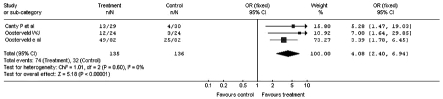
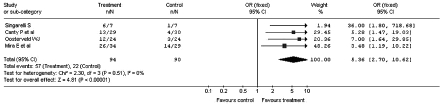
The meta-analysis carried out on the overall sample of the clinical studies selected, calculated a Odds Ratio (OR) in favour of the treatment with betahistine corresponding to 3.52, with a confidence interval between 2.40 and 5.18 (Fig. 1). All the studies showed a significant benefit following treatment with betahistine, except the clinical study of Fischer and Van Elferen 11 where the calculated OR was 2.24 but with a 95% confidence interval (95% CI) between 0.77 and 6.52.
Fig. 1.
Improvement of vertiginous symptomatology after administration of betahistine or placebo: global case study (Odds Ratio).
The total Relative Risk (RR) was 1.78, with a 95% CI between 1.48 and 2.13 (Fig. 2). In this case, for the studies of Singarelli 7, Fischer and Van Elferen 11 and Legent et al. 9, RR and 95% CI were, respectively, 6.00 (0.95-37.76), 1.24 (0.93-1.65) and 1.45 (0.99-2.13)
Meta-analysis of homogeneous sub-groups for experimental design
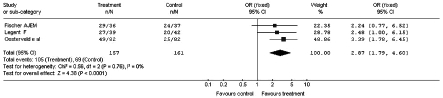
The results of the sub-analysis related to the design used in the clinical trials, carried out by dividing the study into a group (3 studies) in which, besides the double-blind randomisation, also the cross-over was established, are reported in Figure 3. Figure 4 shows the result of the meta-analysis which considered only the double-blind studies without cross-over design (4 studies).
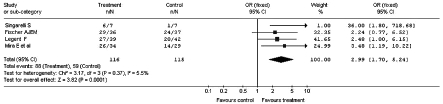
Fig. 4.
Improvement in vertiginous symptomatology after administration of betahistine or placebo: sub-analysis of parallel groups (Odds Ratio).
The meta-analysis for sub-groups, homogeneous for experimental design, totally confirms the results observed with the main meta-analysis carried out on the whole sample.
Meta-analysis of sub-groups homogeneous for dose range
The results of the meta-analysis carried out on the sub-group of 4 studies characterised by the dose of betahistine between 32 and 36 mg/day are reported in Figure 5, while the results of the meta-analysis carried out in the subgroup of 3 studies in which the dose was of 48 mg/day, are reported in Figure 6.
Fig. 5.
Improvement in vertiginous symptomatology: sub-analysis after administration of 32-36 mg/day of betahistine or placebo (Odds Ratio).
Fig. 6.
Improvement in vertiginous symptomatology: sub-analysis after administration of 48 mg/day of betahistine or placebo (Odds Ratio).
Globally, the results of these sub-analyses denote that the maximum effect of betahistine, in the cases of vertigo taken into account, may be reached already with doses of 32-36 mg/day and that higher doses would not lead, on average, to further improvement. Briefly, the sub-analysis does not show any direct relationship with the dose levels and percentage of improvements of vertiginous symptoms.
Meta-analysis of sub-groups homogenous for the range of treatment duration
The results of the meta-analysis, conducted on the sub-group of 4 studies characterized by the treatment duration between 3 and 8 weeks, are shown in Figure 7, while the results of the meta-analysis carried out on the sub-group of 3 studies where the duration was of 3-4 months, are reported in Figure 8.
Fig. 7.
Improvement in vertiginous symptomatology: sub-analysis after administration of betahistine or placebo for 3-8 weeks (Odds Ratio).
Fig. 8.
Improvement in vertiginous symptomatology: sub-analysis after administration of betahistine or placebo for 3-4 months (Odds Ratio).
Globally, the results of this sub-analysis denote that the maximum effect of betahistine, in cases of vertigo, can be reached already after 3-8 weeks of treatment. Increasing duration of treatment up to 4 months does not seem to induce a higher efficacy, although we cannot exclude that prolonged treatment may be useful to maintain and improve the clinical results already achieved.
Conclusions
Since the cause precipitating vertigo, the site of the lesion and, above all, the effects on the subject’s everyday life are difficult to identify, a meticulous analysis of the clinical history and a careful objective examination are mandatory in order to establish the instrumental and laboratory investigations necessary for a correct diagnosis and a beneficial treatment approach.
As the aetiology of balance disorders is often unknown, treatment of vertigo is based on the use of symptomatic drugs and, currently, a combined approach of a pharmacological, rehabilitative and surgical type is adopted. In this field, pharmacological treatment is more frequently used.
Drugs with an antivertiginous effect modulate the activity of neuromediators involved in the control of the vestibular system (GABA, acetylcholine, histamine). In general, they induce a decrease in the nervous activity (vestibuloplegic drugs). Among these, betahistine plays a significant role in the therapeutic approach to the vertiginous patient on account of its mode of action on the histaminergic system. Betahistine causes not only a specific inhibition of the neurons of the lateral vestibular nucleus, but also involves, centrally, the histaminergic neurotransmission and peripherally the microcirculation of the cochleo-vestibular system AS WELL AS the activity of the ampullary ciliated cells 2 5.
The clinical efficacy of betahistine on Ménière’s disease and, more in general, on the vertiginous symptoms was documented in over 100 clinical studies, mainly double-blind verum- or placebo-controlled studies.
The present meta-analysis aimed to reassess the clinical efficacy of betahistine in cases of cupulo-canalolithiasis and in cases secondary to arterial vertebrobasilar insufficiency, excluding vertigo associated with Ménière’s disease. The meta-analysis, carried out on 7, double-blind, placebo-controlled, randomized studies, despite the limits outlined, confirms the therapeutic benefit and the usefulness of betahistine treatment in cupulo-canalolithiasis and in those forms secondary to vertebrobasilar arterial deficit, regardless of the specific causes of the deficit itself.
The clinical efficacy of betahistine could be explained both by the histaminergic-like effect of vasodilation of the cerebral microcirculation and inner ear and by the action at the level of the central histaminergic system as a weak H1 agonist and H3 antagonist enhancing the process of vestibular compensation and reducing the spontaneous activity of peripheral vestibular receptors.
Moreover, the reduction in the number of attacks of positional paroxysmal vertigo, in the case of cupulo-canalolithiasis, is probably associated both with the improvement in the labyrinthine blood flow, with a relative safeguard of macular trophism, and to a modulation of the neuronal activity with a relative reduction of any eccessive vestibular reflectivity of a peripheral or central nature. Probably, this efficacy implies, above all, the presence of microcirculation disorders in a large number of treated cases. These disorders could, actually, induce the above-mentioned conditions facilitating the onset of chronic symptomatology. Certainly, liberatory and repositioning manoeuvres remain the treatment of choice for positional paroxysmal vertigo. In these cases, betahistine is, primarily, a symptomatic treatment and useful tool in recurrent cases or in cases resistant to physical treatment.
Maximum efficacy of betahistine is obtained with long periods of treatment of 3-8 weeks and with daily doses of 32 to 36 mg. High doses, up to 48 mg/day, or treatment periods prolonged up to 4 months do not seem to induce, on average, further benefits. However, this does not exclude the possibility that these high doses can be useful in a selected number of patients to control the vertiginous symptoms, although the present meta-analysis is not technically suitable to demonstrate this benefit. Furthermore, experimental data obtained in animals, even using very high doses (50-100 mg/kg), did not show an univocal relationship between the dose and the clinical efficacy 13, indicating, at the most, that low doses of betahistine inhibit more selectively the synaptic transmission of polysynaptic neurons of the lateral vestibular nucleus 15.
References
- 1.Frew IJC, Menon GN. Betahistine hydrochloride in Ménière’s disease. Postgrad Med J 1976;52:501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacour M, Sterkers O. Histamine and betahistine in the treatment of vertigo. Elucidation of mechanisms of action. CNS Drugs 2001;15:853-70. [DOI] [PubMed] [Google Scholar]
- 3.Arrang JM, Garbarg M, Quach TT, Tuong MDT, Yeramian E, Schwartz JC. Actions of betahistine at histamine receptors in the brain. Eur J Pharm 1985;111:73-84. [DOI] [PubMed] [Google Scholar]
- 4.Wilmot TJ, Menon GN. Betahistine in Ménière’s disease. J Laryngol Otol 1976;9:833-40. [DOI] [PubMed] [Google Scholar]
- 5.The mechanism of action of betahistine: towards a consensus. IMN (International Medical News) 1999;99:1-6. [Google Scholar]
- 6.Singarelli S. Studio in doppio cieco sull’efficacia del cloroidrato di betaistina nel trattamento ambulatoriale di un gruppo di pazienti affetti da vertigine di posizione e acufeni. Nuovo Arch Ital Otol 1979;7(Suppl):69-72. [Google Scholar]
- 7.Canty P, Valentine J, Papworth SJ. Betahistine in peripheral vertigo. A double-blind, placebo-controlled, cross-over study of Serc versus placebo. J Laryngol Otol 1981;95:687-92. [PubMed] [Google Scholar]
- 8.Oosterveld WJ. Betahistine dihydrochloride in the treatment of vertigo of peripheral vestibular origin. A double-blind placebo-controlled study. J Laryngol Otol 1984;98:37-41. [DOI] [PubMed] [Google Scholar]
- 9.Legent F, Calais C, Cellier D. Vertiges paroxystiques itératifs et Serc. Étude clinique contrôlée. Concours Med 1988;110:2539-43. [Google Scholar]
- 10.Oosterveld WJ, Blijleven W, Van Elferen LWM. Betahistine versus placebo in paroxysmal vertigo; a double-blind trial. JDR (J Drug Therapy Res) 1989;14:122-6. [Google Scholar]
- 11.Fischer AJEM, Van Elferen LWM. Betahistine bij de behandeling van aanyalsgewijs optredende duizeligheid. Een dubbelblind onderzoek. JDR (J Drug Therapy Res) 1985;10:933-7. [Google Scholar]
- 12.Mira E, Guidetti G, Ghilardi PL, Fattori B, Malannino N, Maiolino L, et al. Betahistine dihydrochloride in the treatment of peripheral vestibular vertigo. Eur Arch Otorhinolaryngol 2003;260:73-7. [DOI] [PubMed] [Google Scholar]
- 13.Mira E. Betahistine in the treatment of vertigo. History and clinical implications of recent pharmacological researches. Acta Otorhinolaryngol Ital 2001;66(Suppl):1-7. [PubMed] [Google Scholar]
- 14.Tighilet B, Leonard J, Lacour M. Betahistine dihydrochloride treatment facilitates vestibular compensation in the cat. J Vestib Res 1995;5:53-66. [PubMed] [Google Scholar]
- 15.Unemoto H, Sasa M, Takaori S, Ito J, Matsuoka I. Inhibitory effect of betahistine on polysynaptic neurons in the lateral vestibular nucleus. Arch Otorhinolaryngol 1982;236:229-36. [DOI] [PubMed] [Google Scholar]