Summary
The diagnostic approach to patients with dysphagia is well established and relies mainly on videofluoroscopy and endoscopy. Oro-pharyngo-oesophageal scintigraphy permits both a functional and a semi-quantitative study of the various stages of swallowing. Moreover, by means of this investigation, it is possible to estimate the amount of inhaled bolus. Oro-pharyngo-oesophageal scintigraphy with 99mTc-nanocolloid has been found to be easy to use, economical, well tolerated and, supplying precise indications regarding the extent of the swallowing disorder, then permits a better clinical definition of the patient. The limitations of swallowing scintigraphy are: poor definition in visualizing anatomic structures and low specificity when used as the only diagnostic test. Scintigraphy plays an important role in the diagnosis and follow-up of dysphagia, and its use, together with other diagnostic techniques, increases diagnostic accuracy. In this study, the role of oro-pharyngo-oesophageal scintigraphy has been analysed in patients with post-surgical, neurological and oesophageal dysphagia.
Keywords: Deglutition disorder, Diagnosis, Oro-pharyngo-oesophageal scintigraphy
Riassunto
L’approccio diagnostico al paziente disfagico si basa prevalentemente sull’utilizzo della videofluoroscopia e dell’esame endoscopico. Accanto a queste metodiche, la scintigrafia oro-faringo-esofagea con 99mTc-nanocolloide riveste anch’essa un ruolo importante nello studio della disfagia, in quanto fornisce informazioni funzionali e semiquantitative delle varie fasi della deglutizione e permette di identificare l’eventuale presenza di aspirazione tracheo-bronchiale, permettendo di ottenere un migliore inquadramento clinico del paziente disfagico. La scintigrafia oro-faringo-esofagea è una metodica di imaging facile da eseguire, a basso costo e ben tollerata dal paziente, i cui limiti principali sono rappresentati dalla scarsa definizione delle strutture anatomiche e dalla bassa specificità, quando utilizzata come unico test diagnostico. In ambito clinico può essere utilizzata come metodica complementare nella diagnosi della disfagia, aumentando l’accuratezza diagnostica delle altre metodiche e ponendosi come esame molto utile nel follow-up riabilitativo dei pazienti affetti da disturbi della deglutizione. In questo studio abbiamo analizzato il ruolo della scintigrafia oro-faringo-esofagea in soggetti affetti da disfagia post-chirurgica, disfagia neurogena e disfagia esofagea.
Introduction
Dysphagia is a disorder of multi-factorial aetiopathogenesis that leads to several problems regarding its nature, when attempting to define specific deficits related to the structures and to the various stages of swallowing, and differential diagnosis 1 2.
Swallowing is a complex physiological motion that is performed very quickly: in fact, the bolus takes about two seconds to pass from the oral cavity to the cervical oesophagus. For this reason, a morphological examination of the structures involved and a functional assessment of the swallowing itself are aspects that are rather difficult to solve from a technical point of view 1.
According to the American Speech-Language-Hearing Association [ASHA] 3, a clinical-instrumental evaluation of swallowing should demonstrate: structural and functional alterations of the organs involved; the degree of efficacy of swallowing; adequate protection of the lower respiratory tract and co-ordination between breathing and swallowing; the presence of any oesophageal motility disorders and gastro-oesophageal reflux; modifications in the swallowing act itself in relationship to the type of bolus; the efficacy of postures or manoeuvres that aid it.
In clinical practice, the principal events that are studied during the process of swallowing are focused on aspiration of the bolus into the lower respiratory tract, oral and pharyngeal propulsion, and on the efficacy of the upper oesophageal sphincter.
At present, the instrumental method considered the gold standard for studying swallowing is videofluoroscopy, which offers an immediate view of all the stages of swallowing 4 5. Furthermore, the clinical definition of dysphagia often uses other means, such as manometry and pH-metry, which are particularly important for studying functional alterations of the oesophagus 6–9.
Fiberoptic Endoscopic Evaluation of Swallowing (FEES) has recently been adopted. Originally proposed as a complementary tool, this technique is now routinely used, in first instance, due to the fact that it is easy to perform, very well tolerated by patients (bedside examination is possible) and also economical 10–12. Nevertheless, a significant limitation consists in the fact that it does not supply information on the oral and oesophageal stages of swallowing since these areas cannot be explored with the flexible endoscope.
Besides the above-mentioned methods, oro-pharyngo-oesophageal scintigraphy (OPES) is becoming used, more and more, to study swallowing. This method offers qualitative and semi-quantitative information concerning the various stages of swallowing, and consequently a better clinical and diagnostic definition of the diseases and of the anatomical-functional disorders that cause dysphagia.
Despite the fact that, as yet, the method has not been precisely standardised, OPES is easy to use and well tolerated by the patient, it is economical, with low dosimetric exposure, applicable in screening for dysphagia and very useful in performing follow-up examinations since it offers qualitative and semi-quantitative information in patients either lying down or standing up and using physiological boluses of different consistencies 13 14.
In clinical practice, this scintigraphic method is the only tool available to perform a semi-quantitative assessment of food that has been aspirated into the respiratory tract, and an exact evaluation of how long each of the three swallowing stages take, with detailed calculation of transit time and of any bolus retained in the oral cavity, pharynx or oesophagus. The principal limitation of this examination is that it only defines the anatomy of the structures involved 13 14.
In the present study, a review has been made of the clinical applications of OPES used to achieve a better understanding of dysphagic disorders in the oro-pharyngo-oesophageal tract.
Method
OPES produces a rapid sequence of images of individual acts of voluntary swallowing that the patient performs when asked to. It is preferable to perform this scintigraphy at least three hours after the patient has eaten. A test examination is performed prior to the investigation, getting the patient to swallow non-radioactive water; this allows the patient to become familiar with the procedure, enhances compliance and ensures that the patient is capable of swallowing the amount of liquid foreseen (about 10 cc). The investigation starts after approximately five minutes, with the patient standing up and with his/her face in an 80° oblique projection in front of a large-field-of-view γ-camera (LFOV) equipped with a low energy parallel hole collimator (LEHR) and the energy window on 140 KeV (± 10%).
The patient is given a single 10 cc bolus of water labelled with 37 MBq (1 mCi) of 99mTc nanocolloid (Amersham, UK). Eight images per second are acquired (0.125 sec/frame) for one minute, with dynamic acquisitions (64 x 64 matrix and zoom 1) of the visual fields from the oral cavity down to the epigastric area. Two seconds after the start, the patient is invited to swallow the liquid in one swallow. With the patient still in the same position, a static image, lasting 60 seconds, is then acquired at the end of the examination, to evaluate the presence of any tracheo-bronchial aspirate.
The presence of any multiple swallows is assessed at the end of the examination because, if these are involuntary, they do not permit accurate semi-quantitative analysis and, therefore, the test would have to be repeated after inviting the patient to drink several swallows of non-labelled water to wash out the oral cavity of any residual radioactivity.
The investigation is repeated after an interval of 30 minutes, this time with a 10 cc semi-liquid bolus labelled with 37 MBq (1 mCi) of 99mTc nanocolloid. Scintigraphic acquisitions are performed in the same manner as with the liquid bolus.
In our experience, a “jellied drink” (Resource Bevanda Gelificata, Novartis S.A.®) of the type administered to patients with swallowing difficulties while recovering from head-neck surgery has been found to be an excellent gelatinous semi-solid bolus, since its viscosity is constant and there is no possibility of fragmentation.
For studies focusing on oesophageal function, the scintigraphic method is slightly different from that previously described. The patient is administered a 10-15 ml liquid or semi-solid bolus labelled with 99mTechnetium-nanocolloid (Amersham, UK). After an interval of 30 seconds, during which dynamic acquisitions of the swallowing movement are performed 15 16, the patient is asked to perform six single swallows, at intervals of 15 or 30 seconds between them. With this method it is possible to obtain sufficient data to demonstrate any abnormal oesophageal function 17 18.
Another technique foresees the administration of multiple boluses (six boluses 10 cc each) with a sequence of swallow every 30 seconds acquiring 0.5 sec/frame images for the duration of the test 19.
Analysis of the data acquired
OPES permits both qualitative (cine-mode) and semi-quantitative analysis. Cine-mode visual analysis of the images acquired during swallowing of the bolus allows identification of abnormal patterns, such as multiple swallows, retention of the bolus in the oral or pharyngeal cavity, fragmentation of the bolus, gastric-oesophageal reflux, tracheal aspiration and motility abnormalities (uncoordinated and chaotic movement of the bolus).
The semi-quantitative analysis consists in calculation of the oro-pharyngo-oesophageal transit time of the radioactive bolus, with manual definition of the regions of interest (ROI) in the oral cavity, pharynx and oesophagus, respectively (Fig. 1).
Fig. 1.
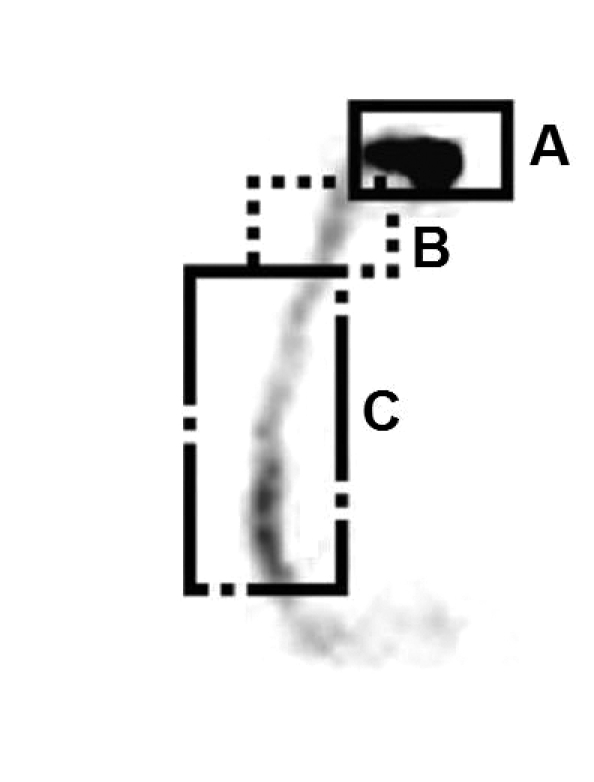
Manual definition of regions of interest (ROI) in oral cavity (A), pharynx (B) and oesophagus (C).
Oral activity is defined in the initial frames, while an external marker on the cricoid delineates the pharyngeal region. The semi-quantitative parameters are derived from the activity/time curves obtained on the marked ROIs. The use of cumulative images enables better positioning of the ROIs. The parameters analysed in our laboratory are as follows:
Oral Transit Time (OTT) – this is the time required for the bolus to leave the oral cavity after the patient has been invited to swallow. The behaviour of the curve shows a rapid decrease in radioactivity, from 100% to less than 5%. This event occurs in less than one second, in normal subjects;
Pharyngeal Transit Time (PTT) – this is the interval of time elapsing between the entrance and the exit of the bolus. The curve behaviour shows a rapid increase in radioactivity, from 0% to 100%, followed by a rapid drop, with the residual radioactivity < 5%. This occurs in normal subjects in less than 1.2 seconds;
Oesophageal Transit Time (ETT) – this takes less than 10 seconds (mean: 5.6 seconds ± 4) and is the interval in time between the entrance and the exit of the bolus through the oesophagus. The curve shows a rapid increase in radioactivity followed by a slower decrease. The residual radioactivity is < 20%;
Retention index – this indicates the quantity of residual bolus, 10 seconds after swallowing. In normal subjects, it is < 5% in the oro-pharyngeal region (OPRI) after the bolus has passed, while it is < 20% in the oesophagus (ERI);
Percentage of oesophageal emptying at 10 seconds (EER10s) – this is achieved through the formula [(Emax – E10sec)/Emax] x 100, where Emax is given by the maximum count of oesophageal radioactivity uptake, while E10s is the radioactivity in the oesophagus 10 seconds after the maximum peak of the activity. This exceeds 80% in normal subjects.
If there is radioactivity in the larynx or in the tracheo-bronchial tract, after the administration of approximately 100 ml of water to eliminate all residual traces from the pharynx, the aspiration percentage (PA) can be calculated by dividing the counts obtained in the aspiration area (RA) by the initial activity detected in the oral cavity before swallowing (activity time 1-AT), subtracted from the residual activity after swallowing (activity time 2-AT2), applying the formula: [PA = RAx100/(AT-AT2)].
In the analysis of the results of the oesophageal scintigraphy, all six swallows are evaluated; it is thus possible to calculate both the overall, and the segmental, transit times as well as the oesophageal retention time 20. In this type of assessment, which refers to multiple swallows, it is very important to pay attention to the interval between each single swallow; in fact, a second swallow occurring less than 4 seconds after the first can inhibit the oesophageal peristaltic wave, whereas a second swallow occurring within 3-8 seconds can stop the striated musculature of the oesophagus from contracting.
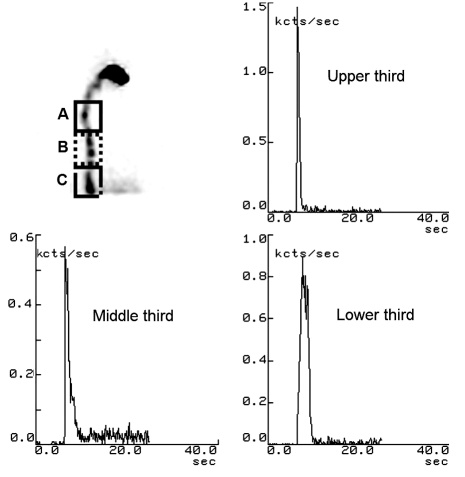
On the other hand, normal peristaltic waves are elicited, in the oesophagus, when the swallows occur at intervals of more than 10-15 seconds 21. The majority of quantitative methods are based on multiple swallows in order to overcome the limits shown by individual variability in a single swallow. The data obtained with this method can be evaluated globally in function of the entire oesophagus, or may supply quantitative information in three distinct areas of the oesophagus (upper, middle, lower regions), previously separated into regions of interest (ROIs) (Fig. 2).
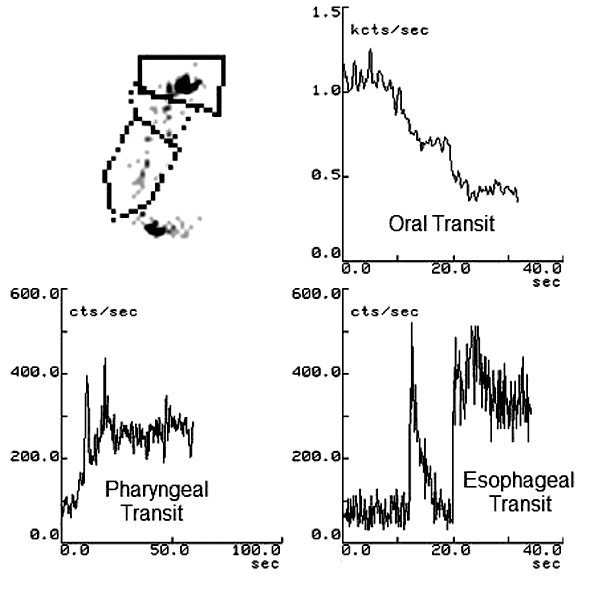
Fig. 2.
Scintigraphic study of three oesophageal regions [upper third (A), middle third (B) and lower third (C)]; static image and typical time-activity curves obtained for oesophageal radionuclide transit study (10 mL liquid bolus) in healthy subject.
Clinical applications
The potential clinical role of OPES in the management of patients affected with dysphagia is its capacity to reveal the physiopathological modifications that are at the root of the motility disorder and to evaluate these modifications in terms both of response to treatment and follow-up.
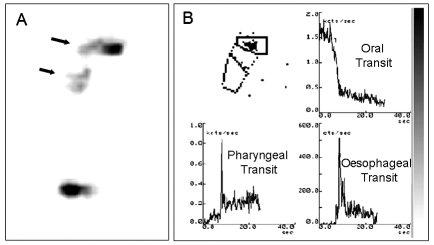
In cases of dysphagia following head-neck surgery, the scintigraphic method has been found to be very efficacious for assessing the capacity of the glottis to close during swallowing when food is resumed orally again, particularly in partially laryngectomised patients. OPES has also been shown to be useful for documenting the onset and the efficacy of compensatory mechanisms (e.g. efficacy of closure of the false chords and/or the glottis plane) as well as the results of strategies for rehabilitating swallowing. Figure 3 refers to a patient who had been submitted to horizontal supraglottic laryngectomy: the activity-time curves relating to the liquid bolus show an increase in OTT and an increase in oro-pharyngeal retention time (OPRT) due to a hindrance in the pharyngeal stage of swallowing, with a consequent reduction in pharyngeal peristalsis, less efficacy of the thrust at the base of the tongue and slowing down of the bolus transit in the hypopharyngeal region (Fig. 3).
Fig. 3.
Oro-pharyngo-oesophageal scintigraphy (10 mL liquid bolus) of subject submitted to horizontal supraglottic laryngectomy. Analysis of scintigraphic images acquired orthostatically shows belated oral and pharyngeal transit (black arrows) (A); time-activity curves show increase in oral and pharyngeal transit time (B).
Figure 4 refers to a patient submitted to subtotal laryngectomy according to Labayle. The patient’s incapacity to fully close the glottis during swallowing, due to the extensive demolition of the larynx, facilitated the entrance both of the liquid and the semi-solid bolus into the lower respiratory tract (Fig. 4).
Fig. 4.
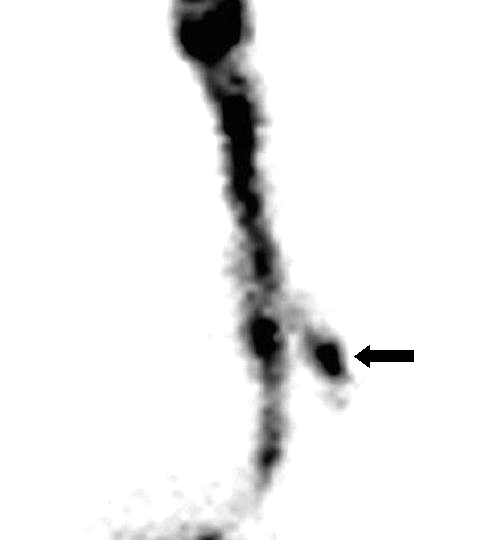
. Patient with subtotal laryngectomy according to Labayle. Static posterior projection image after administration of liquid bolus (10 mL): signs of right tracheo-bronchial aspirate (black arrow).
Assessment of swallowing efficacy, depending on the type of bolus given, is useful for planning the most suitable diet for individual patients and for defining the consistency, type and association of different foods to be given during rehabilitation. The method is also useful for evaluating the patient’s capacity to clear the bolus inhaled, detecting any persisting bolus in the airways after a reflex cough. OPES has been demonstrated to be a very accurate and sensitive method for detecting the presence of bolus particles aspirated into the tracheo-bronchial tract, even in patients with ‘sensitive’ dysphagia following modified sensitivity in the hypopharyngo-laryngeal area. In cases of paralysis of the superior laryngeal nerve as a consequence of, for example, thyroidectomy, scintigraphy has been shown to detect not only bolus inhalation, even in cases previously considered negative with standard radiographic tools, but also to quantify the amount inhaled.
In patients with a neurological disease, dysphagia is often an underestimated invalidating symptom, caused mainly by a deficit in the oral and pharyngeal stages of swallowing. In these cases, scintigraphic examination of the swallowing shows an increase in OTT, PTT and OEPRI (Fig. 5). The oesophageal stage is often altered in neurological patients, though it rarely manifests clinically. On the other hand, scintigraphy detects an increase in EET, ERI and EER, in the presence of oesophageal involvement (Fig. 5).
Fig. 5.
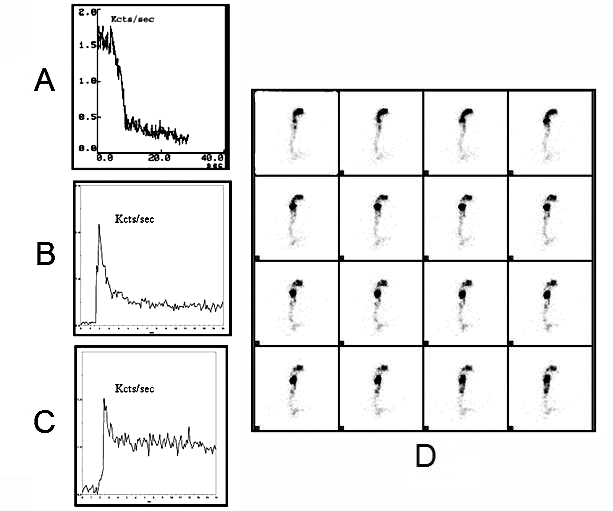
Oro-pharyngo-oesophageal scintigraphy: time-activity curves of liquid bolus (10 mL) in SLA patient with dysphagia: oral phase (A), pharyngeal phase (B) and oesophageal phase (C). Dynamic images (1 frame/sec) show slowed-down transit of bolus with retention particularly in pharyngeal area (D).
As already mentioned, in neurological patients, the oral and pharyngeal stages of swallowing are longer, and this longer oral stage can increase the risk of fragmentation and the bolus can drop from the oral cavity into the pharynx before swallowing takes place. The bolus slips to the glosso-epiglottic vallecular area, causing a double or triple swallowing motion that can increase the risk of inhalation of the bolus before swallowing (Fig. 6). This condition is due to altered motility and deficient thrust in the tongue, together with the incapacity of the palatine veil to keep the bolus within the oral cavity.
Fig. 6.
Patient with Parkinson’s disease. Oro-pharyngo-oesophageal scintigraphy after semi-solid bolus (10 mL): time-activity curves showing considerably lengthy oral phase with double swallowing and significant increase in retention of bolus in oro-pharyngeal area.
A neurological condition can also cause a delay or difficulty in triggering the swallowing motion, together with an increase in the risk of inhaling the bolus into the lower airways because the bolus slips into the pharynx when the sphincter opens again, while the upper sphincter of the oesophagus is still closed. In these situations, the bolus is thrust towards the laryngeal aditus and inhaled either during swallowing or after it [post-swallow inhalation (Fig. 7)].
Fig. 7.
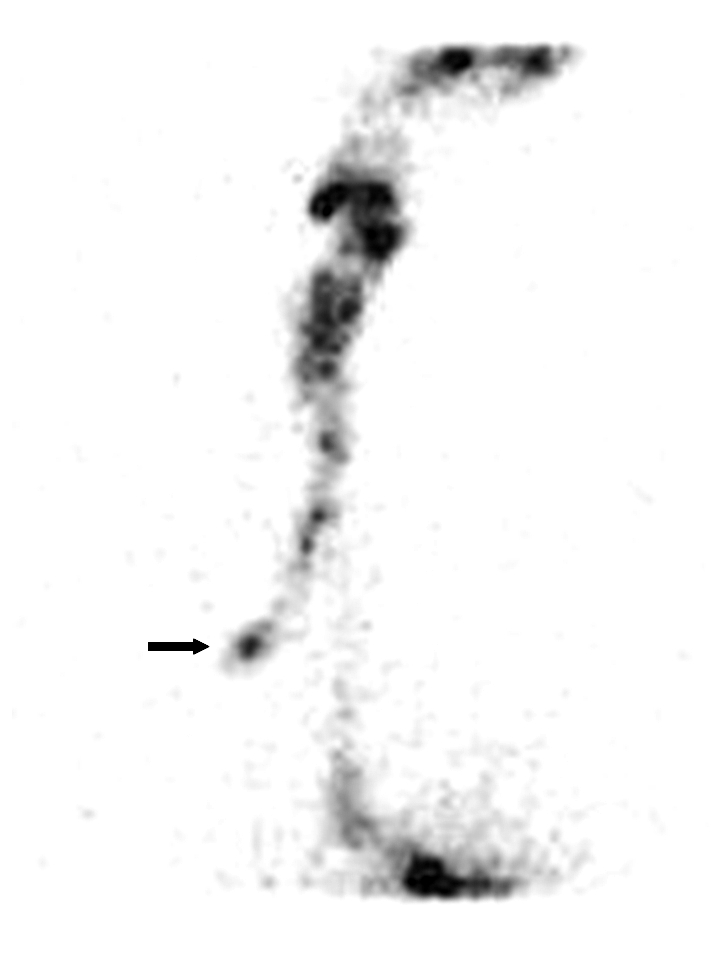
Static anterior projection image after administration of liquid bolus (10 mL) in patient recovering from cerebral ictus: signs of right tracheo-bronchial aspirate (black arrow).
When diagnosing an oesophageal disorder, scintigraphy is indicated if a dynamic and quantitative analysis of the oesophagus is required, particularly if it is necessary to monitor the efficacy of treatment or when other diagnostic methods (manometry and videofluoroscopy) are negative or when results are contradictory. The different sensitivity and specificity encountered in scintigraphic investigation of the oesophagus is attributable, at least in part, to the different conditions taken into consideration. The potential clinical role of oesophageal scintigraphy, including assessment of the oral and pharyngeal stages of swallowing, is certainly greater when managing diseases that are an expression of altered oesophageal motility 19.
Nutcracker Oesophagus
The term “nutcracker esophagus” was introduced by Dalton et al. to describe non-cardiac chest pain or dysphagia, linked with an abnormal genesis of the peristaltic waves in the distal portion of the oesophagus 22. In this abnormality, the high amplitude peristaltic waves do not always interfere with normal clearance of the oesophagus, but can occasionally be strictly related to sporadic episodes of dysphagia or chest pain, or, sometimes, cause retention of the bolus in the oesophagus and induce reflux 22.
The clinical consequences of an isolated episode of hypertonic lower oesophageal sphincter (LES) are still not clear. Three groups of patients can be termed as affected by “isolated hypertonic LES”: those with very high LES tone at rest, those with abnormal contraction of LES after relaxation, and those with incomplete LES relaxation. While the first two can be asymptomatic, an incompletely relaxed LES generally interferes with oesophageal emptying. These functional abnormalities are called “atypical disorders of LES relaxation” 22–24. The role of oro-pharyngo-oesophageal scintigraphy in the study of these subjects is controversial. According to some Authors, oesophageal scintigraphy is not useful for assessing either nutcracker oesophagus or isolated hypertonic LES 25 26. According to others, however, abnormal oesophageal scintigraphic parameters can be found in up to 85% of patients with non-cardiac chest pain, with a motility disorder of the oesophagus or with nutcracker oesophagus, including even those who do not complain of dysphagia 27.
Achalasia
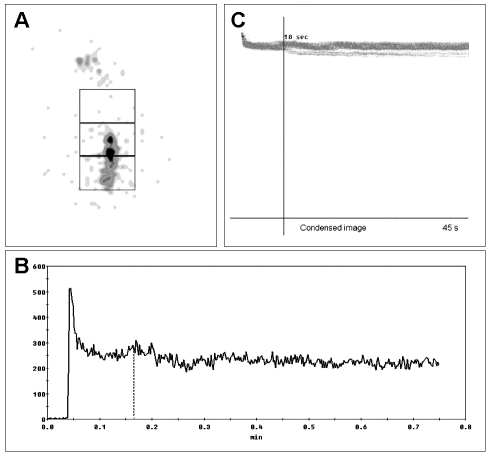
Typical achalasia is caused by a degeneration of the neurons in the oesophageal walls; these neurons are involved in the production of the nitric oxide that contributes to relaxing the smooth muscles of the organ. Scintigraphy, in this classical achalasia, shows a delay in the transit of the bolus – with almost 100% sensitivity – and modifications in the EER and ERI indices 28 29 (Fig. 8). Furthermore, scintigraphic evaluation of oesophageal emptying is commonly accepted as the first-choice method in the follow-up of achalasic patients to monitor the efficacy of the treatment given (myotomy, pneumatic dilatation, botulin toxin injections). Retention of the bolus in the distal portion of the oesophagus does not change when the patient lies down or when he/she drinks liquids. However, to obtain the best results from this scintigraphic examination, the patient should be standing up and should swallow non-labelled water after swallowing a radioactive bolus 28 30–34 (Fig. 9).
Fig. 8.
Scintigraphic study of oesophageal transit (10 mL liquid bolus) in patient suffering from achalasia. Summed image of whole oesophagus, with ROI (A). Time-activity curve shows marked retention of radioactivity in oesophagus (> 60%) (B). Condensed images of oesophageal transit (C).
Fig. 9.
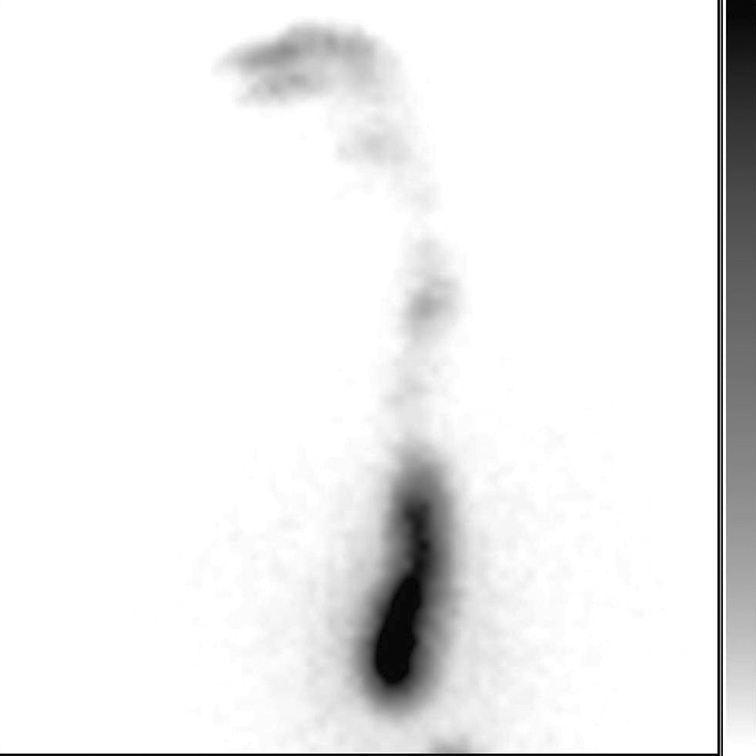
Marked lower oesophageal retention of radioactivity remained unchanged 30 min after end of test and after swallowing 250 cc water. In contrast, oesophageal scintigraphy of patients with scleroderma shows similar pattern of radioactivity retention, which clears upon standing.
Diffuse oesophageal spasm
A diffuse oesophageal spasm is an uncoordinated oesophageal contraction that can interfere with emptying the apparatus. Several manometric criteria have been proposed for diagnosing this condition, including spontaneous and repeated contractions and the presence of prolonged contractions with high amplitude. The most reliable criterion appears to be the presence of simultaneous contractions caused by wet swallows. Apart from the discrepancies seen in manometric diagnostic criteria, diffuse oesophageal spasm is an intermittent phenomenon that is detectable in over 10% of cases (but in far fewer than 100%) during wet swallows 35 36. Hence, oesophageal scintigraphy can be very useful in cases of diffuse oesophageal spasm. In fact, a dynamic scintigraphic study performed on these patients during multiple wet swallows can reveal chaotic movements of the bolus, while the activity/time curves show multiple peaks after a single swallow 19 (Fig. 10).
Fig. 10.
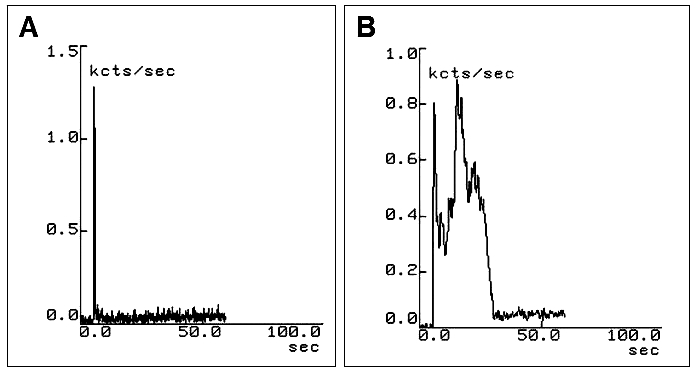
Scintigraphy of oesophageal transit performed after administration of liquid bolus (15 mL) in patient with dysphagia due to diffuse oesophageal spasm. Pharyngeal time-activity curve is within normal limits (A); oesophageal curve shows double peak of radioactivity as consequence of spasm (B).
Scleroderma
Fibrosis and vascular obliteration of the musculature and nerve structures of the oesophagus are at the root of the deficient motility of the organ in patients with scleroderma. Oesophageal scintigraphy demonstrates oesophageal involvement in subjects who, as yet, have no symptoms, showing a typical pattern of radioactivity retention at the third distal of the oesophagus, which resolves only when the patient stands up or when he/she has drunk a glass of water 19. Oesophageal scintigraphy has been shown to be far more sensitive than manometry and radiographic techniques with barium in revealing the abnormal motility of the organ, both at the initial and advanced stages of the disease 37–41.
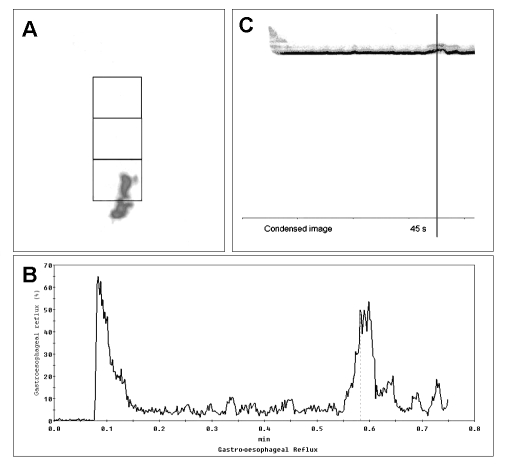
In our experience, and in agreement with the literature, oesophageal scintigraphy is particularly indicated for monitoring the efficacy of medical or surgical treatment of various oesophageal conditions (achalasia, gastro-oesophageal reflux, scleroderma, hiatal hernia) (Fig. 11) and, in this condition, has become the method of choice 19 22 28 40. Moreover, scintigraphy plays an important role in the management of patients complaining of dysphagia but who cannot undergo other investigations (e.g. psychiatric patients) or those who continue to complain of swallowing problems despite repeated negative tests 42 43.
Fig. 11.
Oesophageal scintigraphy transit study (15 mL liquid bolus) of patient with gastro-oesophageal reflux. Summed image of whole oesophagus, with ROI (A). Time/activity curve shows appearance of gastro-oesophageal reflux approximately 35 sec after transit of bolus in oesophagus (B). Condensed images of oesophageal transit (C).
Discussion
The role of OPES is complementary to other diagnostic methods more commonly used in the study of dysphagia 44.
In particular, videofluoroscopy is still considered the gold standard tool for studying swallowing disorders, even though it has certain limitations, such as low accuracy in defining the transit of the bolus from the oral cavity to the pharynx and, in particular, in quantifying oral and pharyngeal clearance 44 45.
Moreover, interpretation of the images obtained with this technique can be difficult since radiographic magnification and/or patient movement can sometimes be the cause of artefacts and distortions 44. Another factor to be considered is that the images are on two planes only whereas the anatomical cavities involved in swallowing are three-dimensional; this creates further difficulty in quantification of oro-pharyngeal clearance. Nevertheless, videofluoroscopy permits an accurate study of the temporal relationship between swallowing and opening of the upper oesophageal sphincter, as well as detection of any tracheal-bronchial aspiration of the bolus.
OPES is a complementary technique permitting a better definition of the dysphagia when associated with other diagnostic methods such as videofluoroscopy and pH-metry since, in comparison to other techniques, it allows a much more accurate calculation of the semi-quantitative parameters in the different stages of swallowing, such as transit time and especially the retention indices of the bolus, supplying important information for planning treatment, rehabilitation and follow-up of the patients 44 45. In particular, OPES allows quantification of the residual bolus in the oral and pharyngeal cavities by means of the retention indices, with a significantly high diagnostic accuracy in the case of oral and pharyngeal dysphagia, whereas, compared to videofluoroscopy, it offers very little further information on pharyngeal dysphagia.
On the other hand, evaluation of transit times in the oro-pharyngo-oesophageal tract is not sufficient, alone, to detect the site of dysphagia with precision 45 46. Shaw et al. 45 calculated that the specificity of the OPES retention indices for liquid boluses is 100% in the oral area and 96% in the pharynx, while sensitivity in these areas is low (being 72% and 57%, respectively); however, these percentages increase when solid boluses are used. The positive predictive values for the oral and pharyngeal retention indices in the case of liquid boluses are 92% and 100%, respectively 45.
An important advantage of OPES is that it allows quantification of any tracheal-bronchial aspiration of the bolus [PA = RAx100/(AT-AT2)] (pulmonary aspiration = radioactivity of the aspirate x 100/radioactivity in the oral area at time 1 (AT) before swallowing minus that after swallowing (AT2) 47. Besides supplying semi-quantitative data, OPES provides the opportunity to study certain qualitative parameters that can help to define swallowing disorders, such as the pre-swallowing drop of the bolus from the oral cavity, fragmentation of the bolus in the oro-pharyngeal and pharyngeal areas causing double/triple swallowing (usually due to reduced thrust of the tongue), pharyngeal-oral and oro-nasal reflux, and functional disorders of oesophageal motility 13 14.
If used as the only method of investigation, the limitations of this scintigraphy are: impossibility to define the anatomy of the structures studied and, therefore, incapacity to detect a precise anatomical site of the dysphagia. Albeit, this scintigraphic approach is: easy to use, economical, well tolerated by patients, and repeatable (regional clearance has excellent test-retest reliability), supplying important information for planning treatment, rehabilitation and follow-up of patients, with, at the same time, exposure to very low dosages of radiation 45 46.
In consideration of the above-mentioned advantages, we maintain that OPES plays an important role in the investigation of all forms of dysphagia, whether post-surgical, neurological or related to oesophageal disorders.
References
- 1.Schindler O, Ruoppolo G, Schindler A. Deglutologia. Torino: Edizioni Omega; 2001. [Google Scholar]
- 2.Tesei F, Caliceti G, Brusoni S, Gavelli GP, Mattioli S, Rinaldi Ceroni A. Lo studio endoscopico e per “imaging” della deglutizione. In: Piemonte M, editor. Fisiopatologia della deglutizione. XIV Giornate Italiane di Otoneurologia. Manifestazione Ufficiale dell’AOOI: Senigallia 18 aprile 1997. p. 87-107. [Google Scholar]
- 3.American Speech-Language-Hearing Association (ASHA) Special interest division 13. Swallowing and swallowing disorders. 1998. [Google Scholar]
- 4.Logemann JA. Swallowing physiology and pathophysiology. Otolaryngol Clin North Am 1988;21:613-22. [PubMed] [Google Scholar]
- 5.Logemann JA. Dysphagia: evaluation and treatment. Folia Phoniatr Logop 1995;47:140-64. [DOI] [PubMed] [Google Scholar]
- 6.Cook IJ, Dodds WJ, Dantas RO, Massey B, Kern MK, Lang IM, et al. Timing of videofluoroscopic, manometric events and bolus transit during the oral and the pharyngeal phases of swallowing. Dysphagia 1989;4:8-15. [DOI] [PubMed] [Google Scholar]
- 7.Dodds WJ, Logemann JA, Stewart ET. Radiologic assessment of abnormal oral and pharyngeal stages of swallowing. Am J Roentgenol 1990;154:965-74. [DOI] [PubMed] [Google Scholar]
- 8.Castell JA, Castell DO. Modern solid state computerized manometry of the pharyngoesophageal segment. Dysphagia 1993;8:270-5. [DOI] [PubMed] [Google Scholar]
- 9.Périé S, Laccoureye L, Flahault A, Hazebroucq V, Chaussade S, St Guily JL. Role of videoendoscopy in assessment of pharyngeal function in oropharyngeal dysphagia: comparison with videofluoroscopy and manometry. Laryngoscope 1998;108:1712-6. [DOI] [PubMed] [Google Scholar]
- 10.Aviv JE, Kaplan ST, Thomson E, Spitzner J, Diamonf B, Close LG. The safety of flexible endoscopic evaluation of swallowing with sensory testing (FEESST): an analysis of 500 consecutive evaluations. Dysphagia 2000;15:39-44. [DOI] [PubMed] [Google Scholar]
- 11.Aviv JE, Murry T, Zschommler A, Cohen M, Gartner C. Flexible endoscopic evaluation of swallowing with sensory testing: patient characteristics and analysis of safety in 1,340 consecutive examinations. Ann Otol Rhinol Laryngol 2005;114:173-6. [DOI] [PubMed] [Google Scholar]
- 12.Tabaee A, Murry T, Zschommler A, Desloge RB. Flexible endoscopic evaluation of swallowing with sensory testing in patients with unilateral vocal fold immobility: incidence and pathophysiology of aspiration. Laryngoscope 2005;115:565-9. [DOI] [PubMed] [Google Scholar]
- 13.Grosso M, Mattone V, Rizza E, Mariani G. Scintigraphy in swallowing disorders. In: Ursino F, editor. Approccio multidisciplinare ai disturbi della deglutizione. Inquadramento diagnostico e terapeutico riabilitativo. Pisa: Plus Ed.; 2005. p. 49-58. [Google Scholar]
- 14.Matteucci F, Fattori B, Grosso M, Bianchi F, Alsharif A, Boni G, et al. La scintigrafia nello studio della fisiopatologia della deglutizione. In: Bruschini P, Mariani G, editors. La scintigrafia in otorinolaringoiatria. Quaderni monografici di aggiornamento Associazione Otorinolaringologi Ospedalieri Italiani (AOOI). Galatina (Lecce): TorGraf; 2003. p. 211-20. [Google Scholar]
- 15.Russell CO, Hill LD, Holmes ER 3rd, Hull DA, Gannon R, Pope CE 2nd Radionuclide transit: a sensitive screening test for esophageal dysfunction. Gastroenterology 1981;80:887-92. [PubMed] [Google Scholar]
- 16.Tolin RD, Malmud LS, Reillely J, Fisher RS. Esophageal scintigraphy to quantitate esophageal transit (quantitation of esophageal transit). Gastroenterology 1979;76:1402-8. [PubMed] [Google Scholar]
- 17.Bartlett RJV. Reproducibility of esophageal transit studies: several single swallows must be performed. Nucl Med Commun 1987;8:317-26. [DOI] [PubMed] [Google Scholar]
- 18.Tatsch K. Multiple swallow test for quantitative and qualitative evaluation of esophageal motility disorders. J Nucl Med 1991;32:1365-70. [PubMed] [Google Scholar]
- 19.Mariani G, Boni G, Barreca M, Bellini M, Fattori B, Alsharif A, et al. Radionuclide gastroesophageal motor studies. J Nucl Med 2004;45:1004-28. [PubMed] [Google Scholar]
- 20.Tatsch K, Voderholzer WA, Weiss MJ, Schrottle W, Hahn K. Re-appraisal of quantitative esophageal scintigraphy by optimizing results with ROC analyses. J Nucl Med 1996;37:1799-805. [PubMed] [Google Scholar]
- 21.Vanek AW, Dominant NE. Responses of human esophagus to paired swallows. Gastroenterology 1987;92:643-50. [DOI] [PubMed] [Google Scholar]
- 22.Dalton CB, Castell DO, Richter JE. The changing faces of nutcracker esophagus. Am J Gastroenterol 1988;83:623-8. [PubMed] [Google Scholar]
- 23.Bassotti G, Alunni G, Cocchieri M, Pelli MA, Morelli A. Isolated hypertensive lower esophageal sphincter: clinical and manometric aspects of an uncommon esophageal motor abnormality. J Clin Gastroenterol 1992;14:285-7. [PubMed] [Google Scholar]
- 24.Garrett JM, Godwin DH. Gastroesophageal hypercontracting sphincter: manometric and clinical characteristics. JAMA 1969;208:992-8. [PubMed] [Google Scholar]
- 25.de Caestecker JS, Blackwell JN, Adam RD, Hannan WJ, Brown J, Heading RC. Clinical value of radionuclide esophageal transit measurement. Gut 1986;27:659-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holloway RH, Lange RC, Plankey MW, McCallum RW. Detection of esophageal motor disorders by radionuclide transit studies: a reappraisal. Dig Dis Sci 1989;34:905-12. [DOI] [PubMed] [Google Scholar]
- 27.Howarth D, Oldfield G, Booker J, Tan P. Esophageal dysfunction in patients with atypical chest pain investigated with esophageal scintigraphy and myocardial perfusion imaging: an outcome study. J Nucl Cardiol 2003;10:490-7. [DOI] [PubMed] [Google Scholar]
- 28.Kim CH, Cameron AJ, Hsu JJ, Talley NJ, Trastek VF, Pairolero PC, et al. Achalasia: prospective evaluation of relationship between lower esophageal sphincter pressure, esophageal diameter and symptoms in response to pneumatic dilatation. Mayo Clin Proc 1993;68:1067-73. [DOI] [PubMed] [Google Scholar]
- 29.Mughal MM, Marples M, Bancewicz J. Scintigraphic assessment of esophageal motility: what does it show and how reliable is it? Gut 1986;27:946-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson CS, Hardy JG, Atkinson M. Qualitative assessment of response to therapy in achalasia of the cardia. Gut 1989;30:768-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonavina L, Nosadini A, Bardini R, Baessato M, Peracchia A. Primary treatment of esophageal achalasia: long term results of myotomy and Dor fundoplication. Arch Surg 1992;127:222-6. [DOI] [PubMed] [Google Scholar]
- 32.Pasricha PJ, Ravich WJ, Hendrix TR, Sostre S, Jones B, Kalloo AN. Intrasphincteric botulinum toxin for treatment of achalasia. N Engl J Med 1995;322:774-8. [DOI] [PubMed] [Google Scholar]
- 33.Cohen S, Parkman HP. Treatment of achalasia: from whale bone to botulinum toxin. N Engl J Med 1995;322:815-6. [DOI] [PubMed] [Google Scholar]
- 34.Chawda SJ, Watura R, Adams H, Smith PM. A comparison of barium swallow and erect esophageal transit scintigraphy following balloon dilatation for achalasia. Dis Esophagus 1998;11:181-7. [DOI] [PubMed] [Google Scholar]
- 35.Spechler SJ, Castell DO. Classification of esophageal motility disorders [review]. Gut 2001;49:145-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richter JE, Castell DO. Diffuse esophageal spasm: a reappraisal. Ann Intern Med 1984;100:242-5. [DOI] [PubMed] [Google Scholar]
- 37.Klein HA. Receiver operating characteristic surfaces for esophageal transit scintigraphy in systemic sclerosis [abstract]. Eur J Nucl Med 1991;16:S31. [Google Scholar]
- 38.Edenbrandt L, Theander E, Hogstrom M, Scheja A, Akesson A, Palmer J. Esophageal scintigraphy in normal subjects and patients with systemic sclerosis. J Nucl Med 1995;36:1533-7. [PubMed] [Google Scholar]
- 39.Geatti O, Shapiro B, Fig LM, Fossaluzza V, Franzon R, De Vita S, et al. Radiolabeled semisolid test meal clearance of esophageal involvement in scleroderma and Sjögren syndrome. Am J Physiol Imaging 1991;6:65-73. [PubMed] [Google Scholar]
- 40.Kaye SA, Siraj QH, Agnew J, Hilson A, Black CM. Detection of early asymptomatic esophageal dysfunction in systemic sclerosis using a new scintigraphic grading method. J Rheumatol 1996;23:297-301. [PubMed] [Google Scholar]
- 41.Cozzi F, Zucchetta P, Durigon N, Marzola MC, Bullo A, Favaro M, et al. Changes in esophageal peristalsis in diverse clinical forms and antibody specificity in scleroderma: a scintigraphic study in 100 cases. Reumatismo 2003;55:86-92. [PubMed] [Google Scholar]
- 42.Roland J, Dhaenen H, Ham HR, Peters O, Piepsz A. Oesophageal motility disorders in patients with psychiatric disorders. Eur J Nucl Med 1996;23:1583-7. [DOI] [PubMed] [Google Scholar]
- 43.Taillefer R, Jadliwalla M, Pellerin E, Lafontaine E, Duranceau A. Radionuclide esophageal transit study in the detection of esophageal motor dysfunction: comparison with motility studies (manometry). J Nucl Med 1990;31:1921-6. [PubMed] [Google Scholar]
- 44.Humphreys B, Mathog R, Rosen R, Miller P, Muz J, Nelson R. Videofluoroscopic and scintigraphic analysis of dysphagia in the head and neck cancer patients. Laryngoscope 1987;94:25-32. [DOI] [PubMed] [Google Scholar]
- 45.Shaw DW, Williams RBH, Cook IJ, Wallace KL, Weltman MD, Collins PJ, et al. Oropharyngeal scintigraphy: a reliable technique for the quantitative evaluation of oral-pharyngeal swallowing. Dysphagia 2004;19:36-42. [DOI] [PubMed] [Google Scholar]
- 46.Argon M, Secil Y, Duygun U, Aydogdu I, Kocacelebi K, Ozkilic H. The value of scintigraphy in the evaluation of oropharyngeal dysphagia. Eur J Nucl Med Mol Imaging 2004;31:94-8. [DOI] [PubMed] [Google Scholar]
- 47.Galli J, Valenza V, D’Alatri L, Gajate Samanes AM, Reale F, La Mura F. Validity of scintigraphy in the study of neurogenic dysphagia. Acta Otorhinolaryngol Ital 2000;20:250-9. [PubMed] [Google Scholar]