Summary
Adenoid cystic carcinoma is a malignant tumour involving the salivary glands, rarely developing in the nasopharynx. The biological behaviour of adenoid cystic carcinoma is characterized by slow growth rate, high tendency to local recurrence and metastatic spread. Its histological features are particularly important for prognostic prediction: solid pattern has the worst outcome. Initial presentation of nasopharyngeal adenoid cystic carcinoma with paresis of cranial nerves and Horner’ s syndrome is infrequent. The Authors present a case of a rare adenoid cystic carcinoma of the nasopharynx, at admission with Horner’ s syndrome, in a 66-year-old male. Magnetic resonance imaging showed an expansive submucosal lesion of the nasopharynx involving the para-pharyngeal space, tensor and levator veli palati muscles and the apex of petrous bone. Positron emission tomography excluded distant metastasis. Definitive histopathological examination revealed an adenoid cystic carcinoma with mixed cribriform and solid pattern. The patient was treated exclusively with radiotherapy (70 Gy) in 35 fractions with partial reduction of the neoplastic mass.
Keywords: Nasopharynx, Adenoid cystic carcinoma, Skull base, Horner’ s syndrome, Radiotherapy
Riassunto
Il carcinoma adenoido-cistico è una neoplasia maligna ad origine dalle ghiandole salivari, che interessa raramente il rinofaringe. Il comportamento biologico del carcinoma adenoido-cistico è caratterizzato da lento accrescimento, alta tendenza alla recidiva locale ed alla diffusione metastatica. Le caratteristiche istologiche di questa neoplasia sono molto importanti ai fini prognostici: la variante solida risulta avere la prognosi peggiore. La presentazione iniziale del carcinoma adenoido-cistico rinofaringeo con paralisi dei nervi misti ed in particolare con una sindrome di Horner è rara. Gli Autori presentano un raro caso di carcinoma adenoido-cistico del rinofaringe, manifestatosi con una sindrome di Horner, in un uomo di 66 anni. La RMN mostrava una lesione sottomucosale del rinofaringe coinvolgente lo spazio parafaringeo, i muscoli tensore ed elevatore del palato nonché l’ apice della rocca petrosa. La PET escludeva metastasi a distanza. L’ esame istopatologico definitivo mostrava un carcinoma adenoido-cistico variante mista, cribriforme e solida. Il paziente è stato sottoposto esclusivamente a trattamento radioterapico (70 Gy) in 35 sedute, con una parziale riduzione di volume della massa neoplastica.
Introduction
Adenoid cystic carcinoma (ACC) is a malignant tumour of the exocrine glands. It most commonly arises in the salivary glands (it accounts, in fact, for 12% to 14% of malignant parotid neoplasms, for 31% to 58% of malignant tumours of the submandibular gland and for 33% to 55% of malignant tumours of the minor salivary glands), even if localizations have been described in prostate, lacrymal glands, cervix uteri, breast and bronchial mucosa 1–5.
Nasopharyngeal localization is uncommon, accounting for approximately 0.5% to 4% of all the carcinomas of the nasopharynx and for 2.4% to 3.7% of all head and neck ACC 1 6–8.
Typical features of ACC are: slow growth rate, high propensity for perineural spread, for local recurrence and for distant metastasis, usually involving lung, bones and liver 3 4 9 10.
Histologically, three growth patterns can be recognized for ACC: cribriform, tubular and solid. This is particularly important for prognostic purposes since the predominant tubular pattern has the best prognosis, the predominant solid pattern the worst, being associated with the highest incidence of distant metastasis and perineural infiltration with, consequently, 15 years survival rate of 5% 2 3 11–13.
The reported incidence of invasion of the skull base ranges from 4% to 22% and can realize through various ways of spread: the peritubaric space, the branches of the trigeminal nerve and the internal carotid artery 2 14–16.
These tumours have a long natural history characterized by a typical slow growth rate responsible for the delay in seeking early medical consultation. The interval between onset of the disease and onset of the first symptoms is estimated to be between 2 and 5 years 1 3 17.
The symptoms most commonly found are epistaxis, progressive nasal stenosis, dysfunction of the Eustachian tube and, in relation to the invasion of the skull base, disorders of ocular motility, diplopia, facial pain, dysfunction of IX, X, XI and XII pairs of cranial nerves and, more rarely, Horner’ s syndrome 1 2 17.
Imaging of ACC is based on computed tomography (CT) scan, particularly helpful in detecting bony erosions of the skull base, and on Magnetic Resonance Imaging (MRI) with gadolinium, effective in demonstrating possible involvement of infra-temporal fossa, cavernous sinus, and perineural or perivascular infiltration 3.
Case report
U.R.B., a 66-year-old male, was referred to the ENT Department, complaining of a visual deficit in the lateral look associated with progressive hoarseness, dysphonia and dysphagia both for liquid and solid food which had been present for 2 months.
Clinical history showed chronic lung emphysema. More-over, the patient was in follow-up at the Department of Gastroenterology for a gastric carcinoid tumour successfully removed by endoscopic surgery two years earlier.
Otolaryngologic examination revealed paralysis of the left soft palate and of the left vocal fold. Investigation with flexible endoscopy was negative for macroscopic disease of the nasopharynx.
Neurological evaluation revealed anisocoria due to constriction (miosis) of the left pupil, drooping of the left upper eyelid (ptosis) and light sinking of the eyeball into the bony cavity of the eye (enophthalmos). All these signs led to the diagnosis of Horner’ s syndrome.
High resolution computed tomography (HRCT) scan of the skull base revealed vast erosion of the foramen lacerum extended to the foramen rotundum and ovale, to the apex of petrous bone and to the internal carotid artery.
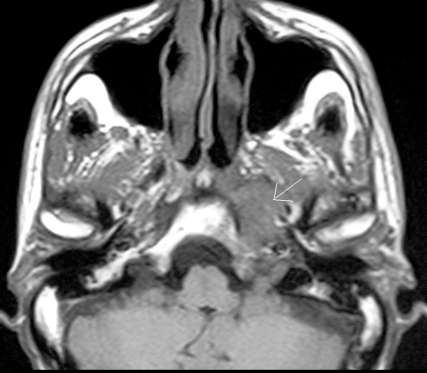
MRI of the skull base confirmed the presence of an expansive submucosal lesion with involvement of the left para-pharyngeal space reaching the omolateral petrous bone with infiltration of tensor and levator veli palati muscles (Fig. 1).
Fig. 1.
MRI of skull base with evidence of expansive naso-pharyngeal submucosal lesion extended to left para-pharyngeal space and reaching omolateral petrous bone with infiltration of tensor and levator veli palati muscles.
The presence of distant metastasis was excluded by means of positron emission tomography (PET).
According to the indications of the HRTC scan and of MRI, multiple biopsies were performed, deeply into the mucosal surface which was healthy in appearance, following the posterior margin of the left Eustachian tube. Intra-operative frozen section examination of nasopharyngeal tissue demonstrated an infiltrating adenocarcinoma. Definitive histological examination confirmed the diagnosis; in particular, the morphological pattern (mixed cribriform and tubular) and immunohistochemical profile (CK7+, CK20-, S100+), indicated an adenoid cystic carcinoma of the minor salivary glands (Fig. 2).
Fig. 2.
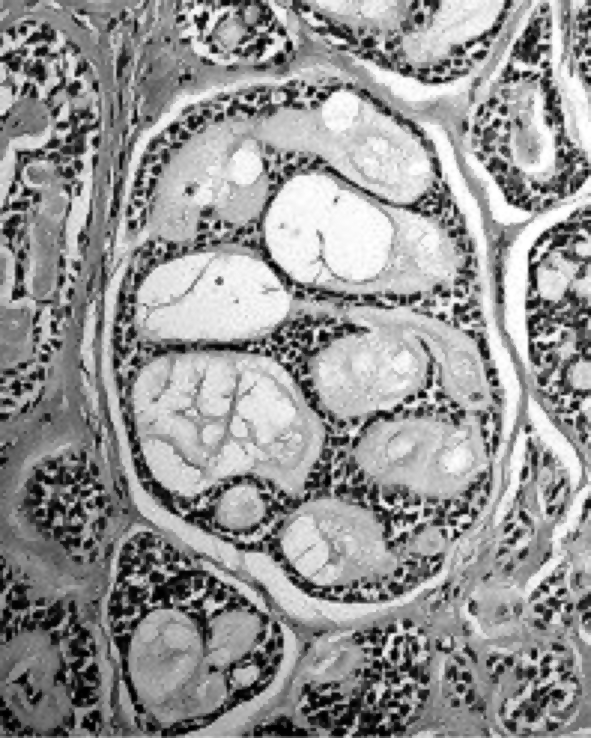
Definitive histologic examination indicating adenoid cystic carcinoma of minor salivary glands with mixed cribriform and tubular pattern.
Considering the site and the extension of the lesion, treatment with exclusive radiation therapy was prescribed. The patient was immobilized with a thermoplastic mask. Treatment was delivered with conformal high energy photons (6 MV) that included the nasopharynx and skull base. A total dose of 70 Gy, in 35 fractions, was administered.
Treatment was not interrupted with good clinical tolerance; we detected grade 1 mucositis (WHO scale) and weight loss, resolved with adequate therapy.
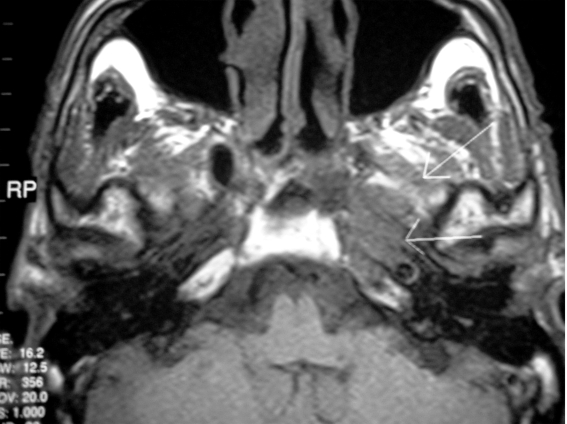
Post-treatment MRI, performed 6 months after the radiation therapy, showed a reduction of the neoplastic mass (Fig. 3).
Fig. 3.
Post-treatment MRI, performed 6 months after radiation therapy, showing a slight reduction of neoplastic mass.
This radiological finding was the same as that at the MRI control performed 18 months after radiotherapy.
The patient reported improvement in swallowing function.
Otolaryngologic revaluation did not show any significant variations in the clinical aspects found at the time of diagnosis.
Discussion
The best treatment for ACC is unanimously considered radical surgical resection followed by radiotherapy 1 3 5 13 16–20.
In cases of nasopharyngeal ACC, the frequent perineural and perivascular infiltrations, associated with the anatomical characteristics of the nasopharynx, however, make the surgical approach risky on account of technical difficulties, due substantially to the proximity of surgical margins to critical neural and vascular structures 8 13.
Surgical treatment, therefore, is often characterized by incomplete oncological radicality, consequently with increased frequency of local recurrence within 3 years of the initial treatment 3 9 13 21.
This aspect is of great importance considering the study of Sur et al., who, analysing the prognostic factors influencing the clinical control of the disease, indicate, in the portion of the residual tumoural mass, the only parameter indicating therapeutic effectiveness 20.
In cases of ACC, moreover, it should be emphasized that, also when good local control of the disease has been reached (complete resection of the tumoural mass), the presence of distant metastases is possible in 39% of the patients 3.
Another aspect to be taken into consideration is that surgical treatment of these neoplasms with extension to the skull base is associated with a significant morbidity rate, due to the frequent appearance of sequels and complications, due to vascular and nervous lesions 1–8 22.
In the case of ACC with intracranic extension, it, therefore, appears important to evaluate the real benefits of surgery, also considering that this pathological condition has a slow clinical progression which allows long-term survival of many patients, even with advanced or metastatic disease 1 13 14 22–24.
In the literature, moreover, many studies have shown that survival rates, over a long period of time, in patients with nasopharyngeal ACC do not seem to be significantly influenced by different (more or less aggressive) types of treatment 2 3 13 18 21.
Given the proven radiosensitivity of the ACC, this tumour is, in fact, regarded as a radiosensitive, even if not radiocurable, neoplasm, thus exclusive radiotherapy can determine a reduction in tumour volume with a meaningful improvement in the clinical symptoms 1 2 5 8 13 17 20 25 26.
Vikram et al. reported regression of the tumoural mass in 96% of 49 patients treated only with radiotherapy, although in 93% of the cases they observed recurrence of the disease within 5 years 24.
Although the surgical approach with attempts of oncological radicality, followed by radiotherapy, remains the treatment of choice for ACC, also in the case of a nasopharyngeal localization, exclusive radiotherapy, in those cases in which surgery is contraindicated, for general reasons or for technical difficulties, offers a valid therapeutic alternative guaranteeing good control of the disease and minimal side-effects.
References
- 1.Lee DJ, Smith RR, Spaziani JT, Rostock R, Holliday M, Moses H. Adenoid cystic carcinoma of the nasopharynx. Case reports and literature review. Ann Otol Rhinol Laryngol 1985;94:269-72. [PubMed] [Google Scholar]
- 2.Alleyne CH, Bakay RA, Costigan D, Thomas B, Joseph GJ. Intracranial adenoid cystic carcinoma: case report and review of the literature. Surg Neurol 1996;45:265-71. [DOI] [PubMed] [Google Scholar]
- 3.Gormley WB, Sekhar LN, Wright DC, Olding M, Janecka IP, Snyderman CH, et al. Management and long-term outcome of adenoid cystic carcinoma with intracranial extension: a neurosurgical perspective. Neurosurgery 1996;38:1105-12. [DOI] [PubMed] [Google Scholar]
- 4.Koka VN, Tiwari RM, van der Waal I, Snow GB, Nauta J, Karim AB, et al. Adenoid cystic carcinoma of the salivary glands: clinicopathological survey of 51 patients. J Laryngol Otol 1989;103:675-9. [DOI] [PubMed] [Google Scholar]
- 5.Saadi I, El Marfany M, Hadadi K, Amaoui B, Kebdani T, Errihani H, et al. Adenoid cystic carcinoma of the nasopharynx: a case report. Cancer Radiother 2003;7:190-4. [DOI] [PubMed] [Google Scholar]
- 6.Spiro RH, Koss LG, Hajdu SI, Strong EW. Tumours of minor salivary origin. A clinicopathologic study of 492 cases. Cancer 1973;3:117-29. [DOI] [PubMed] [Google Scholar]
- 7.Resta L, Ricco R, Santangelo A. Morphologic and classificatory consideration about 140 cases of carcinoma of the nasopharynx. Tumori 1983;69:313-21. [DOI] [PubMed] [Google Scholar]
- 8.Aloulou S, Merad-Boudia Z. Adenoid cystic carcinoma (cylindroma) of the nasopharynx with extension into the cavernous sinus. Presse Med 2002;31:1653-6. [PubMed] [Google Scholar]
- 9.Vincentelli F, Grisoli F, Leclercq TA, Ardaud B, Diaz-Vasquez P, Hassoun J. Cylindromas of the base of the skull. Report of four cases. J Neurosurg 1986;65:856-9. [DOI] [PubMed] [Google Scholar]
- 10.Witt RL. Adenoid cystic carcinoma of the minor salivary glands. Ear Nose Throat J 1991;70:218-22. [PubMed] [Google Scholar]
- 11.Batsakis JG, Luna MA, el-Naggar A. Histopathologic grading of salivary gland neoplasms: III. Adenoid cystic carcinomas. Ann Otol Rhinol Laryngol 1990;99:1007-9. [DOI] [PubMed] [Google Scholar]
- 12.Carrau RL, Petruzzelli G, Cass SP. Adenoid cystic carcinoma of the nasopharynx. Otolaryngol Head Neck Surg 1995;112:501-2. [DOI] [PubMed] [Google Scholar]
- 13.Bradley PJ. Adenoid cystic carcinoma of the head and neck: a review. Curr Opin Otolaryngol Head Neck Surg 2004;12:127-32. [DOI] [PubMed] [Google Scholar]
- 14.Conley J, Dingman DL. Adenoid cystic carcinoma in the head and neck (cylindroma). Arch Otolaryngol 1974;100:81-90. [DOI] [PubMed] [Google Scholar]
- 15.Vrielinck LJ, Ostyn F, van Damme B, van den Bogaert W, Fossion E. The significance of perineural spread in adenoid cystic carcinoma of the major and minor salivary glands. Int J Oral Maxillofac Surg 1988;17:190-3. [DOI] [PubMed] [Google Scholar]
- 16.Shotton JC, Schmid S, Fisch U. The infratemporal fossa approach for adenoid cystic carcinoma of the skull base and nasopharynx. Otolaryngol Clin North Am 1991;24:1445-64. [PubMed] [Google Scholar]
- 17.Wang CC, See LC, Hong JH, Tang SG. Nasopharyngeal adenoid cystic carcinoma: five new cases and a literature review. J Otolaryngol 1996;25:399-403. [PubMed] [Google Scholar]
- 18.Pitman KT, Prokopakis EP, Aydogan B, Segas J, Carrau RL, Snyderman CH, et al. The role of skull base surgery for the treatment of adenoid cystic carcinoma of the sinonasal tract. Head Neck 1999;21:402-7. [DOI] [PubMed] [Google Scholar]
- 19.Schramm VL Jr, Imola MJ. Management of nasopharyngeal salivary gland malignancy. Laryngoscope 2001;111:1533-44. [DOI] [PubMed] [Google Scholar]
- 20.Sur RK, Donde B, Levin V, Pacella J, Kotzen J, Cooper K, et al. Adenoid cystic carcinoma of the salivary glands: a review of 10 years. Laryngoscope 1997;107:1276-80. [DOI] [PubMed] [Google Scholar]
- 21.Howard DJ, Lund VJ. Reflections on the management of adenoid cystic carcinoma of the nasal cavity and paranasal sinuses. Otolaryngol Head Neck Surg 1985;93:338-41. [DOI] [PubMed] [Google Scholar]
- 22.Wiseman SM, Popat SR, Rigual NR, Hicks WL Jr, Orner JB, Wein RO, et al. Adenoid cystic carcinoma of the paranasal sinuses or nasal cavity: a 40-year review of 35 cases. Ear Nose Throat J 2002;81:510-4, 516-7. [PubMed] [Google Scholar]
- 23.Ellis ER, Million RR, Mendenhall WM, Parsons JT, Cassisi NJ. The use of radiation therapy in the management of minor salivary gland tumours. Int J Radiat Oncol Biol Phys 1988;15:613-7. [DOI] [PubMed] [Google Scholar]
- 24.Vikram B, Strong EW, Shah JP, Spiro RH. Radiation therapy in adenoid-cystic carcinoma. Int J Radiat Oncol Biol Phys 1984;10:221-3. [DOI] [PubMed] [Google Scholar]
- 25.Rounthwaite FJ, Frei JV, Wallace AC, Watson TA. The effect of radiotherapy in the treatment of adenoid cystic carcinoma of the head and neck arising in minor salivary glands. J Otolaryngol 1977;6:297-308. [PubMed] [Google Scholar]
- 26.Takahashi T, Mitsuhashi N, Sakurai H, Takahashi M, Hirato J, Niibe H. A case report of adenoid cystic carcinoma of the nasopharynx with aggressive systemic bone metastases. Radiat Med 1995;13:301-3. [PubMed] [Google Scholar]