Summary
Aim of this prospective preliminary study was to assess effectiveness and reliability of autofluorescence endoscopy in the diagnosis of laryngeal lesions, in particular, evaluating whether it could represent an improvement in comparison to standard endoscopy alone. A total of 81 laryngeal biopsy specimens, taken from 46 consecutive patients who underwent intra-operative endoscopic assessment in a period of 2 years, were examined. Thirteen patients underwent the procedure for presumed benign lesions; the other 33 cases for pre-operative endoscopic suspicion of pre-cancerous or cancerous lesions. In our experience, autofluorescence evaluation seemed to accurately delineate the limits of the tumour and the possible presence of second primary, proving a useful guide in the choice of sites to make a biopsy. In particular, this endoscopic method has proved to be characterized by higher sensitivity and specificity not inferior to standard endoscopy, both in the discrimination between benign and preneoplastic/neoplastic lesions and between pre-neoplastic and neoplastic. Correct choice of the application field is mandatory in order to obtain the maximum effectiveness of this method. Autofluorescence endoscopy, in fact, was found to be very useful in the evaluation of untreated tissues with suspected pre-cancerous or cancerous lesions. On the contrary, in the study of “frankly benign” laryngeal lesions, this exam does not improve upon the results obtained by standard endoscopy but increases the risk of false positives. The best results can be obtained only by integration of data provided by both white-light and accurate auto-fluorescence endoscopic assessment.
Keywords: Larynx, Precancerous lesions, Diagnosis, Laryngeal endoscopy, Autofluorescence
Riassunto
Lo scopo di questo studio preliminare prospettico è stato di valutare l’utilità dell’endoscopia ad autofluorescenza nella diagnosi delle lesioni laringee considerando, in particolare, se questa metodica possa rappresentare un miglioramento rispetto alla sola endoscopia standard. Complessivamente sono stati esaminati 81 campioni bioptici prelevati da 46 pazienti consecutivi sottoposti a studio endoscopico intraoperatorio nell’arco di 2 anni. Tredici pazienti sono stati sottoposti alla procedura per neoformazioni presumibilmente benigne; gli altri 33 con il sospetto di lesioni precancerose o cancerose. Nella nostra esperienza l’endoscopia ad autofluorescenza ha dimostrato ottime capacità nell’identificare e demarcare lesioni preneoplastiche e neoplastiche così come la possibile presenza di lesioni multiple primitive rivelandosi un’utile guida nella scelta delle sedi da sottoporre a biopsia. In particolare questa metodica endoscopica ha dimostrato una maggior sensibilità, ed una specificità non inferiore, rispetto all’endoscopia standard, sia nella discriminazione tra lesioni benigne e lesioni preneoplastiche/neoplastiche, sia nella capacità di distinguere tra lesioni preneoplastiche e neoplastiche. Una scelta corretta dei campi d’applicazione è fondamentale perché questa metodica garantisca la massima efficacia. L’endoscopia ad autofluorescenza, infatti, è risultata particolarmente utile nello studio di tessuti non trattati con il sospetto di lesioni precancerose o cancerose; al contrario, l’esame di neoformazioni francamente benigne non migliora i risultati ottenibili con l’endoscopia standard aumentando il rischio di falsi positivi. In ogni caso, i risultati migliori possono essere ottenuti solo dall’integrazione dei dati emersi da un accurato studio endoscopico eseguito sia con luce bianca che con fluorescenza.
Introduction
Head and neck squamous cell carcinoma is associated with poor prognosis with 5-year survival rates of approximately 50% for all stages of the disease 1.
An epidemiological study on laryngeal carcinoma 1, performed on a large population, with 9 years follow-up, revealed a survival rate of 87.5% for early cancer (T1-T2), that drops to 76.0% for locally advanced cancer (T3-T4), and is drastically reduced to 46.2% in the presence of cervical metastases. Distant metastasis are clinically evident only in 10-15% of cases 2.
Delayed diagnosis, leading to loco-regional failure, and a high incidence of second primary are the two main reasons for poor outcome 3.
From these observations, it is obvious that an instrument offering the possibility to detect pre-cancerous-early cancerous lesions, and satellite foci or second primaries would be the key to improving the survival rate in head and neck cancer.
Previous studies on the fluorescence characteristics of normal and neoplastic tissues 4 5, represent an attempt to solve this problem. These studies are based on the considerations that certain tissutal molecules in tissue, known as fluorophores, are able to absorb electromagnetic waves increasing their energy and thus becoming excited. A high energy state is unstable, therefore, these molecules tend to loose their energy through different mechanisms. They may release part of their energy as heat and then remaining energy as photons. These radiations represent the fluorescence light that, according to Plank law, has a lower frequency and a higher wavelength than the exiting radiations.
The aspect and the degree of fluorescence of each tissue depend on three factors: amount of fluorophores, morphological aspects and wavelength of exciting light.
Auto-fluorescence (AF) was first observed by Policard in 1924 6, while in 1984 Alfano et al. 4 showed that it can distinguish normal and neoplastic human tissue.
So far, several studies have been carried out on the fluorescence characteristics of human epithelium of the urothelial and aerodigestive tract 7–9. However, fluorescence spectro-scopy alone 4 10 11, not associated with a device that “translates” into images the fluorescence characteristics, has limited its diagnostic potential and its applicability in routine evaluation.
Aim of the present investigation was to assess the effect-iveness and reliability of AF endoscopy in the diagnosis of laryngeal lesions, evaluating, in particular, whether it could represent an improvement in comparison to the standard endoscopy alone.
Material and methods
A prospective preliminary study was designed. The D-light AF system (Karl Storz, Tuttlingen, Germany) was employed. In this device, the excitatory blue light source is provided by a xenon arc lamp with an exciting wavelength ranging from 375 to 440 nm, with the possibility of commutation between AF and white light (WL) modality. The images were captured by a high resolution 3CCD camera. The light source and the camera were connected to an optic system that consists of rigid telescopes (diameter 5 mm, length 24 cm), with direct vision and angled at 30° and 70°. This system allows real-time WL endoscopy and switches over to AF modality by pressing a button.
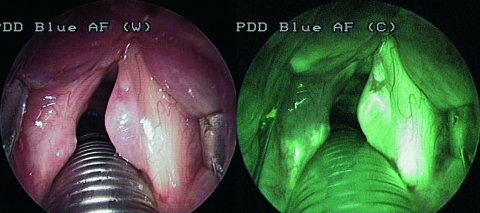
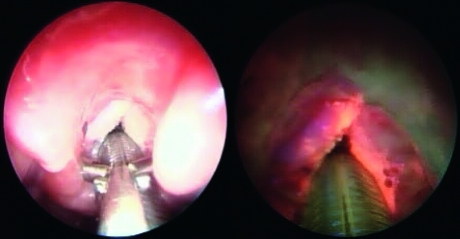
The study was carried out, in all cases, during intra-operative video-laryngo-tracheoscopy under general anaesthesia. written informed consent was obtained from each patient prior to the study. All areas of the upper aerodigestive tract were subsequently inspected with both the endoscopic modalities. In AF, one camera was positioned as follows: exposure 1:50, gain medium-high, enhancement off. A lesion was suspected to be benign if no variations in the bright green fluorescence (g.1), typical of normal laryngeal tissue, were observed (Fig. 1). A slight reduction in AF with a shift towards red/light-violet (g.2) was interpreted as a possible precancerous lesion (mild or moderate dysplasia) (Fig. 2). Absence or a marked reduction of green fluorescence, with a shift to blue/dark-violet (g.3) was considered as suspicious of a malignant lesion (severe dysplasia/in situ carcinoma or infiltrating carcinoma) (Fig. 3).
Fig. 1.
Reinke’s oedema. No colorimetric variations in normal bright green fluorescence are evident. Intensification of vascular pattern is detectable.
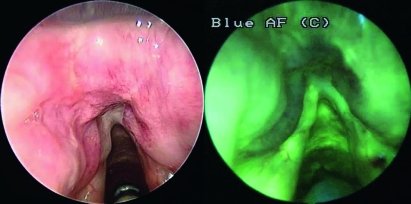
Fig. 2.
Moderate dysplasia (DL II): slight reduction of endogenous fluorescence with an increase in fluorescence at inferior margin of lesion.
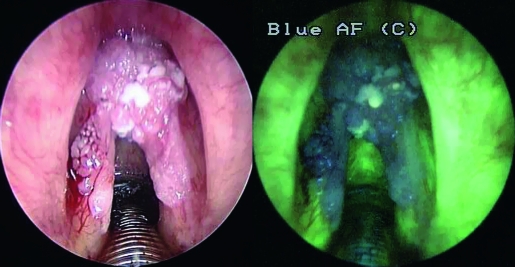
Fig. 3.
G2 infiltrating carcinoma. A marked reduction of green fluorescence, with a shift to blue/dark-violet is evident. Some “light spots”, due to hyperkeratosis, are visible in “dark area”.
All images have been recorded on a digital, custom-implemented, video system (MS Software, Treviso, Italy) for documentation and analysis. This procedure required about 10-15 minutes more than standard endoscopy alone.
Depending upon the type, size and extension of the lesions, an incisional biopsy or mucosal decortication or in toto exeresis were performed. The specimens were then routinely fixed in 4% formalin and studied by a pathologist not involved in the study.
The suspicion provided by WL and AF endoscopy was compared with the histological findings, considered as gold standard. All recorded data underwent statistical analysis. The sensitivity (SE) and specificity (SP) of the two endoscopic modalities were calculated comparing, at first, benign lesions vs. pre-neoplastic plus neoplastic (phase 1), and then benign plus pre-neoplastic vs. neoplastic (phase 2). To compare accuracy and effectiveness of each test, the confidence interval (CI) and Youden’s index (J) are then calculated and compared.
Results
A total of 81 laryngeal biopsy specimens, taken from 46 consecutive patients (40 male, 6 female, age range: 31-80 years) who had undergone intra-operative endoscopic assessment in a period of 2 years (September 2002 - September 2004), were examined.
Of these, 13 patients underwent the procedure for presumed benign lesions, subsequently confirmed by histology in 12 cases (4 vocal fold polyps, 3 papillomas, 2 Reinke’s oedema, 2 laryngeal cysts, 1 granulatous tissue). In 5 of these, AF endoscopy showed a moderate (g.2) reduction of fluorescence (2 papillomas, 2 angiomatous polyps, 1 granuloma). In one patient, treated for presumed laryngeal papilloma, AF endoscopy showed a marked reduction of green fluorescence (g.3), thereafter histologically confirmed as verrucous carcinoma.
The other 33 patients underwent the procedure for pre-operative endoscopic suspicion of precancerous or cancerous lesions, 7 of these already had a positive histology for carcinoma. In 14 cases, previous laryngeal treatment (surgery, radiotherapy or both) had been carried out. Complete correspondence in the data revealed by WL and AF endoscopy was found in 15/33 patients. In the other cases, the differences, qualitative and quantitative, between the two endoscopic methods were tested by histological evaluation of multiple incisional biopsy specimens. A total of 68 biopsy samples, from this group of 33 patients were examined with the following results: 1 papillomatosis; 1 necrosis; 5 para-keratosis; 22 chronic phlogosis; 1 DL I; 5 DL II; 10 DL III/Ca is; 4 verrucous Ca; 7 Ca GI; 11 Ca G2; 1 Ca GIII.
Overall, 81 laryngeal biopsy samples were taken and examined.
In the first phase of the study (p.1), discrimination between benign and pre-neoplastic/neoplastic lesions has been evaluated, for both the endoscopic methods, with histopathology as a control: WL videoendoscopy showed a SE of 82.5% (CI 70.5-94.5%), whereas the AF method achieved a rate of 97.5% (CI 92.5-100%). SP rate was 51.2% (CI 36.2-66.8%) both for standard WL endoscopy and for AF endscopy, with only one false-negative (FN) in our series (Table I).
In the second phase (p.2) the reliability of detection between benign/pre-neoplastic and neoplastic lesions has been tested: standard endoscopy revealed a SE significantly lower compared to that with AF (57.1 vs. 94.3%) with a significant difference demonstrated by the CI (40.3-73.9% vs. 86.5-100%). A lesser difference resulted in SP (84.8 vs. 91.3% – CI 74.2-95.4% vs. 83.0%-99.6%) (Table II).
Table I. Benign vs. preneoplastic + neoplastic laryngeal lesions.
| WL | AF | |
| TP | 33 | 39 |
| TN | 21 | 21 |
| FP | 20 | 20 |
| FN | 7 | 1 |
| SE | 0.825 (95 CI 70.5-94.5) | 0.975 (CI 92.5%-100%) |
| SP | 0.512 (CI 36.2%-66.8%) | 0.512 (CI 36.2%-66.8%) |
| J | 0.337 | 0.487 |
WL = white light endoscopy; AF = autofluorescence endoscopy; TP = true positive; TN = true negative; FP = false positive; FN = false negative; SE = sensitivity; SP = specificity; CI = confidence interval; J = Youden’s index.
Table II. Benign + preneoplastic vs. neoplastic laryngeal lesions.
| WL | AF | |
| TP | 20 | 33 |
| TN | 39 | 42 |
| FP | 7 | 4 |
| FN | 15 | 2 |
| SE | 0.571 (CI 40.3%-73.9%) | 0.943 (CI 86.5%-100%) |
| SP | 0.848 (CI 74.2%-95.4%) | 0.913 (CI 83.0%-99.6%) |
| J | 0.419 | 0.856 |
WL = white light endoscopy; AF = autofluorescence endoscopy; TP = true positive; TN = true negative; FP = false positive; FN = false negative; SE = sensitivity; SP = specificity; CI = confidence interval; J = Youden’s index.
Overall, in the first phase, WL endoscopy revealed a Youden index of 33.7% vs. 48.7% with the AF test. In p.2, WL revealed a J of 41.9% while AF endoscopy reached 85.6% (Tables I–II).
Discussion
In our series, AF videoendoscopy demonstrated a higher sensitivity than the standard technique (97.5 vs. 82.5%) in the discrimination between benign and pre-neoplastic/neoplastic laryngeal lesions (p.1) with an extremely low rate of false-negatives (FNs). These results correspond to data reported by Malzahn et al. 11 on a series of 127 patients, for whom a sensitivity of 97.3% was observed FNs were attributed to a diffuse hyperkeratosis covering neoplastic tissue below. In these cases, intensity of green fluorescence can be found; the investigated mucosa appears bright-green to white, depending on the thickness of the hyperkeratosis 11–13. In our experience, intensity of fluorescence was sometimes noted as small spots in the context of a wider “dark area” without completely covering the neoplastic tissue (Fig. 3).
The high sensitivity of the AF method is also revealed by the presence of demarcation of the cancer limits, with an increase in contrast between the normal and pathological mucosa. According to Freyen et al. the colorimetric differences, in the AF study, make the tumour borders more visible, sometimes exalting the fluorescence at cancer limits (Fig. 2). This phenomenon has been correlated with the presence of packed elastic fibres at the margins of the neoplasm 14. Furthermore, in 5 cases in our series, AF indicated a wider extension of the suspected area than standard endoscopy or a reduction of endogenous fluorescence in macroscopically negative sites. In all these cases, the presence of carcinoma was confirmed by biopsy. On the other hand, no colorime-tric differences were observed in relation to the progressive grade of neoplastic dedifferentiation (well-, moderately-, poorly- or un-differentiated carcinoma).
The gap in sensitivity, between WL and AF endoscopy, resulted even more evident in p.2 (discrimination between pre-neoplastic and neoplastic lesions). In this test, in fact, comparing confidence intervals, fluorescence endoscopy revealed a significantly higher SE (p < 0.05) than standard endoscopy. Nevertheless, in our opinion, the assignment of a predictive value to fluorescence, in the distinction between dysplasic and neoplastic lesions, requires great experience; in fact, in this case, the endoscopist should not only recognize a reduction in the green colour, but should also assign a clinical value to the different blue-violet intensities. Obviously, this operation requires a long learning phase and, moreover, risks being greatly operator-dependent.
Moreover, as with other endoscopic methods (e.g. contact “micro-endoscopy”), an intrinsic limit of the fluorescence study is the impossibility to distinguish between in situ, and an invasive, carcinoma; AF endoscopy, in fact, evaluating the surface of the tissues, is unable to estimate the in-depth extension of the tumour.
Despite the high sensitivity of the method, specificity is rather low, in particular in the p.1 test where WL and AF endoscopy show the same number of FPs both reaching a SP rate of 51.2%. Due to the high absorption of excitation light by haemoglobin, FPs occurred more frequently in the presence of highly vascularized lesions such as telangiectasic polyps, granulation tissue, papillomas 11. We obtained a better SP rate in discrimination between pre-neoplastic and neoplastic lesions (p.2) with both endoscopic methods but, nonetheless, higher with AF evaluation (84.8 vs. 91.3%). These considerations are basic as far as concerns the application field of this method. In accordance with Delank et al. 15, we consider that AF endoscopy is not useful in the evaluation of “frankly benign” laryngeal lesions. In cases of nodules, cysts, polyps, or granulomatous tissue, this examination does not improve the results obtained from the clinical evaluation and standard endoscopy but increases the risk of FPs. On the contrary, AF is very useful in the evaluation of suspected neoplastic and pre-neoplastic lesions such as leukoplakia, erythroplakia and hyperkeratosis. In these cases, in fact, neither standard endoscopy, nor microlaryngoscopy reach a SE comparable to the endogenous fluorescence method, maintaining a similar SP.
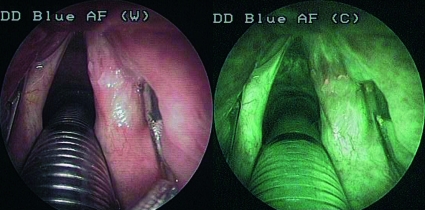
In our experience, a large number of FPs (9/14) have been found also in patients previously submitted to surgery and/or radiotherapy for laryngeal carcinoma (Fig. 4). Irradiation, in particular, causes deep alterations in the tissue structure, leading to unpredictable modifications in the endogenous fluorescence. Not only hyperaemia, presence of dilated vessels and inflammatory cells, contribute to the reduction of endogenous fluorescence, but also the presence of bacteria producing exogenous fluorophores which can modify the characteristics of fluorescence, in irradiated tissues, causing complex colorimetric pictures which are difficult to interpret 11 (Fig. 5). Our experience confirmed these considerations and, therefore, in our opinion, fluorescence endoscopy is not very suitable for the follow-up of patients submitted to radiotherapy for head-neck carcinoma.
Fig. 4.
False positive in patient who underwent radiotherapy for a anterior supraglottid carcinoma.
Fig. 5.
Recurrence of carcinoma after radiotherapy. Aspecific alterations of endogenous fluorescence, due to hyperkeratosis and alterations in architectural tissue structure.
Conclusions
AF endoscopy is a low invasive method that can favour the identification and demarcation of precancerous and cancerous lesions. In particular, it accurately defines the limits of the tumour and the possible presence of a second primary thus being a useful guide in the choice of sites where to make a biopsy.
On the other hand, an intrinsic limit of the method is the impossibility to evaluate the extension of the carcinoma in the deeper tissues and, therefore, to differentiate between carcinoma in situ and invasive carcinoma.
In our experience on laryngeal lesions, AF evaluation has proved to be characterized by higher SE and SP, not inferior to standard endoscopy. The risk of a low SP, can be reduced by the correct choice of the field of application. The method is particularly useful in the evaluation of “virgin” lesions with suspected dysplasia or carcinoma; on the contrary, it is not suitable in the study of “frankly benign” laryngeal lesions (nodules, cysts, polyps and granulomas), since it does not add any useful information with respect to standard endoscopy and, on the other hand, presents a high risk of FPs.
The low SP in the tissues of patients submitted to radio therapy, must be taken into consideration in the follow-up of these cases.
Nevertheless, the best results can be obtained only by integrating the data provided both by accurate WL and AF endoscopy assessment.
Acknowledgements
Authors are grateful to Prof. Antonio Piccoli for precious statistical advice.
References
- 1.Arens C, Malzahn K, Dias O, Andrea M, Glanz H. Endoscopic imaging techniques in the diagnosis of laryngeal carcinoma and its precursor lesions. Laryngol Rhinol Otol 1999;78:685-91. [DOI] [PubMed] [Google Scholar]
- 2.Shah JP, Karnell LH, Hoffman HT, Ariyan S, Brown GS, Fee WE, et al. Patterns of care for cancer of the larynx in the United States. Arch Otolaryngol Head Neck Surg 1997;123:475-83. [DOI] [PubMed] [Google Scholar]
- 3.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1996. CA Cancer J Clin 1996;65:5-27. [DOI] [PubMed] [Google Scholar]
- 4.Alfano R, Tata D, Cordero J. Laser induced fluorescence spectroscopy for native cancerous and normal tissue. IEEE J Quant Electron 1984;20:1507-11. [Google Scholar]
- 5.Kolli VR, Savage HE, Yao TJ, Schantz SP. Native cellular fluorescence of neoplastic upper aerodigestive mucosa. Arch Otolaryngol Head Neck Surg 1995;121:1287-92. [DOI] [PubMed] [Google Scholar]
- 6.Policard A. Etude sur les aspects offerts par des tumeurs expérimentales examinées à la lumière de Wood. Comptes Rendues Hebdomadaires des Séances Mémories la Société Biologie ses Filiales, 1924;91:1423-4. [Google Scholar]
- 7.Anidjar M, Cussenot O, Blais J, Bourdon O, Avrillier S, Ettori D, et al. Argon laser induced autofluorescence may distinguish between normal and tumour urothelial cells: a microspectrofluorometric study. J Urol 1996;155:1771-4. [PubMed] [Google Scholar]
- 8.D’Hallewin M, Bezdetnaya L, Guillemin F. Fluorescence detection of bladder cancer: a review. Eur Urol 2002;42:417-25. [DOI] [PubMed] [Google Scholar]
- 9.Dhingra JK, Zang X, McMillan K, Kabani S, Manoharan R, Itzkan I, et al. Diagnosis of head neck precancerous lesions in an animal model using fluorescence spectroscopy. Laryngoscope 1998;108:471-5. [DOI] [PubMed] [Google Scholar]
- 10.Zellweger M, Grosjean P, Goujon D. In vivo autofluorescence spectroscopy of human bronchial tissue to optimize the detection and imaging of early cancers. J Biomed Opt 2001;6:41-51. [DOI] [PubMed] [Google Scholar]
- 11.Malzahn K, Dreyer T, Glanz H, Arens C. Autofluorescence endoscopy in the diagnosis of early laryngeal cancer and its precursor lesions. Laryngoscope 2002;112:488-93. [DOI] [PubMed] [Google Scholar]
- 12.Arens C, Dreyer T, Glanz H, Malzahn K. Indirect autofluorescence laryngoscopy in the diagnosis of laryngeal cancer and its precursor lesions. Eur Arch Otorhinolaryngol 2004;261:71-6. [DOI] [PubMed] [Google Scholar]
- 13.Zargi M, Fajdiga I, Smid L. Autofluorescence imaging in the diagnosis of laryngeal cancer. Eur Arch Otorhinolayngol 2000;257:17-23. [DOI] [PubMed] [Google Scholar]
- 14.Freyen A, Glanz H, Lohmann W, Dreyer T, Bohle M. Significance of autofluorescence for the optical demarcation of field cancerisation in the upper aerodigestive tract. Acta Otolaryngol (Stockh) 1997;117:316-9. [DOI] [PubMed] [Google Scholar]
- 15.Delank W, Khanavkar B, Nakhosteen JA, Stoll W. A pilot study of autofluorescent endoscopy for the in vivo detection of laryngeal cancer. Laryngoscope 2000;110:368-73. [DOI] [PubMed] [Google Scholar]