Summary
The Child Hearing Early Assessment Programme (CHEAP) regional project, was a combined departmental approach (Audiology, Neonatology) of the University Hospital of Ferrara, aimed at identifying neonatal hearing impairment and defining early intervention strategies. Aims of this project have been: i. construction of a neonatal screening programme using evoked otoacoustic emission and auditory brainstem responses; ii. the calculation of a precise estimate of cost-benefits for every child tested; iii. the development of an information flow instrument (database) for the storage of data and the statistical analysis of the results. The present report refers only to the results of the project related to the otoacoustic emission data from well-babies and intensive care unit residents. In the period January 2000-December 2004, 4269 full-term newborns and 654 Neonatal Intensive Care Unit babies were tested at the Neonatology Department. The cost of the Universal Neonatal Hearing Screening was estimated at € 9,20 per child, considering the use of the ILO-292 apparatus, and € 8,28 per child in the case of an automatic screener. In this screening model, the initial hardware costs can be re-iterated into budget in a period of two years, if 1000 children per year are tested.
Keywords: Newborn Hearing Screening, Otoacoustic emissions, Distortion products, Auditory Brainstem Responses
Riassunto
Il CHEAP (Child Hearing Early Assessment Programme) è stato un progetto regionale e multi-dipartimentale (Audiologia, Neonatologia) dell’Università di Ferrara mirato all’identificazione della ipoacusia infantile e ad un intervento precoce. Gli obiettivi del progetto sono stati la costruzione di un programma di screening neonatale con emissioni otoacustiche (OAE) e potenziali del tronco, il calcolo di una stima precisa dei costi-benefici per ogni bambino testato e lo sviluppo del software per l’archiviazione e l’analisi statistica delle risposte ottenute. Nel presente articolo, gli Autori riportano solo i risultati relativi a quattro anni (2000-2004) di screening uditivo neonatale con le OAE; al Nido sono stati testati 4269 neonati in totale, 654 in terapia intensiva. Una stima dei costi associati allo screening neonatale è di 9,20 € per bambino se si considera come apparecchio l’ILO-292 e 8,28 € per bambino nel caso si utilizzi un apparecchio automatico; tali costi/investimenti iniziali possono essere azzerati in un periodo di due anni se vengono testati almeno 1000 bambini all’anno.
Introduction
In 2001, according to the World Health Organization (WHO) data, 250 million individuals in the world were affected by an auditory impairment (about 28 million in the USA). Hearing impairment is the most common congenital disease (3.5-9%) considering all degrees of permanent uni- or bilateral deafness 1–5. Today, early detection of permanent infant hearing impairment is of great importance, since appropriate procedures involving hearing aids and rehabilitation can be taken to ensure better language development and superior cognitive functions 2 6. Recent evidence indicates that timely hearing aid and rehabilitation treatment are decisive for the positive management of hearing impaired children: with the use of a suitable hearing aid within the first 3-6 months, speech and language development, psychological balance, and school and social integration can become similar as may reach levels similar to those of children of the same age with normal hearing 2 4–6.
The main objective of neonatal hearing screening is the early identification of all cases with mild-severe hearing loss (> 35-40 dB HL). Recently, numerous studies 8–10 have proven the feasibility of newborn hearing screening strategies based on the acquisition of otoacoustic emission responses (OAE). These responses are non-linear acoustic signals generated by the outer hair cells of the organ of Corti. They are always present in subjects with normal hearing thresholds and are currently considered as indices of regular cochlear function. The OAEs are absent in the case of sensorineural hearing losses ≥ 40 dB HL. Clinically, the OAEs can be classified in two types; transient emissions (TEOAEs), evoked from transient stimuli 11–16 and distortion product emissions (DPOAEs) evoked from two pure tones of different frequency simultaneously received by the cochlea 17. Despite the strength of the clinical OAE protocols used clinically, the OAE community has always been interested in the development of new protocols and procedures which could minimize the time required for an OAE recording. An example is the Maximum Length Sequence (MLS) method, where it is possible to obtain reliable data (i.e., cochlear responses) in times ≥ 2s 10. Nevertheless, a commercial implementation of this technique is not currently available.
In neonatal screening, it is preferable to first consider populations with a higher probability of developing a hearing impairment. The risk factor criteria proposed by the Joint Committee on Infant Hearing (JCIH) (Table I) are very useful for the recognition of this category of subjects 18. It has been reported that, unfortunately, about 50% of the infantile cases identified with a hearing impairment, do not show any of the JCIH factors 19 20. Therefore, a screening programme based only on “babies with JCIH risk factors” could potentially miss a significant percentage of cases presenting hearing impairment. In addition, assessment of risk-factor cases only with OAEs could potentially miss cases presenting retrocochlear hearing complications (i.e., Auditory Neuropathy). In order to reduce these critical errors, and in order to correctly identify the maximum number of infants with mild to moderate hearing impairment, several Authors have proposed the combination of two main strategies:
Table I. JCIH criteria for the identification of hearing impairment risks.
|
Newborn audiological risk factors (0-28 days) (JCIH, 1990, 1994, 2000) |
Intrauterine infections
|
Cranio-Facial abnormalities
|
| Familiarity for infantile sensorineural hypoacusis |
| Low birth weight (< 1500 g) |
Disorders usually associated with sensorineural and/or conductive hypoacusis
|
Severe dysfunction at birth
|
| Icterus |
Hospitalisation NICU > 48 hours
|
|
Post-natal audiological risk factors (29 days - 2 years) (JCIH, 1990, 1994, 2000) |
Post-natal infections associated with sensorineural hypoacusis
|
| Recurrent or persistent otitis media |
| Cranial Trauma |
screening on all newborns (well-babies) with TEOAEs or DPOAEs;
screening with multiple protocols (using OAEs and automated ABR) all newborns with risk indicators (NICU residents). With this set of protocols, it is also possible to obtain estimates of auditory neuropathy cases (OAEs present, ABR asynchronous or not present).
The CHEAP project was designed to collect data from a large sample size in the greater area of Ferrara (Emilia Romagna). The aims of the project can be summarized in the following three areas:
set up a neonatal screening programme using OAE and ABR responses; the precise estimate of administration costs for every child tested; the clinical comparison/efficacy of the OAE and ABR devices employed;
early identification of infantile hearing loss (within the VI-VII month of life); estimate of the degree of parental anxiety related to a child with a hearing impairment;
creation of an instrument to more easily divulge the flow of neonatal screening information, from data collection to data reporting.
In the present article (Part 1), an overview of findings emerging during the setting up of the project are reported. Further information will be presented in Parts 2 and 3.
Material and methods
The recordings from well-babies and Neonatal Intensive Care Unit (NICU) residents were collected during the period January 2000 - December 2004, at the University Hospital of Ferrara.
Various instruments have been used in the collection of OAE data ranging from third generation devices (ILO-292 from Otodynamics) to fourth generation palm-type instruments (Accuscreen from Fischer-Zoth; Eclipse from Labat; Otoread from Interacoustics; Audioscreener from Viasys). Numerous devices were used in the acquistion of Automated ABR data, such as Natus (Algo portable), AccuScreen and Audioscreener.
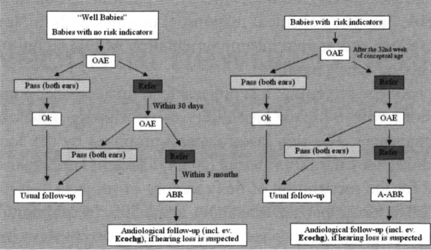
Screening of well-babies (newborns with no risk factors) was carried out within the first 48 hours of life, using a three-phase (OAE-OAE-ABR) procedure (Fig. 1). TEOAEs or DPOAEs were employed in the assessment of cochlear function; TEOAEs were used primarily in the linear protocol mode with an eliciting stimulus of 75 dB SPL. DPOAEs were elicited by asymmetrical protocols (75-65 dB SPL) testing the frequencies 1.5, 2.0, 3.0, 4.0 and 6.0 kHz. The DPOAEs were found to be more immune to noise than TEOAEs and were very useful in PASS border-line cases. The rationale for the choice of OAE parameters and additional details can be found elsewhere 13 15. The presence of an OAE response in both ears was considered as a “PASS”. The criteria for a PASS were based on previously established signal and signal to noise ratio (S/N) values 13–15. In the event, an acceptable OAE response was not present, even in one ear, a second test with OAEs was performed within 15-30 days. If the result was again a “REFER”, an ABR test within the third month of age was performed, and if the suspected impairment was confirmed, a complete audiological evaluation was carried out (ABR and where necessary electrocochleography).
Fig. 1.
Newborn Hearing Screening using a three-stage procedure.
For screening performed at the NICU, a three-phase procedure (OAE-OAE-AABR) was used (Fig. 1). OAE screening was performed in pre-term babies after the 32nd week of conceptual age, and an acceptable OAE response, in both ears, was necessary for a PASS. If the outcome of the second OAE test (mainly DPOAE) was a REFER then an automated-ABR (AABR) test was performed within 24 hours. In the case of suspected impairment, a complete audiological evaluation with clinical ABR was carried out (in most cases, with electrocochleography).
For the comparison of devices, we set the ILO-92 as the gold standard and the PASS-REFER rates of this device were compared with the performance of the other devices. The format of the tests was device X (ILO-292) vs. device Y. A table was constructed with the number of PASSes and REFERs of each device. To evaluate performance, McNemar’s test based on matched-pair data (devices used on same subjects) were used. The statistical procedure considers the distribution of discordant pairs (off-diagonal entries in the table). Due to the time required to perform the OAE test, only two devices were compared each time, maintaining the ILO-292 as the reference device. Each group comprised 120 recordings, 80 from well-babies and 40 from NICU residents.
The PASS cases (TEOAE responses only) were pooled (except the Accuscreen data) in order to investigate whether the PASS parameters of device X were significantly similar to the PASS parameters of device Y. For the numerical comparison of devices, mean differences were estimated per variable of interest and tolerance intervals were constructed with p ≤ 0.05. The parameters investigated were: correlation and signal to noise at 2.0, 3.0, and 4.0 kHz.
Results
Well-babies
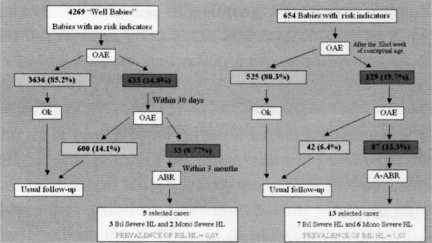
A total of 4269 full-term newborns were tested during the period January 2000 - December 2004. Of these, 633 (14.8%) failed the first OAE screening, and were re-tested within 30 days; 33 cases (0.77%) failed the second stage OAE test and were studied using ABR and electrocochleography. For that population, 5 cases (0.12%) were found with hearing impairment. Of these, 3 babies presented bilateral profound hearing loss, one of whom (born in 2001) has already received a cochlear implant; the remaining 3 presented unilateral severe deafness and are still in follow-up. The prevalence of bilateral hearing loss, in this group, was estimated as 0.07%.
NICU babies
Of the 654 NICU babies tested, January 2000 - December 2004, 129 (19.7%) failed the first OAE session (Fig. 2), 87 of whom (13.3%) resulted as REFER, in the second OAE test and underwent further audiological tests (ABR and electrocochleography). In the latter group, 13 cases (2%) were found to have hearing impairment. Of these, 6 presented unilateral severe deafness, while the remaining 7 had severe bilateral hearing loss. One of these babies is waiting for a cochlear implant. Another 3 babies have concomitant severe psycho-neurological retardation, and are presently in a follow-up programme; one baby, born in 2002, is using bilateral hearing aids and is also enrolled in a follow-up programme; the 2 remaining cases came from outside the Ferrara area and were offered assistance in another hospital in the Emilia-Romagna Region. The prevalence of bilateral hearing loss in this group was, therefore, 1.07%. Despite the combined use of OAEs and AABR, in the NICU population, since 2003, no cases of Auditory Neuropathy have been identified.
Fig. 2.
Hearing Screening results at the Ferrara University Hospital.
Screening Costs
For the screening costs, we have considered two possible scenarios, one with the purchase of the ILO-292 and another with the purchase of a portable device. Additional costs were related to the salary of the technician/s performing the clinical OAE tests, the cost of maintaining the database and various computer items. The data from well-babies and NICU residents were pooled and are presented analytically in Table II A,B. The average cost per tested infant varies from € 8.28 to € 9.20. The costs of the screening of infants presenting hearing impairment (a total of 10 bilateral cases) are much higher, ranging from € 4030 to € 4530.
Table II A. Costs of the screening protocol using ILO-292 (A), and an automatic apparatus (B).
| Parameter | Costs in € (VAT incl) |
| OAE apparatus (ILO-292) | 10.000,00 |
| Database and data tracking software | 4.000,00 |
| Salary of OAE operator (48 mos) | 24.000,00 |
| PC with Laser printer | 1.500,00 |
| Collection and storage of data (48 mos) | 4.800,00 |
| Phone calls, letters, other paper material (48 mos) | 1000,00 |
| Total | 45.300,00 |
| For every tested baby (45300/4923) | 9,20 |
| For every identified baby (45300/10) | 4530,00 |
Comparison of Devices
For the well-babies, the performance of devices was very similar and no significant differences were found in the counts of PASS or REFER. The order, in terms of best performance, was: Accuscreen, Eclipse & Otoread, Audioscreener, ILO-292. The order (best performance) differed for the NICU data, being: Audioscreener, Otoread, Eclipse & Accuscreen, ILO-292. In the NICU data, significant differences in performance (with the exception of PASSes) were observed between the first two devices and the other three.
The comparison of the PASS responses between devices indicated that the PASS variables have significantly different values across the various devices. Analytically: (i) between ILO-292 and Audioscreener, differences were found in the correlation and S/N, at 4.0 kHz; (ii) between ILO-292 and Eclipse, differences were found at the S/N of 4.0 kHz; (iii) between ILO-292 and OtoRead, differences were found at the S/N values, at 3.0 and 4.0 kHz.
Discussion
The main objective of CHEAP project was to set up a local screening programme (Ferrara and neighbourhood) on well-babies and pre-term infants with the possibility of offering intervention services to those in need, before the age of 6 months.
The percentages of territorial coverage and efficiency have been close to the limit established before the beginning of the project (i.e., ≥ 90%). Assuming the availability of regional funds, this percentage might increase significantly in the future by using a larger number of screening devices and network technologies. The increase of territorial coverage does not always coincide with increased screening efficiency. Data from larger screening programmes in the US 3 21 have indicated that as the territorial coverage increases, the percentage of non-returning cases can rise exponentially to estimates close to 20% or 30% of the total number of subjects tested.
The data from the project related to the well-babies indicated that this population is easily assessed and that the incidence of bilateral hearing loss is low. The data indicate that less than one child (0.7) per thousand is suffering from hearing impairment. Earlier studies in the literature 7 18 22 have reported values ranging from 1.2 to 2.0 cases per thousand. We postulate that the difference between those estimates and that from our study, depends on two factors: (i) the very homogeneous population of the greater Ferrara area; (ii) the impact of using newer and state-of-the-art screening technology (fourth generation palm OAE devices).
The percentage of bilateral hearing impairment, detected by our screening programme in the NICU population (1.07%), is also below the estimates presented in the literature. The probable cause for this apparent discrepancy (again excluding issues related to population homogeneity) is that the majority of the NICU testing (at least in the first two years of the project) has been on cases with specific risk factors. These are low birth weight, prolonged mechanical ventilation, hyperbilirubinaemia and low Apgar score. Prolonged mechanical ventilation has been considered a risk indicator for hearing loss by the Joint Committee on Infant Hearing since 1990, although in 1994, the risk indicator was changed to mechanical ventilation of 5 days or longer. The spectrum of risk factors, identified in the NICU population, is part of a dynamic process that has continued to change since the early 1990s. In fact, there are more extremely low birth weight infants surviving at the limits of viability, and new technology and treatment continue to emerge, such as high frequency ventilation and surfactants. Some diseases, which previously had high prevalence rates, such as congenital rubella, have been effectively reduced. We contemplate that the constant change in the characteristics of the NICU population will also affect the specificity of neonatal screening.
One of the most important issues in neonatal screening practice is the policy related to false positive cases (i.e., normal hearing infants evaluated as hearing impaired by OAEs). In our experience, this aspect is minimal in the well-baby population (25/4269 = 0.5%) due to various factors, mainly proper acoustic conditions during the tests. The NICU environment is more corrupt, from an acoustic and electro-magnetic point of view, and the number of false positives rises to 74/654 (11.3%). This estimate can be minimized by the application in situ of AABR measurements or by numerous repetitions of OAE or automated OAE (AOAE) measurements. The data from the CHEAP project show that AABR measurements probably influence the false positive (FP) estimate. During the first two years of the project, the FP was 15.2% and in the last two years (where AABR and multiple AOAEs were used), the estimate dropped to 11.4%. The difference in FP are marginally significant and from the data available it cannot be established which factor (AABR, AOAE, or the tracking queries of the database) contributed the most.
The anxiety induced, in the family, by a “false positive” response is one of the disadvantages associated with any screening programme, even if several Authors have already demonstrated that most relatives are in favour of these projects 18 23–25. As in every screening programme, the presence of “false positive” results is possible, even if it should be < 3-4%, also with a tendency to decrease as soon as the expertise of the operators improves. Moreover, factors that can influence the “false positive” rate include the presence of the baby’s movements or regurgitation during the exam, or obstruction of the external auditory canal 23 24 26. Other emotional factors include anxiety (71%), stress (29%), impatience (25%), search for aid (24%), depression (13%), sense of guilt (7%). These feelings could remain present, in the baby’s family, even for months or years 29, even if other audiological exams then proved the absence of hearing impairment. However, reasons in favour of a screening programme are high 18 25 27 28 even if the long-term effects induced by those emotional states are presently unknown. Data in the literature support our experience that neonatal hearing screening is well accepted by families 18 25 29 30 and, in the light of these data, the importance of sharing qualitative information with the families of infants is considered as one of the most fundamental factors for a screening programme aiming to achieve an efficient intervention policy.
We also estimated the screening costs of the CHEAP project, using several simplistic scenarios (Table IIA, B). The initial financial investment can be recovered in a relatively short period of 24 months, assuming the possibility to clinically assess 1000 infants per year with a cost of the screening test equal to € 24. Considering the time span of the project, the neonatal screening costs using ILO-292 have been estimated to be € 9.20 per child, while costs for the use of an automatic apparatus are lower (€ 8.28). The estimates presented do not include the costs for the diffusion of screening results (via web or printed paper); the final cost is less than the estimate of € 23 per child, that we had considered as the maximum attainable value at the beginning of the project.
The evaluation of 5 AOAE devices suggested that, in terms of technology, the performance of most screening equipment was comparable. This finding may be misleading since it refers to data from the well-baby clinic. Testing, in a silent room, an infant which is not agitated does not challenge the OAE technology, we have today, and the results obtained reflect the pleasant surroundings and subject conditions (necessary for an OAE test). In the NICU, the acoustic and electromagnetic noise level is significantly higher and present a suitable challenge to current OAE technology. The gold standard (ILO-292) did not perform very well as the diameter of the probe of this device (plus the neonatal tip) was wider than the external meatus of the infants tested and adds considerable external noise to the OAE recording. These issues were completely eliminated by the use of the smaller probes from the fourth generation devices. The neonatal probes currently supplied by Otodynamics have tips of smaller dimensions to address the fitting problems in the NICU. It is also worthwhile mentioning that the fourth generation devices have shown a superior performance in terms of immunity to electromagnetic interference which is quite common in the NICU facilities, as we have experienced with earlier versions of the ILO equipment.
Comparison of the TEOAE variables from the 4 devices (Accuscreen excluded), for PASS cases, indicated that the PASS responses differ significantly between devices. This is not an issue for clear-cut PASS well-baby or NICU cases, but we postulate that it could be a problem in borderline cases. The ILO-292 responses appear to enhance the high frequency components of the TEOAE response in comparison to the data structures recorded with the other portable devices.
The complexity of the CHEAP project suggested, from the initial design stages, the need of a tool to make the flow of information easier, i.e., from the OAE registration to the actual performance reports. As the number of subjects tested increases, the need to track individual cases (mostly REFERs) also increases. Without such an instrument, it is difficult to further develop a Universal Neonatal Screening programme and to offer good intervention strategies to the infants identified with various hearing deficits. In the context of the CHEAP programme, the small number of REFERs did not permit a definite analysis of the impact of our tracking system. A number of recent studies, however, have tested our hypothesis concerning the importance of a database in an Early Hearing Detection and Intervention programme 31–33. The database served not only as an archive of data and a tracking monitor of the REFER activity, but was also used to analyze and search the data for various important patterns. For more information on this structure see Appendix.
Conclusions
The present data indicate that the well-baby population and the NICU residents can be easily assessed for estimates of hearing and that hearing screening is only the first step in the care of a hearing-impaired infant. In our opinion, a database for the statistical analysis of the data and eventual patient tracking is a tool able to efficiently manage the flow of information. One of the most important issues in a universal screening programme is to reduce the “false positive” rate that can generate anxiety in the infant’s family. The transition to early intervention should be as unemotional as possible, with a cohesive team of health care professionals capable of providing medical, audiologic, communication, and educational management for the infant, as well as emotional support for the family.
Appendix: software development
The term database relates primarily to the term Information management and plays an important role in the current Early Hearing Detection and Intervention programmes (EHDI), which are the evolution of the UNHS models we have developed over the last few years. The software for data tracking is not supplied by any of the manufacturers of OAE devices and, at present, can be found only in the US with prices equivalent to the cost of the AOAE devices themselves. In this context, it was considered very advantageous to develop a European tracking platform which could be distributed, with minimal costs, to all the centres taking part in the screening. Thus, the development of software for tracking-data structure (database) became one of the main aims of the CHEAP project. The database was designed with the aim of improving:
collection of OAE data from different OAE equipment (i.e., data directly downloaded into the database or introduced manually);
statistical analysis of the data collected with pre-design forms (patterns);
tracking and monitoring of REFER cases;
creation of messages/letters to invite REFER subjects for re-testing;
generation of reports presenting screening performance of the project per population or per time interval;
possibility to run on an INTERNET server and connect many screening sites with a central coordination station.
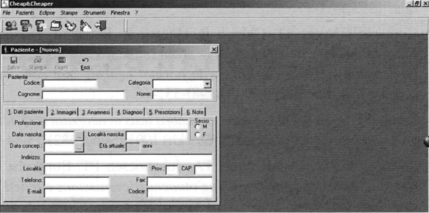
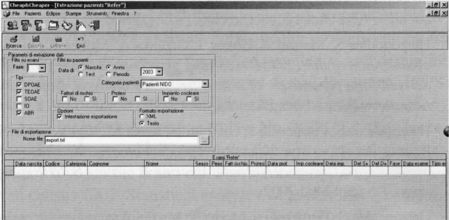
Data are inserted in the database by filling out a pre-specified form (Fig. 3); the database contains personal data of each infant, the number of OAE/AOAE/ABR/AABR tests carried out, and also the eventual hearing aid prescription or eventual date of cochlear implantation. It is even possible to estimate the territorial coverage or the screening performance, or even the number of PASS and REFER results through the various phases of the screening. Figure 4 shows a query of REFER patients; it is possible to choose the period of interest, select patients with risk factors, or even the phase or type of examination.
Fig. 3.
Database software: the screen-shot shows the input module for inserting data related to a subject.
Fig. 4.
Database software: the screen-shot shows the query which identifies the REFER patients during phases 2 and 3 or during the whole screening procedure.
The database has been designed with funds from the CHEAP regional project and maintained and updated with contributions from the LABAT company (for information see http://www.labat.it). For more detailed information on the structure of the database see the Otoacoustic Emissions Portal http://www.otoemissions.org
Table II B.
| Parameter | Costs in € (VAT incl) |
| Automatic OAE Apparatus | 5.500,00 |
| Database and data tracking software | 4.000,00 |
| Salary of OAE operator (48 mos) | 24.000,00 |
| PC with Laser printer | 1.500,00 |
| Collection and storage of data (48 mos) | 4.800,00 |
| Phone calls, letters, and other paper material (48 mos) | 1000,00 |
| Total | 40.800,00 |
| For every tested baby (40800/4923) | 8,28 |
| For every identified baby (40800/10) | 4080 |
Acknowledgments
These project was supported by a grant from the Emilia Romagna region (cheaper).
References
- 1.Parving A. Prevalence of congenital hearing impairment and risk factors. Neonatal hearing screening. In: Grandori F, Lutman M, editors. The European Consensus Development Conference on Neonatal Hearing Screening. Milan: 1998. p. 6-10. [Google Scholar]
- 2.Moeller MP. Early intervention and language development in children who are deaf and hard of hearing. Pediatrics 2000;106:43-51. [DOI] [PubMed] [Google Scholar]
- 3.Hayes D. Newborn hearing screening: selected experience in the United States. Scand Audiol 2001;30(Suppl 53):29-32. [DOI] [PubMed] [Google Scholar]
- 4.Yoshinaga-Itano C, Sedey AL, Coulter BA, Mehl AL. Language of early and later-identified children with hearing loss. Pediatrics 1998;102:1168-71. [DOI] [PubMed] [Google Scholar]
- 5.Apuzzo ML, Yoshinaga-Itano C. Early identification of infants with significant hearing loss and the Minnesota Child Development Inventory. Semin Hear 1995;16:124-39. [Google Scholar]
- 6.Molini E, Ricci G, Baroni S, Ciorba A, Bellocci A, Simoncelli C. Identifying congenital hearing impairment. Personal experience based on selective hearing screening. Acta Otorhinolaryngol Ital 2004;24:109-16. [PubMed] [Google Scholar]
- 7.Mauk GW, Beherens TR. Historical political and technological context associated with early identification of hearing loss. Sem Hear 1993;14:1-17. [Google Scholar]
- 8.Gorga MP, Neely ST, Ohlrich B, Hoover B, Redner J, Peters J. From laboratory to clinic: A large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear 1997;18:440-55. [DOI] [PubMed] [Google Scholar]
- 9.Kemp DT, Ryan S. Otoacoustic emissions tests in neonatal screening programmes. Acta Otolaryngol (Stockh) 1991;(Suppl 482):73-84. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen AN, Osterhammel PA, Johannesen PT, Borgkvist B. Neonatal hearing screening using otoacoustic emissions elicited by maximum length sequences. Br J Audiol 1998;32:355-66. [DOI] [PubMed] [Google Scholar]
- 11.Vohr BR, Maxon AB. Screening infants for hearing impairment. J Pediatr 1996;128:710-4. [DOI] [PubMed] [Google Scholar]
- 12.Hatzopoulos S, Petruccelli J, Pelosi G, Martini A. An optimized neonatal TEOAE screening protocol based on linear stimulus sequences. Acta Otolaryngol (Stockh) 1999;119:135-9. [DOI] [PubMed] [Google Scholar]
- 13.Hatzopoulos S, Martini A, Cheng J, Grzanka A, Morlet T. On the optimization of the TEOAE recording protocols. A linear protocol derived from parameters of a time-frequency analysis. Data from neonatal subjects. Scand Aud 2000;29:21-7. [DOI] [PubMed] [Google Scholar]
- 14.Hatzopoulos S, Petruccelli J, Giarbini N, Rossi M, Vigi V, Chierici R, et al. A comparison of distortion product otoacoustic emissions protocols in a Universal Neonatal Hearing Screening (UNHS) program. J Audiol Med 2002. [Google Scholar]
- 15.Hatzopoulos S, Petruccelli J, Morlet T, Martini A. Otoacoustic emission protocols revised. Data from adult subjects. IJA 2002;42:339-47. [DOI] [PubMed] [Google Scholar]
- 16.Kemp DT, Ryan S. Use of transiently evoked otoacoustic emissions in neonatal screening programmes. Sem in Hear 1993;14:33-6. [Google Scholar]
- 17.Huang JM, Berlin CI, Keats JB, Lin ST, Money M. The application of distortion product otoacoustic emissions to identify carriers of recessive hereditary deafness. In: Berlin C, editor. Hair Cells and Hearing Aids. San Diego: Singular Publishing Group; 1996. p. 57-72. [Google Scholar]
- 18.Reuter G, Bordgen F, Dressler F, Schafer S, Hemmanouil I, Schonweiler R, et al. Neonatal hearing screening with the Echosensor automated device for otoacoustic emissions. A comparative study. HNO 1998;46:932-41. [DOI] [PubMed] [Google Scholar]
- 19.Joint Committee on Infant Hearing 1990 position statement. ASHA 1991;33:3-6. [PubMed]
- 20.Mauk GW, White KR, Mortensen LB, Beherens TR. The effectiveness of hearing programs based on high-risk characteristic in early intervention of hearing impairment. Ear Hear 1991;12:312-9. [DOI] [PubMed] [Google Scholar]
- 21.Vohr BR, Widen JE, Cone-Wesson B, Sininger Y, Gorga MP, Folson RC, et al. Identification of neonatal hearing impairment: characteristis of infants in the neonatal intensive care unit and well-baby nursery. Ear Hear 2000;25:373-82. [DOI] [PubMed] [Google Scholar]
- 22.Proceedings of the European Consensus Development Conference on Neonatal Hearing Screening. Milan, May 15-16, 1999 da http://www.nhs.polimi.it
- 23.de Uzcategui C, Yoshinaga-Itano C. Parent’s reactions to newborn hearing screening. Audiology Today 1997;9:24-7. [Google Scholar]
- 24.Polukakis Z, Barker M, Wake M. Six month impact of false positive in an Australian infant hearing screening programme. Arch Dis Child 2003;88:20-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watkin PM, Balwin M, Dixon S, Beckam A. Maternal anxiety and attitude to universal neonatal hearing screening. Br J Audiol 1998;32:27-37. [DOI] [PubMed] [Google Scholar]
- 26.Proceedings of the European Concerted Action HEAR. Bibione 1999, HEAR infoletter 6.
- 27.Mason JA, Herrmann KR. Screening universale dell’udito nel bambino mediante misurazione automatizzata delle risposte dei potenziali evocati uditivi. Pediatr 1998;10:42-9. [Google Scholar]
- 28.Parving A. Aetiological diagnosis in hearing-impaired children- clinical value and application of a modern examination programme. Int J Pediatr Otorhinol 1984;7:29-38. [DOI] [PubMed] [Google Scholar]
- 29.White KR, Culpepper B, Maxon AB, Vohr BR, Mauk GW. Transient evoked otoacoustic emission-based screening in typical nurseries: a response to Jacobson and Jacobson. Int J Pediatr Otorhinol 1995;33:17-21. [DOI] [PubMed] [Google Scholar]
- 30.Magnunson M, Hergils L. The parents’ view on hearing screening newborns. Feelings, thoughts and opinions on otoacustic emissions screening. Scand Audiol 1999;28:47-56. [DOI] [PubMed] [Google Scholar]
- 31.White KR. The current status of EHDI programs in the United States. Ment Retard Dev Disabil Res Rev 2003;9:79-88. [DOI] [PubMed] [Google Scholar]
- 32.Helfer TM, Lee RB, Maris DC, Shields AR. Wanted: a national standard for early hearing detection and intervention outcomes data. Am J Audiol 2003;12:23-30. [DOI] [PubMed] [Google Scholar]
- 33.Delb W, Merkel D, Pilorget K, Schmitt J, Plinkert PK. Effectiveness of a TEOAE-based screening program. Can a patient-tracking system effectively be organized using modern information technology and central data management? Eur Arch Otorhinolaryngol 2004;261:191-6. [DOI] [PubMed] [Google Scholar]