Summary
Over the last fifteen years, increasing public demand for minimally-invasive surgery and recent technological advances have led to the development of a number of conservative options for the therapeutic management of obstructive salivary disorders such as calculi and duct stenosis. These include extracorporeal shock-wave lithotripsy, sialoendoscopy, laser intra-corporeal lithotripsy, interventional radiology, the video-assisted conservative surgical removal of parotid and sub-mandibular calculi and botulinum toxin therapy. Each of these techniques may be used as a single therapeutic modality or in combination with one or more of the above-mentioned options, usually in day case or one-day case under local or general anaesthesia. The multi-modal approach is completely successful in about 80% of patients and reduces the need for gland removal in 3%, thus justifying the combination of, albeit, time-consuming and relatively expensive techniques as part of the modern and functional management of salivary calculi. With regard to the management of salivary duct anomalies, such as strictures and kinkings, interventional radiology with fluoroscopically controlled balloon ductoplasty seems to be the most suitable technique despite the use of radiation. Operative sialoendoscopy alone is the best therapeutic option for all mobile intra-luminal causes of obstruction, such as microliths, mucous plugs or foreign bodies, or for the local treatment of inflammatory conditions such as recurrent chronic parotitis or autoimmune salivary disorders. Finally, in the case of failure of one of the above techniques and regardless of the cause of obstruction, botulinum toxin injection into the parenchyma of the salivary glands using colour Doppler ultrasonographic monitoring should be considered before deciding on surgical gland removal.
Keywords: Salivary glands, Salivary calculi, Salivary duct stenosis, Surgical treatment, Extracorporeal lithotripsy, Sialoendoscopy, Botulinum toxin therapy
Riassunto
Negli ultimi quindici anni la sempre maggiore richiesta di terapie minimamente invasive ed i recenti progressi tecnologici hanno favorito l’affermazione di tecniche conservative nel management dei disordini ostruttivi salivari quali la scialolitiasi e le stenosi duttali. Tali nuove opzioni terapeutiche includono la litotrissia extracorporea, l’endoscopia salivare, la litotrissia intracorporea laser, la radiologia interventistica, la rimozione chirurgica di calcoli parotidei e sottomandibolari video-assistita con preservazione ghiandolare ed il trattamento con tossina botulinica. Le tecniche menzionate possono essere impiegate singolarmente oppure combinate tra loro, generalmente in regime ambulatoriale o di Day Surgery e One-Day Surgery in anestesia locale o generale. L’elevato successo terapeutico, pari a circa l’80%, garantito dall’approccio multimodale e l’abbattimento al 3% dei casi destinati alla scialoadenectomia tradizionale, giustifica l’utilizzo in modo combinato di tali tecniche costose e time-consuming come parte integrante del moderno management della litiasi salivare. La radiologia interventistica, in particolar modo la riabilitazione duttale plastica mediante catetere a palloncino sotto controllo fluoroscopico, appare, nonostante l’impiego di radiazioni, la migliore opzione terapeutica nella gestione delle stenosi e dei kinkings duttali. L’endoscopia salivare operativa, da sola, rappresenta il trattamento di scelta per tutte le ostruzioni endoluminali mobili (microliti, mucous plugs o corpi estranei) e per il trattamento locale di patologie infiammatorie tra cui parotiti ricorrenti croniche o disordini salivari di origine autoimmune. In aggiunta a ciò, in caso di fallimento delle precedenti opzioni terapeutiche, indipendentemente dalla causa dell’ostruzione, l’infiltrazione di tossina botulinica nel parenchima salivare sotto controllo ecocolor Doppler dovrebbe essere considerata prima di optare per l’asportazione chirurgica della ghiandola salivare.
Introduction
Salivary gland diseases are relatively common. The most frequent non-neoplastic salivary disorder is obstructive sialadenitis 1, which may be due to calculi, fibromucinous plugs, duct stenosis, foreign bodies, anatomic variations, or malformations of the duct system leading to a mechanical obstruction associated with stasis 2.
Patients with obstructive sialadenitis present with a history of recurrent painful periprandial swelling of the involved gland, best known as the “meal-time syndrome” 3, which is often complicated by recurrent bacterial infections, with fever and a purulent discharge at the papilla 2 4.
Sialolithiasis
Sialolithiasis is the main cause of obstructive salivary diseases, being involved in 66% of cases 5 and accounting for about 50% of major salivary gland diseases 6. Post-mortem studies have shown a 1.2% prevalence of salivary calculi in the general population 7, although Escudier and McGurk described the incidence of symptomatic salivary calculi as being about 59 cases per million per annum 8, for a clinical prevalence of 0.45% 9.
Sialolithiasis is more frequent in male patients 10. Incidence peaks between the age of 30 and 60 years 11, and it is uncommon in children as only 3% of all sialolithiasis cases occur in the paediatric population 12.
Sialolithiasis affects the submandibular gland in 80-90% of cases 13, mainly unilaterally 10 but without a preferred side; this finding is partly explained by recent post-mortem morphometric studies which found a symmetry between the right and left gland 14. In our experience, the mean size of submandibular stones is about 7.3 mm, although giant sialoliths measuring up to 7 cm have occasionally been described 15–19. The majority of calculi are located in the distal third of the duct or at the hilum of the gland; pure intraparenchymal stones are infrequent 20.
Between 5% and 10% of cases occur in the parotid gland 13. The striking difference between parotid and submandibular stones is partially related to the ascendent and sharper angled duct system of the submandibular gland and the type of (mainly mucous) secretion 3. The sublingual and other minor salivary glands are rarely affected (about 0-5% of cases) 13.
The traditional aetiopathogenetic factors associated with stone formation are obstruction, reduced salivary flow rate, dehydration, change in salivary pH associated with oropharyngeal sepsis and impaired crystalloid solubility 3; physiologically, microliths may be detected following precipitation in a supersaturated solution of mucous plugs 3 or membrane phospholipids within redundant secretory vescicles 21 22, which become symptomatic and act as a nidus in which successive layers of inorganic and organic substances are deposited.
In addition to these classic hypotheses, Marchal et al. 4 have recently suggested a retrograde theory in lithogenesis, according to which a retrograde migration of foods, bacteria or foreign bodies from the oral cavity to the duct system may lead to stone formation, being facilitated by variations in the sphincter-like mechanism reported in 90% of cases 23. This hypothesis has been supported by Teymoortash et al. 24, who used polymerase chain reaction (PCR) to extract gene fragments belonging to oral bacteria from salivary calculi, most of which related to Streptococcus species (the same as those found in gingival bacterial plaque).
The traditional diagnostic approach consists of standard radiography, which does not reveal radiolucent 25, intraglandular or small stones in about 20% of cases 7 26, and computed tomography (CT), which is limited by the fact that the stone can be occulted by thick radiological slices and that scans do not provide the precise localisation of a sialolith within the duct system 27. Colour Doppler sonography has also been considered useful in patients with sialolithiasis 28.
Ultrasonography currently represents an excellent first-level diagnostic technique 29 insofar as, in experienced hands, it reveals ductal and highly mineralised stones with a diameter of at least 1.5 mm with a accuracy of 99% 30.
Recent advances in optical technology have led to the development of sialoendoscopy, a new diagnostic means of directly visualising intra-ductal stones that has bridged the diagnostic gap between the clinical suspicion of salivary obstruction and the limitations of conventional radiology. Appropriately miniaturised instruments for the mean diameters of the excretory ducts of the major salivary glands (0.5-1.4 mm for Stensen’s duct and 0.5-1.5 mm for Wharton’s duct, as suggested by histological studies 31), allow an almost complete exploration of the duct system in most patients.
Duct anomalies
Strictures and kinks are the second most frequent cause of obstructive sialadenitis 32 and, unlike sialolithiasis, frequently involve the parotid ductal system (75.3% 33) and mainly affect females 33.
Sialographic findings indicate that salivary duct stenosis accounts for about 23-30% of recurrent parotid swellings 34 35 and 3% of recurrent submandibular swellings 34. Other anatomic variations have been described in the case of salivary gland obstructions; these include accessory ducts 27, sphincter-like mechanisms located near the papilla in Wharton’s duct or posteriorly in Stensen’s duct 36 37, pelvis-like formations which are basin-like structures at the hilum instead of a bifurcation or trifurcation 1 38 39, and intraductal evaginations 1 38.
Strictures are usually a result of epithelial duct injuries following recurrent infections or traumas caused by sialoliths or surgical procedures, although congenital strictures have also been described 2 34 40. In this context, bilateral parotid duct sialectasia in patients with parotid obstruction but no signs of chronic parotitis may be considered a congenital anomaly.
Concerning the origin of kinks, Nahlieli et al. 41 have described the involvement of the sharp bend in Wharton’s duct above the lingual nerve and the mylohyoid muscle in the region known as the “knee area”, in addition to herniation of the surrounding tissue through the mylohyoid muscle or the loosening of the same 41 42.
The traditional diagnostic approach to duct stenosis includes sialography, which is still considered the diagnostic gold standard, and also plays a therapeutic role by stretching duct walls as a result of contrast medium injection 43. Sialo-CT has also been proposed for the diagnosis of abnormalities in the duct system. However, these imaging modalities visualise the salivary duct system indirectly 27, expose patients to radiation, and may be complicated by infections or iatrogenic lesions of the duct wall 44.
Magnetic resonance (MR) sialography has more recently been introduced as a new diagnostic tool for visualising the duct system up to the tertiary branches and the parenchymal tissue 45 46. It has the advantages that it does not require contrast medium, there is no radiation and no need for ductal cannulation, it can also be performed during acute gland infection and, finally, the use of citric acid to stimulate salivary secretion (dynamic sialo-MR) allows a functional evaluation of the affected gland 47 (Fig. 1).
Fig. 1.
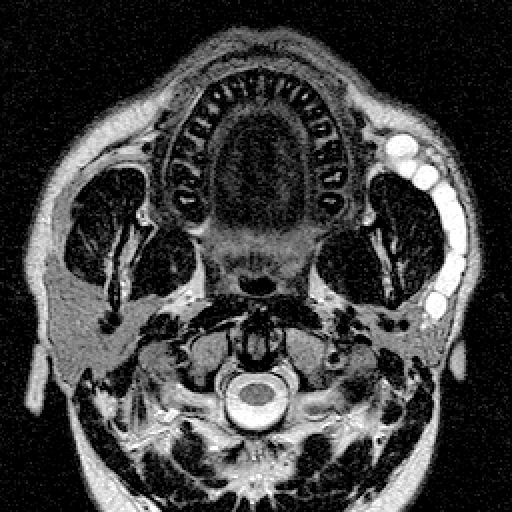
Sialo-MR image of parotid duct stenosis of distal third.
MR sialographic 3-dimensional reconstruction imaging and MR virtual endoscopy for salivary gland ducts have recently been proposed on the basis of experiences using MR virtual endoscopy in other systems such as the gastrointestinal, urinary and biliary tracts, and vascular structures 48–51. This new diagnostic modality has been proposed as a non-invasive pre-surgical procedure in order to fit conventional surgical endoscopy to the patient’s individual anatomy on the basis of the endoluminal views provided 52.
Sialoendoscopy is useful in detecting ductal anomalies that may not be detected by means of either traditional or new imaging techniques.
Other causes of obstruction
Salivary duct obstruction may be caused by mucous plugs, foreign bodies, sialodochitis, ab estrinseco compression due to a neoplasm or reactive intraparenchymal parotid lymph nodes, intraductal polyps, or the granulation tissue sometimes associated with immunological disorders such as Sjögren’s syndrome 1 27 38.
Obstructive symptoms can also follow dose- and time-dependent damage to salivary glands exposed to radioiodine therapy administered to patients with thyroid carcinoma, because the salivary glands, and especially the serous parotid cells, selectively concentrate iodine 53.
Salivary duct obstruction of the parotid gland due to a lack of neuromuscular masseteric coordination has also been described 54, as well as obstruction due to traumatic erupting teeth 34 or denture-induced compression of the salivary duct orifice.
In most of such cases in which traditional and modern imaging techniques cannot visualise the cause of the obstruction, sialoendoscopy provides immediate and direct information.
Traditional management
The traditional approach to obstructive salivary disorders suggests duct dilatation, incision and dissection in the case of distal stones (sometimes followed by marsupialisation, with the risk of post-operative stenosis), and sialadenectomy in the case of proximal, hylar or intraparenchymal sialoliths. Sialolithiasis is still the main indication for sialadenectomy 55, although common post-operative complications include nerve injury, recrudescent symptoms due to stones retained in the remaining duct 55 56, and unsatisfactory aesthetic outcomes. With regard to parotid stones, total conservative parotidectomy has been considered better than superficial parotidectomy in order to avoid recurrences 57. The main complication reported after superficial parotidectomy for obstructive salivary disorders is facial nerve palsy (temporary nerve weakness in 16-38% of cases, permanent in up to 9% 58–60); Frey’s syndrome is rare 61 62. After sub-mandibular gland excision, there is a 1-8% risk of permanent marginal mandibular nerve palsy 63–66 and a 1-5% risk of lingual nerve injury 63–65. Sialocele, salivary fistulas or cyst formation, neurinomas, infections and haematomas are rarely encountered after sialoadectomy for obstructive disease 67.
In the case of ductal anomalies, traditional management suggests surgical derivation of salivary flow or by-pass with the creation of a new excretory duct proximal to the stenosis, or ductal sialodochoplasty 68–70.
Current management
Although sialolithiasis has been associated with a high incidence of chronic inflammation suggesting that the obstruction of the duct of the salivary glands led to irreversible parenchymal damage, recent scintigraphic 71 72 and histopathological studies 73 have shown that recovery of secretory function after stone removal is guaranteed in most cases. For example, on the basis of scintigraphic examination, Yoshimura et al. 72 assessed functional restoration in 78% of salivary glands after sialolithotomy, and Marchal et al. 73 found that at least half of his patients who underwent sialoadenectomy showed a normal histological pattern.
Herewith, a review is presented of the main minimally invasive gland-preserving techniques currently used in the management of obstructive salivary disease, including shock-wave lithotripsy, sialoendoscopy, interventional radiology, endoscopically video-assisted trans-oral and cervical surgical retrieval of stones, and botulinum toxin therapy.
Shock-wave lithotripsy
In 1989, Iro et al. 74 introduced the application of extra-corporeal shock-wave lithotripsy (ESWL) (previously used in the urological and gastroenterological fields) for the management of sialolithiasis. Sialolithotripsy is a non-invasive method of fragmenting salivary stones into smaller portions in order to favour their possible flushing out from the salivary duct system spontaneously or after salivation induced by citric acid or other sialogogues. Exploiting the change in impedance at the stone/water interface, lithotripsy leads to stone fracture by producing a compressive wave that spreads through the calculus and an expansive wave that pits it and induces its cavitation 75. The shock-waves may be generated extra-corporeally using piezoelectric 76 and electromagnetic techniques 77–79, or intra-corporeally using electro-hydraulic 80, pneumatic 81 82 or laser endoscopic devices 83 84.
Extra-corporeal shock-wave lithotripsy
Extra-corporeal electromagnetic shock-wave lithotripsy
Dedicated lithotripters with a mobile arm (Minilith SL-1, Storz Medical, Kreutzlingen, Switzerland) are currently used for the treatment of salivary calculi (Fig. 2). The ultrasound-guided shock-wave generated by a small-diameter, cylindric, electromagnetic source focuses on the salivary stones by means of a parabolic reflector within the cushion, while the patients remain supine in a semi-reclined position in a dentist’s chair 85. The 2.4 mm size of the shock-wave focus permits the treatment of stones with diameters of ≥ 2.4 mm 85. The pulse frequency of the wave may vary from 0.5 to 2 Hz and no more than 4000 shock-waves may be administered per session. Continuous sonographic monitoring allows direct visualisation of the degree of fragmentation during treatment and avoids lesions to the surrounding tissues 9 85.
Fig. 2.

Dedicated miniaturised extracorporeal lithotripter for fragmentation of salivary stones.
The exclusion criteria for ESWL are stones with a diameter of < 2 mm or which cannot be identified using an ultrasound probe, and the presence of complete distal duct stenosis; the procedure is contra-indicated in patients with acute sialadenitis or acute inflammation in the head and neck region, as well as in patients with cardiac pacemakers 85 86.
The main limitation of ESWL is that it does not always completely clear the calculus but leaves stone fragments inside the duct system that may subsequently become the nidus of recurrent sialolithiasis 73. In fact, ESWL completely eliminates 34-69% of parotid calculi 9 78 79 85 87 and 32-42% of submandibular calculi 78 79 85 87 98. Capaccio et al. 87 analysed a series of 322 patients, and found statistically significant associations between favourable outcomes and parotid stones and intraductal sub-mandibular stones, calculi < 7 mm, age < 46 years, and fewer than 2000 shock-waves. On the basis of these experiences, ESWL is currently considered the treatment of choice for all parotid calculi and submandibular perihilar or intraparenchymal stones < 7 mm 9 88.
The reported untoward effects are skin pain over the treated area (in 79.5% of cases 87), glandular swelling (35.2% 87), duct haemorrhage (36.8% 87), and cutaneous petechiae (22.7% 87).
Extra-corporeal piezoelectric shock-wave lithotripsy
The piezoelectric technique exploits the pressure wave produced in water by the expansion of crystals due to the application of voltage. The crystals are placed on a concave disk that converges the wave on a 3 mm area to a depth of 11 mm 75. Iro et al. 76 89–92 have documented results of 50-58% of stone-free cases, and 76-100% of patients experiencing symptom relief.
Intra-corporeal shock-wave lithotripsy
In intra-corporeal lithotripsy, the shock-waves reach the stone surface through a lithotripsy probe placed inside the salivary duct system under endoscopic guidance 93. The energy needed to fracture the stone is usually provided by means of a laser beam, pneumatic devices, or electro-hydraulic probes.
Endoscopically guided intra-corporeal laser lithotripsy
In 1990, Gundlach et al. 94 reported the first successful application of endoscopically guided intra-corporeal lithotripsy for salivary stones using a laser beam, achieving 92% of stone clearance. Intra-corporeal laser lithotripsy using Holmium YAG (yttrium-aluminum-garnet) or pulsed dye lasers has also been reported in limited series of patients 43 84: the former, the efficacy of which, for urolithiasis, is well known 95 96, is associated with a high risk of soft tissue damage, and their difficulty of use is attributable both to their thermal effects and absorption by the surrounding tissues 5 43; the latter are harmless, but extremely expensive 43 97.
Raif et al. 98 recently proposed the development of an erbium (Er) fibre delivery system for endoscopic lithotripsy of salivary stones: hollow metal wave guides optimised for an Er: YAG laser were end-sealed with a polished sapphire rod of 0.63 mm, designed to adapt to the laser and the sialoendoscope. Complete stone fracture was achieved in 5/21 calculi treated.
Endoscopically controlled intra-corporeal electro-hydraulic lithotripsy
In 1993, Konigsberger et al. 80 used endoscopically controlled intra-corporeal electro-hydraulic lithotripsy by placing a flexible fibroscope with an additional probe inside the ductal system: the shock wave was generated by a sparkover at the tip of the probe electrode placed 1 mm in front of the stone. The clinical trial led to complete stone fragmentation in 20/29 patients with sub-mandibular sialolithiasis 80. On the basis of the results of in vitro and experimental animal studies, Iro et al. 99 criticised this procedure as having a high risk of ductal iatrogenic injuries and being scarcely efficacious at low voltage, and it has now been abandoned on account of possible tissue damage.
Endoscopically controlled intra-corporeal pneumatic lithotripsy
In 1996, Arzoz et al. 82 introduced a rigid 2.1 mm urethroscope with a 1 mm working channel in order to perform intraductal stone fracture using both a pneumoblastic lithotriptor and a laser device under endoscopic control in 12 patients. Pneumatic lithotriptors work by means of ballistic energy and can be likened to a biological “pneumatic hammer” 100. However, despite the encouraging results achieved in the urological field, the use of pneumoballistic devices in the treatment of human sialolithiasis is considered unjustified because the results in in vitro studies suggest a high risk of ductal perforation 100.
Sialoendoscopy
Initially used for diagnostic purposes, sialoendoscopy is now scheduled interventionally in the case of obstructive salivary gland disease 23.
Sialoendoscopy was first described, in 1991, by Katz 101, who used a 0.7 mm flexible endoscope to remove salivary stones with Dormia baskets. Since then, various rigid 82 102 103, semi-rigid and moderately flexible 104 devices, with different diameters, and equipped with working channels and irrigation ports have been developed, and a new more flexible semi-rigid instrument in nitinol has recently been described 105. According to anatomic studies, 1.2 mm should be the upper limit of the diameter of a sialoendoscope in order to avoid iatrogenic lesions 31. As the main problem with sialoendoscopy is entering the ductal ostium, various techniques have been proposed to overcome the ostium, including dilatation with lacrimal probes or bougies on guide wires, papillotomy using a CO2 laser, a sialolithotomy opening, or microsurgical dissection of the anterior ductal portion (the “ductal cut-down” technique) 38 106 107. The mean duration of diagnostic and operative sialoendoscopy is, respectively, 26 ± 14 and 73 ± 43 minutes 5; the ductal lumen is irrigated with isotonic saline fluid through the irrigation port during the procedure in order to permit advancement of the endoscopic device and free movement of the operative instruments.
The only absolute contraindication to the procedure is complete distal obliteration of the duct that is impenetrable by the endoscope. The most frequent side-effect is a transient glandular swelling due to the irrigation with physiological solution in 80-100% of cases 1 37 39 104 107, but ductal strictures (2-4% 39 104) or lacerations (1-8% 5 27), basket block (6% 5), infections (2-3% 37 39 104 107), temporary lingual nerve paresthesia (0.4-0.6% 37 39 104 107), ranula formation (0.6-0.9% 37 39 104 107) and bleeding (0.5% 37) have also been described.
Operative sialoendoscopy in the case of salivary stones
Graspers, miniforceps, Dormia baskets and balloons are mainly used for the endoscopically-controlled retrieval of stones or their fracture into smaller pieces through the working channel or by pushing them forward in parallel to the endoscopic device (Fig. 3). Intra-corporeal laser litho-tripsy may be alternatively adopted to fragment the stone before using the graspers or baskets 38.
Fig. 3.
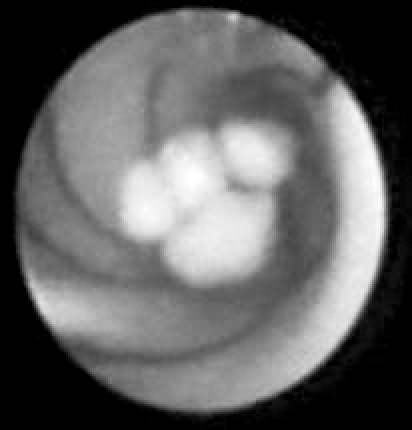
Sialoendoscopic removal of submandibular stone by basket.
Endoscopic stone removal is not indicated in the case of deep intra-glandular sialoliths 108 or stones embedded in the ductal wall. The published success rates are 89% for sub-mandibular stones 37 107 and 83-86% for parotid sialoliths 37 107. Sialoendoscopy is also effective in removing mucus plugs, foreign bodies, polyps, and granulation tissue.
Operative sialoendoscopy in the case of ductal anomalies
Use of saline pressure irrigation, during sialoendoscopy, is usually enough to stretch strictures that are less than half of the duct diameter 41 up to the hilar area 32. In the case of severe strictures, a sialoballoon with a diameter of < 1 mm (2.5-3 Fr) is inserted through the diagnostic unit 32 38 41: it is usually inflated to 18 Bar for 90 sec. up to a maximum of 3 mm, then deflated and re-inflated 38 41. Miniature grasping forceps can also be used in a retrograde fashion along the inner wall towards the stricture 32 38. An intraductal injection of hydrocortisone and the insertion of a sialostent to avoid recurrences are recommended 38 41.
Nahlieli et al. 38 41 have described an “anti-kink procedure” in which the kink is envelopped by means of a balloon before performing an advancement ductoplasty: the duct is stripped, some millimetres are removed from the anterior portion, and then a stent is positioned, with its anterior edge being sutured to the mucosa and the periostium in order to extend the angle 38 41.
According to Nahlieli et al. 37 41, the endoscopic treatment of strictures has a success rate of 80-81%; with regard to kinks, they documented the complete remission of symptoms in all nine patients submitted to the anti-kink procedure in 2001 41.
Interventional radiology
Interventional radiology in the case of salivary stones
Interventional radiology was first reported by Kelly et al. 109, who removed a sub-mandibular duct stone using Dormia baskets under fluoroscopic control in 1991. Since then, various techniques have been proposed for the removal both of parotid and sub-mandibular stones, including the use of a coronary angioplasty balloon, a wire loop vascular snare, or an embolectomy catheter under fluoroscopic control.
Fluoroscopically guided stone retrieval is currently the best therapeutic option in interventional radiology, and is indicated for mobile stones located in the middle and proximal portion of the sub-mandibular ductal system 9 110 and parotid duct stones 110. The reported success rates range from 40% to 100% 9 110–115, and failures are related to unsuccessful stone identification and the presence of fixed or unreachable stones 93 110 113. The main complications described after radiological stone retrieval include gland swelling (100% 110), infections (8% 110), and a gland-impacted basket requiring surgical intervention 110. The main limitation of the procedure is the administration of ionising radiation.
Interventional radiology for stricture dilatation
In 1992, Buckenham et al. 116 pioneered the use of a coronary angioplasty balloon catheter to dilate parotid strictures using digital subtraction imaging; subsequently, sub-mandibular duct dilatations were also performed 2 111 117.
In 2006, Brown 118 completely eliminated duct stricture in 71.5% of a series of 125 patients by means of balloon ductoplasty, under fluoroscopic control; 9.6% of this group showed residual stenosis at post-operative sialography.
Conservative trans-oral surgical removal of sub-mandibular stones
Seward 119 120 first attempted trans-oral sub-mandibular stone removal from the anterior floor of the mouth in 1968, but it is only recently that conservative trans-oral release has been judged feasible enough to replace traditional sialoadenectomy in the management of sialoliths located inside the proximal duct or at the hilum 9 16 88 121 122. Trans-oral surgical stone removal is currently considered the treatment of choice for deeply sited hilar sub-mandibular stones fixed to the ductal wall that are bimanually palpable and have a diameter of at least 8 mm 9 16 122. The only contra-indication is limited mouth opening.
Stones can be retrieved trans-orally using various techniques: Zenk et al. 88 have proposed an expanding duct excision from the papilla until the stone is visible and then a hilar marsupialisation of the duct after stone release, whereas McGurk et al. 9 121 122 and Capaccio et al. 16 preserve the entire Wharton’s duct until the hilum and make an incision directly over the palpated stone 9 16 121 122 (Fig. 4); the ductal opening can be sutured with stitches or using a net of Surgicel in order to cover the incision area.
Fig. 4.
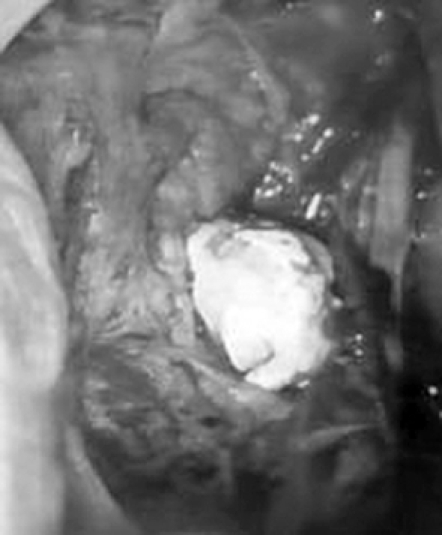
Delivery of hylar submandibular stone during transoral conservative approach.
The success rate of trans-oral surgery in removing sub-mandibular stones is 82-98% 9 16 88 122. Post-operative complications include tingling at the tip of the tongue 16 122, swelling of the floor of the mouth (5% 88) and lingual nerve injury (1% 88), and ranulas (2% 88 122), strictures (2-5% 88 122) and infections (5% 122) may develop during follow-up. Recurrences have been reported in about 1-10% of cases 88 123.
Endoscopically assisted removal of parotid and sub-mandibular stones
Baurmarsh and Dechiara 124 were the first to retrieve a parotid stone extra-orally in 1991 and, a few years later, Nahlieli et al. 125 proposed an endoscopically assisted parotid stone retrieval technique. Since then, a number of new endoscopically assisted procedures have been proposed for extra-ductal sialolithotomies, including both intra- and extra-oral techniques: intra-oral sialolithotomy (the so-called “ductal stretching technique” 38) can be used for both parotid and sub-mandibular stones, and consists of conservative trans-oral stone release by means of an extended dissection under endoscopic control 38; the extra-oral technique is reserved for impacted or intraparenchymal parotid stones or sialoliths posteriorly situated in the parotid duct system with proximal duct obstruction 38. It is contra-indicated for severe stenoses or stones located deeper than 6 mm from the outer skin surface 38 126. Once the stone has been visualised through the endoscope, trans-illumination is used as a guide to mark its exact location on the outer skin before the stone is exposed and delivered through a 1 cm incision above it or through the creation of a pre-auricular skin flap which preserves the buccal branch of the facial nerve 38 126.
This combined technique has led to high success rates according to McGurk et al. 126 127 and Marchal 128, who documented symptomatic relief in 92% of 37 patients with parotid stones 128. The post-operative complications have been described as: swelling and paresthesia of the periauricular skin, infections, post-operative strictures, and damage to the ductal system, sometimes requiring duct ligation 126 127.
Botulinum toxin therapy
Botulinum toxin therapy, which is already used in the neurological field, has recently been introduced in the management of otorhinolaryngological disorders clinically characterised by an increased salivary flow rate such as drooling, sialorrhea 129–131, and salivary fistulas 132–134; the rationale underlying the use of botulinum toxin is the selective chemical denervation obtained by blocking neurotransmitter release at the cholinergic parasympathetic nerve terminals of the salivary glands 135. An injection of botulinum toxin reduces the secretory capacity of the gland while avoiding xero-stomia as basal secretory activity is maintained through the adrenergic pathway. Botulinum toxin therapy has also been successfully used to treat sialoceles 136–138, and chronic and recurrent parotitis 131, responsible for obstructive salivary symptoms.
The most frequently used botulinum toxin type A can be injected under electromyographic 134 or colour Doppler US control 131 138, the latter being preferred since it is thus possible to avoid intra-vessel toxin penetration.
The rare complications reported in the literature are transient paresis of the upper lip, loss of the naso-labial fold, and numbness of the upper cheek. The major limitation of the treatment is the relatively brief duration of its effect (3-4 months, in most cases), which is why patients require a second injection 4-7 months after the first 131.
Conclusions
Over the last fifteen years, increasing public demand for minimally invasive treatment together with the rapid development in medical technology have led to various minimally invasive and conservative methods becoming available for the management of obstructive salivary disease with preservation of the salivary glands.
The use of ESWL under ultrasonographic monitoring began in 1989, and the long-term experience since acquired in centres throughout the world show that it has become the preferred minimally invasive treatment for all parotid stones and may also be used as a primary treatment modality for intraductal and intraparenchymal sub-mandibular stones < 7 mm.
Since its introduction in 1990, considerable progress has been made in diagnostic and operative sialoendoscopy as a result of the development of improved optical systems and endoscopic units. Flexible, rigid, and semi-rigid endoscopes have been used with outer diameters ranging from 0.8 to 2.7 mm, and the latest highly flexible semi-rigid sialoendoscopes appear to be able to adjust to the anatomical landmarks of the salivary duct system. All of these sialoendoscopes have a working channel that allows the introduction of microforceps, a basket or a balloon catheter for the operative removal of single or multiple stones; however, on the basis of published results and personal experience, the major limitation of sialoendoscopy alone is the difficulty in removing stones with a diameter > 4 mm, or located in a secondary branch of the ductal system or after an acute bend in the main duct. Future progress in the field of endoscopic laser lithotripsy, such as the use of erbium laser, will probably soon bridge this therapeutic gap.
Interventional radiology with the basket retrieval of stones under fluoroscopic imaging is currently used (especially in the UK) and has a complete success rate of 71.5%. The long-term experience acquired by the main European and Middle Eastern centres has shown that up to 30% of patients undergoing ESWL (particularly those with large hilo-parenchymal stones) have not had successful results with this therapeutic approach, which has prompted clinicians to investigate new conservative and gland-preserving surgical approaches.
The recently proposed trans-oral removal of palpable hilar sub-mandibular stones by means of extended duct dissection or direct hilar incision (possibly under endoscopic control) now represents one of the main therapeutic options for sub-mandibular stones. Finally, the video-assisted surgical removal of palpable and ultrasonographically superficial stones of the parotid gland has recently been described.
All these minimally invasive procedures are carried out mainly under local anaesthesia and general anaesthesia in Day Surgery or One-Day Surgery and it is likely that further improvement in this field will definitely shift the treatment of salivary stones from an in-patient to an outpatient setting.
The multimodal approach to salivary calculi based on litho-tripsy, sialoendoscopy and gland-preserving surgical techniques (Tables I, II) leads to a high overall success rate (about 80%) in terms of stone elimination, and only 3% of patients require gland excision; this justifies combining these time-consuming and relatively expensive techniques as part of the modern and functional management of salivary calculi.
Table I. Management of parotid obstruction caused by calculi.
Table II. Management of submandibular obstruction caused by calculi.
With regard to the management of salivary duct anomalies such as strictures and kinkings (Table III), interventional radiology with balloon ductoplasty under fluoroscopic control seems to be the most adequate technique notwithstanding the use of radiation; in this regard, the use of sialoendoscopy for the rehabilitation of the ductal system is to be preferred, especially in paediatric patients.
Table III. Management of salivary ductal stenosis.
Finally, sialoendoscopy alone is to be considered the best therapeutic option for all mobile intraluminal causes of obstruction, such as microliths, mucous plugs, foreign bodies, or polyps. Moreover, sialoendoscopy is useful in the management of inflammatory conditions, such as recurrent chronic parotitis 32 or autoimmune salivary disorders (e.g. the presence of intraluminal granulation tissue in Sjögren’s syndrome), by means of ductal lavage and irrigation with steroids and antibiotics.
In the case of failure of any one of the above techniques, and regardless of the cause of obstruction, botulinum toxin injection into the parenchyma of salivary glands using colour Doppler US monitoring should also be considered before deciding on surgical removal of the affected gland.
References
- 1.Capaccio P, Minetti AM, Manzo R, Palazzo V, Ottaviani F. The role of the sialoendoscopy in the evaluation of obstructive salivary disease. Int J Maxillo Odontostomatol 2003;2:9-12. [Google Scholar]
- 2.Brown AL, Shepherd D, Buckenham TM. Per oral balloon sialoplasty: results in the treatment of salivary duct stenosis. Cardiovasc Intervent Radiol 1997;20:337-42. [DOI] [PubMed] [Google Scholar]
- 3.Escudier MP. The current status and possible future for lithotripsy of salivary calculi. In: Pregrel M, editor. Atlas of oral and maxillofacial surgery clinics of North America. Philadelphia, PA: Saunders; 1998. p. 117-32. [PubMed] [Google Scholar]
- 4.Marchal F, Kurt AM, Dulguerov P, Lehmann W. Retrograde theory in sialolithiasis formation. Arch Otolaryngol Head Neck Surg 2001;127:66-8. [DOI] [PubMed] [Google Scholar]
- 5.Marchal F, Dulguerov P, Becker M, Barki G, Disant F, Lehmann W. Specificity for parotid sialendoscopy. Laryngoscope 2001;111:264-71. [DOI] [PubMed] [Google Scholar]
- 6.Epker BN. Obstructive and inflammatory diseases of the major salivary glands. Oral Surg Oral Med Oral Pathol 1972;33:2-27. [DOI] [PubMed] [Google Scholar]
- 7.Rauch S, Gorlin RJ. Diseases of the salivary glands. In: Gorlin RJ, Goldman HM, editors. Thoma’s Oral Pathology (6th edn), vol 2. St Louis: Mosby; 1970. p. 997-1003. [Google Scholar]
- 8.Escudier MP, McGurk M. Symptomatic sialadenitis and sialolithiasis in the English population, an estimate of the cost of hospital treatment. Br Dent J 1999;186:463-6. [DOI] [PubMed] [Google Scholar]
- 9.McGurk M, Escudier MP, Brown JE. Modern management of salivary calculi. Br J Surg 2005;92:107-12. [DOI] [PubMed] [Google Scholar]
- 10.Haubrich J. Klinik der nicht Tumor bedingten Erkrankungen der Speicheldrusen. Arch Otorhinolaryngol 1976;213:1-59. [DOI] [PubMed] [Google Scholar]
- 11.Lustmann T, Regev E, Melamed Y. Sialolithiasis; a survey of 245 patients and review of the literature. Int J Oromaxillofac Surg 1990;19:135-8. [DOI] [PubMed] [Google Scholar]
- 12.Nahlieli O, Eliav E, Hasson O, Zagury A, Baruchin A. Pediatric sialolithiasis. Oral Surg Oral Med Oral Pathol 2000;90:709-12. [DOI] [PubMed] [Google Scholar]
- 13.Bodner L. Salivary gland calculi: Diagnostic imaging and surgical management. Compendium 1993;14:572-86. [PubMed] [Google Scholar]
- 14.Stimetic B, Nikolic S, Rakocevic Z, Bulajic M. Symmetry of the submandibular glands in humans – a postmortem study assessing the linear morphometric parameters. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:391-4. [DOI] [PubMed] [Google Scholar]
- 15.Ledesma-Montes C, Garces-Ortiz M, Salcido-Garcia JF, Hernandez-Flores F, Hernandez-Guerrero JC. Giant sialolith: case report and review of the literature. J Oral Maxillofac Surg 2007;65:128-30. [DOI] [PubMed] [Google Scholar]
- 16.Paul D, Chauhan MS. Salivary megalith with a sialo-cutaneous and sialo-oral fistula: a case report. J Laryngol Otol 1995;109:767-9. [DOI] [PubMed] [Google Scholar]
- 17.Bodner L. Giant salivary gland calculi: diagnostic imaging and surgical management. Oral Surg Oral Med Oral Pathol Oral Radio Endod 2002;94:320-3. [PubMed] [Google Scholar]
- 18.Raksin SZ, Gould SM, William AC. Submandibular gland sialolith of unusual size and shape. J Oral Surg 1975;33:142-5. [PubMed] [Google Scholar]
- 19.Cavina C, Santoli A. Su alcuni casi di calcolosi salivare di particolare interesse. Minerva Stomatol 1965;14:90-5. [PubMed] [Google Scholar]
- 20.Capaccio P, Bottero A, Pompilio M, Ottaviani F. Conservat-ive transoral removal of hilar submandibular salivary calculi. Laryngoscope 2005;115:750-2. [DOI] [PubMed] [Google Scholar]
- 21.Epivatianos AJ, Harrison D, Dimitriou T. Ultrastructural and histochemical observations on microcalculi in chronic submandibular sialadenitis. J Oral Pathol 1987;16:514-7. [DOI] [PubMed] [Google Scholar]
- 22.Harrison JD, Triantafyllou A, Garrett JR. Ultrastructural localization of microliths in salivary glands of cat. J Oral Pathol Med 1993;22:358-62. [DOI] [PubMed] [Google Scholar]
- 23.Marchal F, Becker M, Dulguerov PO, Lehmann W. Interventional sialendoscopy. Laryngoscope 2000;110:318-20. [DOI] [PubMed] [Google Scholar]
- 24.Teymoortash A, Wollstein AC, Lippert BM, Peldszus R, Werner JA. Bacteria and pathogenesis of human salivary calculus. Acta Oto-Laryngol 2004;122:210-4. [DOI] [PubMed] [Google Scholar]
- 25.Yuasa K, Nakhyama E, Ban S, Kawazu T, Chikui T, Shimizu M, et al. Submandibular gland duct endoscopy. Diagnostic value for salivary duct disorders in comparison to conventional radiography, sialography and ultrasonography. Oral Surg Oral Med Oral Pathol 1997;84:578-81. [DOI] [PubMed] [Google Scholar]
- 26.Schratter M, Steiner E, Imhof H. Konventionelle röntgendiagnostik der Speicheldrusen: immer noch klinischer Stellenwert oder “Traditionspflege”? Radiologe 1994;34:248-55. [PubMed] [Google Scholar]
- 27.Koch M, Zenk J, Bozzato A, Bumm K, Iro H. Sialoscopy in case of unclear swelling of the major salivary glands. Otolaryngol Head Neck Surg 2005;133:863-8. [DOI] [PubMed] [Google Scholar]
- 28.Ariji Y, Yuasa H, Ariji E, Japan N. High-frequency color Doppler sonography of the submandibular gland. Relationship between salivary secretion and blood flow. Oral Maxillofac Radiol 1998;86:476-81. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura Y, Inoue Y, Odagawa T. Sonographic examination of sialolithiasis. J Oral Maxillofac Surg 1989;47:907-12. [DOI] [PubMed] [Google Scholar]
- 30.Fodra C, Kaarmann, Iro H. Sonographie und Röntgennativaufnahme in der Speichelsteindiagnostik – experimentelle Untersuchungen. HNO 1992;40:25-8. [PubMed] [Google Scholar]
- 31.Zenk J, Hosemann WG, Iro H. Diameters of the main excretory ducts of the adult human submandibular and parotid gland. A histologic study. Oral Surg Oral Med Oral Pathol 1998;85:576-80. [DOI] [PubMed] [Google Scholar]
- 32.Nahlieli O, Bar T, Shacham R, Eliav E, Hecht-Nakar L. Management of chronic recurrent parotitis: current therapy. J Oral Maxillofac Surg 2004;62:1150-5. [DOI] [PubMed] [Google Scholar]
- 33.Ngu RK, Brown JE, Whaites EJ, Drage N, Ng S, Makdissi J. Salivary duct strictures – nature and incidence in benign salivary obstruction. Dento Maxillofac Radiol 2007;36:63-7. [DOI] [PubMed] [Google Scholar]
- 34.Rose SS. A clinical and radiological survey of 192 cases of recurrent swellings of the salivary glands. Ann R Coll Surg Engl 1954;15:370-401. [PMC free article] [PubMed] [Google Scholar]
- 35.Patey DH. Inflammation of the salivary glands with particular reference to chronic and recurrent parotitis. Ann R Coll Surg Engl 1965;36:26-44. [PMC free article] [PubMed] [Google Scholar]
- 36.Mason DK, Chisholm DM. Salivary glands in health and disease. London: Saunders; 1970. [Google Scholar]
- 37.Nahlieli O, Hecht-Nakar L, Nazarian Y, Turner MD. Sialoendoscopy. J Am Dent Assoc 2006;137:1394-400. [DOI] [PubMed] [Google Scholar]
- 38.Nahlieli O. Endoscopic techniques for diagnosis and treatment of salivary gland diseases. Tüttlingen: Endo-Press; 2005. [Google Scholar]
- 39.Nahlieli O, Baruchin A. Endoscopic technique for the diagnosis and treatment of obstructive salivary gland diseases. J Oral Maxillofac Surg 1999;57:1394-401. [DOI] [PubMed] [Google Scholar]
- 40.Miglets AW. The salivary glands. In: Cummings CW, editor. Otolaryngology: Head and Neck Surgery (1st ed, vol. 2). Toronto: CV Mosby; 1986. p. 1005. [Google Scholar]
- 41.Nahlieli O, Shacham R, Yoffe B, Eliav E. Diagnosis and treatment of strictures and kinks in salivary gland ducts. J Oral Maxillofac Surg 2001;59:484-90. [DOI] [PubMed] [Google Scholar]
- 42.Geisthoff UW, Lehnert BKW, Verse T. Ultrasound guided mechanical intraductal stone fragmentation and removal for sialolithiasis. Surg Endosc 2006;20:690-4. [DOI] [PubMed] [Google Scholar]
- 43.Marchal F, Dulguerov P. Sialolithiasis management: the state of art. Arch Otolaryngol Head Neck Surg 2003;129:951-6. [DOI] [PubMed] [Google Scholar]
- 44.Cockrell DG, Rout P. Adverse reactions following sialography. Dentomaxillofac Radiol 1993;22:41-2. [DOI] [PubMed] [Google Scholar]
- 45.Lomas DJ, Carroll NR, Johnson G, Antoun NM, Freer CEL. MR sialography: work in progress. Radiology 1996;200:129-33. [DOI] [PubMed] [Google Scholar]
- 46.Becker M, Marchal F, Becker CD, Dugerov P, Georgakopoulos G, Lehmann W, et al. Sialolithiasis and salivary ductal stenosis: diagnostic accuracy of MR sialography with a three-dimensional extended-phase conjugate-symmetry rapid spin-echo sequence. Radiology 2000;217:347-58. [DOI] [PubMed] [Google Scholar]
- 47.Morimoto Y, Ono K, Tanaka T, Kito S, Inoue H, Shinohara Y, et al. The functional evaluation of salivary glands using dynamic MR sialography following citric acid stimulation: A preliminary study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;100:357-64. [DOI] [PubMed] [Google Scholar]
- 48.Schreyer AG, Rath HC, Kikinis R, Völk M, Schölmerich J, Feuerbach S, et al. Comparison of magnetic resonance imaging colonography with conventional colonoscopy for the assessment of intestinal inflammation in patients with inflammatory bowel disease: a feasibility study. Gut 2005;54:250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neri E, Boraschi P, Caramella D, Battolla L, Gigoni R, Armillotta N, et al. Virtual endoscopy of the upper urinary tract. Am J Roentgenol 2000;175:1697-702. [DOI] [PubMed] [Google Scholar]
- 50.Cirillo S, Bonamini R, Gaita F, Tosetti I, De Giuseppe M, Longo M, et al. Magnetic resonance angiography virtual endoscopy in the assessment of pulmonary veins before radiofrequency ablation procedures for atrial fibrillation. Eur Radiol 2004;14:2053-60. [DOI] [PubMed] [Google Scholar]
- 51.Simone M, Mutter M, Rubino F, Dutson E, Roy C, Soler L, et al. Three dimensional virtual cholangioscopy: a reliable tool for the diagnosis of common bile duct stones. Ann Surg 2004;240:82-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su YX, Liao GQ, Kang Z, Zou Y. Application of magnetic resonance virtual endoscopy as a presurgical procedure before sialoendoscopy. Laryngoscope 2006;116:1899-906. [DOI] [PubMed] [Google Scholar]
- 53.Nahlieli O, Nazarian Y. Sialadenitis following radioiodine therapy – a new diagnostic and treatment modality. Oral Dis 2006;12:476-81. [DOI] [PubMed] [Google Scholar]
- 54.Barsony T. Kasuistische Mitteilung. Idiopathischestenongang-dilatation. Klin Wochenschr 1925;4:2500-1. [Google Scholar]
- 55.Hald J, Andreassen UK. Submandibular gland excision: short- and long-term complications. ORL J Otorhinolaryngol Relat Spec 1994;56:87-91. [DOI] [PubMed] [Google Scholar]
- 56.Dulguerov P, Marchal F, Lehmann W. Postparotidectomy facial nerve palsy: possible etiologic factors and results with routine facial nerve monitoring. Laryngoscope 1999;109:754-62. [DOI] [PubMed] [Google Scholar]
- 57.Beahrs OH. The facial nerve in parotid surgery. Surg Clin North Am 1963;43:973-7. [DOI] [PubMed] [Google Scholar]
- 58.Mra Z, Komisar A, Blaugrund SM. Functional facial nerve weakness after surgery for benign parotid tumours: A multivariate statistical analysis. Head Neck 1993;15:147-52. [DOI] [PubMed] [Google Scholar]
- 59.Owen ER, Banerjee AK, Kissin M, Kark AE. Complications of parotid surgery: the need for selectivity. Br J Surg 1989;76:1034-5. [DOI] [PubMed] [Google Scholar]
- 60.Bates D, O’Brien CJ, Tikaram K, Painter DM. Parotid and submandibular sialadenitis treated by salivary gland excision. Aust N Z J Surg 1998;68:120-4. [DOI] [PubMed] [Google Scholar]
- 61.Laskawi R, Ellies M, Rodel R, Schoenebeck C. Gustatory sweating: clinical implications and etiologic aspects. J Oral Maxillofac Surg 1999;57:642-8. [DOI] [PubMed] [Google Scholar]
- 62.Moody AB, Avery CM, Taylor J, Langdon JD. A comparison of one hundred and fifty consecutive parotidectomies for tumours and inflammatory disease. Int J Oral Maxillofac Surg 1999;28:211-5. [PubMed] [Google Scholar]
- 63.Milton CM, Thomas BM, Bickerton RC. Morbidity study of submandibular gland excision. Ann R Coll Surg Engl 1986;68:148-50. [PMC free article] [PubMed] [Google Scholar]
- 64.Berini-Aytes L, Gay-Escoda C. Morbidity associated with removal of the submandibular gland. J Craniomaxillofac Surg 1992;20:216-9. [DOI] [PubMed] [Google Scholar]
- 65.Ellies M, Laskawi R, Araglebe C, Schott A. Surgical management of non-neoplastic diseases of the submandibular gland. A follow-up study. Int J Oral Maxillofac Surg 1996;25:285-9. [DOI] [PubMed] [Google Scholar]
- 66.Kenefick JS. Some aspects of salivary gland disorders. Proc R Soc Med 1975;68:283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amin MA, Bailey BMW, Patel SR. Clinical and radiological evidence to support superficial parotidectomy as the treatment of choice for chronic parotid sialadenitis: a retrospective study. Br J Oral Maxillofac Surg 2001;39:348-52. [DOI] [PubMed] [Google Scholar]
- 68.Labrunie G, Lair J, Touzet C. Whartonstomie de derivation. Rev Stomat Chir Maxillofac 1981;82:70-5. [PubMed] [Google Scholar]
- 69.Mandel L, Kaynar A. Surgical bypass of submandibular duct stricture. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:532-3. [DOI] [PubMed] [Google Scholar]
- 70.Rontal M, Rontal E. The use of sialodochoplasty in the treatment of benign inflammatory obstructive submandibular gland disease. Laryngoscope 1987;97:1417-21. [DOI] [PubMed] [Google Scholar]
- 71.Makdissi J, Escudier MP, Brown JE, Osailan S, Drage N, McGurk M. Glandular function after intra-oral removal of salivary calculi from the hilum of the submandibular gland. Br J Oral Maxillofac Surg 2004;42:538-41. [DOI] [PubMed] [Google Scholar]
- 72.Yoshimura Y, Morishita T, Sugihara T. Salivary gland function after sialolithiasis: scintigraphic examination of submandibular glands with 99m Tc-pertechnetate. J Oral Maxillofac Surg 1989;47:704-10. [DOI] [PubMed] [Google Scholar]
- 73.Marchal F, Kurt AM, Dulguerov P, Becker M, Oedman M, Lehmann W. Histopathology of submandibular glands removed for sialolithiasis. Ann Otol Rhinol Laryngol 2001;110:464-9. [DOI] [PubMed] [Google Scholar]
- 74.Iro H, Nitsche N, Schneider TH, Ell C. Extracorporeal shockwave lithotripsy of salivary gland stones. Lancet 1989;2:115. [DOI] [PubMed] [Google Scholar]
- 75.Ashby RA, deBurgh Norman JE, Iro H, McGurk M. Salivary calculi and obstructive sialadenitis. In: deBurgh Norman JE, McGurk M, editors. Color atlas and text of the salivary glands diseases, disorders and surgery. Barcelona: Mosby-Wolfe; 1995. p. 243-66. [Google Scholar]
- 76.Iro H, Schneider T, Nitsche N, Waitz G, Ell C. Extracorporeal piezoelectric lithotripsy of salivary calculi: initial clinical experiences. HNO 1990;38:251-5. [PubMed] [Google Scholar]
- 77.Wehrmann T, Kater W, Marlinghaus EH, Peters J, Caspary WF. Shock wave treatment of salivary duct stones: substantial progress with a minilithotripter. Clin Invest 1994;72:604-8. [DOI] [PubMed] [Google Scholar]
- 78.Ottaviani F, Capaccio P, Campi M, Ottaviani A. Extracorporeal electromagnetic shock-wave lithotripsy for salivary gland stones. Laryngoscope 1996;106:761-4. [DOI] [PubMed] [Google Scholar]
- 79.Escudier MP, Brown JE, Drage NA, McGurk M. Extracorporeal shockwave lithotripsy in the management of salivary calculi. Br J Surg 2003;90:482-5. [DOI] [PubMed] [Google Scholar]
- 80.Konigsberger R, Freyh J, Goetz A, Kastenbauer E. Endoscopically-controlled electrohydraulic intracorporeal shock wave lithotripsy (EISL) of salivary stones. J Otolaryngol 1993;22:12-3. [PubMed] [Google Scholar]
- 81.Iro H, Benzel W, Gode U, Zenk J. Pneumatic intracorporeal lithotripsy of salivary stones: an in vitro and in vivo animal investigation. HNO 1995;43:172-6. [PubMed] [Google Scholar]
- 82.Arzoz E, Santiago A, Esnal F, Palomero R. Endoscopic intracorporeal lithotripsy for sialolithiasis. J Oral Maxillofac Surg 1996;54:847-50. [DOI] [PubMed] [Google Scholar]
- 83.McGurk M, Prince MJ, Jang ZX, King TA. Laser lithotripsy: a preliminary study on its application for sialolithiasis. Br J Oral Maxillofac Surg 1994;32:218-21. [DOI] [PubMed] [Google Scholar]
- 84.Ito H, Baba S. Pulsed dye laser lithotripsy of submandibular gland salivary calculus. J Laryngol Otol 1996;110:218-21. [DOI] [PubMed] [Google Scholar]
- 85.Ottaviani F, Capaccio P, Rivolta R, Cosmacini P, Pignataro L, Castagnone D. Salivary gland stones: US evaluation in shock wave lithotripsy. Radiology 1997;204:437-41. [DOI] [PubMed] [Google Scholar]
- 86.Eggers G, Chilla R. Ultrasound guided lithotripsy of salivary calculi using an electromagnetic lithotriptor. J Oral Maxillofac Surg 2005;34:890-4. [DOI] [PubMed] [Google Scholar]
- 87.Capaccio P, Ottaviani F, Manzo R, Schindler A, Cesana B. Extracorporeal lithotripsy for salivary calculi: a long-term clinical experience. Laryngoscope 2004;114:1069-73. [DOI] [PubMed] [Google Scholar]
- 88.Zenk J, Constantinidis J, Al-Kadah B, Iro H. Transoral removal of submandibular stone. Arch Otolaryngol Head Neck Surg 2001;127:432-6. [DOI] [PubMed] [Google Scholar]
- 89.Iro H, Schneider T, Fodra C, Waitz G, Nitsche N, Heinritz HH, et al. Shockwave lithotripsy of salivary duct stones. Lancet 1992;339:1333-6. [DOI] [PubMed] [Google Scholar]
- 90.Iro H, Waitz G, Nitsche N, Benninger J, Schneider T, Ell C. Extracorporeal piezoelectric shock-wave lithotripsy of salivary gland stones. Laryngoscope 1992;102:492-4. [DOI] [PubMed] [Google Scholar]
- 91.Iro H, Benzel W, Zenk J, Fodra C, Heinritz HH. Minimally invasive treatment of sialolithiasis using extracorporeal shock waves. HNO 1993;41:311-6. [PubMed] [Google Scholar]
- 92.Iro H, Zenk J, Waldfahrer F, Benzel W, Schneider T, Ell C. Extracorporeal shock-wave lithotripsy of parotid stones: Results of a prospective trial. Ann Otol Rhinol Laryngol 1998;107:860-4. [DOI] [PubMed] [Google Scholar]
- 93.Brown JE. Minimally invasive techniques for the treatment of benign salivary gland obstruction. Cardiovasc Intervent Radiol 2002:25;345-51. [DOI] [PubMed] [Google Scholar]
- 94.Gundlach P, Scherer H, Hopf J, Leege N, Muller G, Hilst L, et al. Endoscopic-controlled laser lithotripsy of salivary calculi: In vitro studies and initial clinical use. HNO 1990;38:247-50. [PubMed] [Google Scholar]
- 95.Larizgoitia I, Pons JM. A systematic review of the clinical efficacy and effectiveness of the holmium: YAG laser in urology. BJU Int 1999;84:1-9. [DOI] [PubMed] [Google Scholar]
- 96.Wollin TA, Denstedt JD. The holmium laser in urology. J Clin Laser Med Surg 1998;16:13-20. [DOI] [PubMed] [Google Scholar]
- 97.Gundlach P, Hopf J, Linnaz M, Leege N, Tschepe J. Einsatz kurzgepulster Lasersysteme zur Speichelstein-lithotripsie. Lasermedizin 1994;10:75-81. [Google Scholar]
- 98.Raif J, Vardi M, Nahlieli O, Gannot I. An Er: YAG laser endoscopic fiber delivery system for lithotripsy of salivary stones. Lasers Surg Med 2006;38:580-7. [DOI] [PubMed] [Google Scholar]
- 99.Iro H, Zenk J, Hosemann WG, Benzel W. Electrohydraulic intracorporeal lithotripsy of salivary calculi. In vitro and in animal experimented studies. HNO 1993;41:389-95. [PubMed] [Google Scholar]
- 100.Iro H, Benzel W, Gode U, Zenk J. Pneumatic intracorporeal lithotripsy of salivary calculi. In vitro and in animal experimented studies. HNO 1995;43:172-6. [PubMed] [Google Scholar]
- 101.Katz P. Endoscopy of the salivary glands. Ann Radiol (Paris) 1991;34:110-3. [PubMed] [Google Scholar]
- 102.Nahlieli O, Neder A, Baruchin AM. Salivary gland endoscopy: a new technique for diagnosis and treatment of sialolithiasis. J Oral Maxillofac Surg 1994;52:1240-2. [DOI] [PubMed] [Google Scholar]
- 103.Nahlieli O, Baruchin AM. Sialoendoscopy: three years’ experience as a diagnostic and treatment modality. J Oral Maxillofac Surg 1997;55:912-8. [DOI] [PubMed] [Google Scholar]
- 104.Nahlieli O, Baruchin AM. Long-term experience with endoscopic diagnosis and treatment of salivary gland inflammatory diseases. Laryngoscope 2000;110:988-93. [DOI] [PubMed] [Google Scholar]
- 105.Zenk J, Koch M, Bozzato A, Iro H. Sialoscopy: initial experience with a new endoscope. Br J Oral Maxillofac Surg 2004;42:293-8. [DOI] [PubMed] [Google Scholar]
- 106.Chossegros C, Guyton L, Richard O, Barki G, Marchal F. A technical improvement in sialoendoscopy to enter the salivary ducts. Laryngoscope 2006;116:842-4. [DOI] [PubMed] [Google Scholar]
- 107.Nahlieli O, Schacham R, Bar T, Eliav E. Endoscopic mechanical retrieval of sialoliths. Oral Surg Oral Med Oral Pathol Radiol Endod 2003;95:396-402. [DOI] [PubMed] [Google Scholar]
- 108.Ziegler CM, Steveling H, Seubert M, Muhling J. Endoscopy: a minimally invasive procedure for diagnosis and treatment of diseases of the salivary glands. Six years of practical experience. Br J Oral Maxillofac Surg 2004;42:1-7. [DOI] [PubMed] [Google Scholar]
- 109.Kelly IMG, Dick R. Technical report. Interventional sialography: Dormia basket removal of a Wharton’s duct calculus. Clin Radiol 1991;43:205-6. [DOI] [PubMed] [Google Scholar]
- 110.Drage N, Brown JE, Escudier M, McGurk M. Interventional radiology in the removal of salivary calculi. Radiology 2000;214:139-42. [DOI] [PubMed] [Google Scholar]
- 111.Buckenham TM, George CD, McVicar D, Moody AR, Colles GS. Digital sialography: imaging and intervention. Br J Radiol 1994;67:524-9. [DOI] [PubMed] [Google Scholar]
- 112.Kim RH, Strimling AM, Grosch T, Feider DE, Varanth JJ. Nonoperative removal of sialoliths and sialodochoplasty of salivary duct strictures. Arch Otolaryngol Head Neck Surg 1996;122:974-6. [DOI] [PubMed] [Google Scholar]
- 113.Yoshino N, Hosokawa A, Sasaki T, Yoshioka T. Interventional radiology for the nonsurgical removal of sialoliths. Dentomaxillofac Radiol 1996;25:242-6. [DOI] [PubMed] [Google Scholar]
- 114.Nixon P, Payne M. Conservative surgical removal of a submandibular duct calculus following interventional sialography. Clin Radiol 1999;54:337-8. [DOI] [PubMed] [Google Scholar]
- 115.North E. Submandibular sialoplasty for stone removal and treatment of a stricture. Br J Oral Maxillofac Surg 1998;36:213-4. [DOI] [PubMed] [Google Scholar]
- 116.Buckenham TM, Page JE, Jeddy T. Technical report: Interventional sialography – Balloon dilatation of a Stensen’s duct stricture using digital subtraction sialography. Clin Radiol 1992;45:34. [DOI] [PubMed] [Google Scholar]
- 117.Davies RP, Whyte AM, Lui CL. Interventional sialography: a single-center experience. Cardiovasc Intervent Radiol 1997;20:331-6. [DOI] [PubMed] [Google Scholar]
- 118.Brown JE. Interventional sialography and minimally invasive techniques in benign salivary gland obstruction. Semin Ultrasound CT MRI 2006;27:465-75. [DOI] [PubMed] [Google Scholar]
- 119.Seward GR. Anatomic surgery for salivary calculi. 3. Calculi in the posterior part of the submandibular duct. Oral Surg Oral Med Oral Pathol 1968;25:525-31. [DOI] [PubMed] [Google Scholar]
- 120.Seward GR. Anatomic surgery for salivary calculi. II. Calculi in the anterior part of the submandibular duct. Oral Surg Oral Med Oral Pathol 1968;25:287-93. [DOI] [PubMed] [Google Scholar]
- 121.McGurk M. Surgical release of a stone from the hilum of the submandibular gland: a technique note. Int J Oral Maxillofac Surg 2005;34:208-10. [DOI] [PubMed] [Google Scholar]
- 122.McGurk M, Makdissi J, Brown JE. Intra-oral removal of stones from the hilum of the submandibular gland: report of technique and morbidity. Int J Oral Maxillofac Surg 2004;33:683-6. [DOI] [PubMed] [Google Scholar]
- 123.Seldin HM, Seldin D, Rakower W. Conservative surgery for the removal of salivary calculi. Oral Surg Oral Med Oral Pathol 1953;6:579-87. [DOI] [PubMed] [Google Scholar]
- 124.Baurmarsh H, Dechiara SC. Extracorporeal parotid sialolithotomy. J Oral Maxillofac Surg 1991;46:127-32. [DOI] [PubMed] [Google Scholar]
- 125.Nahlieli O, London D, Zagury A, Eliav E. Combined approach to impacted parotid stones. J Oral Maxillofac Surg 2002;60:1418-23. [DOI] [PubMed] [Google Scholar]
- 126.McGurk M, MacBean AD, Fan KFM, Sproat C, Darwish C. Endoscopically assisted operative retrieval of parotid stones. Br J Oral Maxillofac Surg 2006;44:157-60. [DOI] [PubMed] [Google Scholar]
- 127.McGurk M. Endoscopically assisted surgical removal of parotid stones. In: Salivary gland disease. 2nd International Course. Gargnano, 2006. Proceedings Book p. 72. [Google Scholar]
- 128.Marchal F. A combined endoscopic and external approach for extraction of large stones with preservation of parotid and submandibular glands. Laryngoscope 2007;117:373-7. [DOI] [PubMed] [Google Scholar]
- 129.Ellies M, Laskawi R, Rohrbach-Volland S, Arglebe C, Beuche W. Botulinum toxin to reduce salivary flow: selected indications for ultrasound-guided toxin application into salivary glands. Laryngoscope 2002;112:82-6. [DOI] [PubMed] [Google Scholar]
- 130.Ellies M, Laskawi R, Rohrbach-Volland S, Arglebe C. Up-to-date report of botulinum toxin therapy in patients with drooling caused by different etiologies. J Oral Maxillofac Surg 2003;61:454-7. [DOI] [PubMed] [Google Scholar]
- 131.Ellies M, Gottstein U, Rohrbach-Volland S, Arglebe C, Laskawi R. Reduction of salivary flow with botulinum toxin: extended report on 33 patients with drooling, salivary fistulas, and sialadenitis. Laryngoscope 2004;114:1856-60. [DOI] [PubMed] [Google Scholar]
- 132.Marchese Ragona R, De Filippis C, Staffieri A, Tugnoli V, Restivo DA. Parotid gland fistula: treatment with botulinum toxin. Plast Reconstr Surg 2001;107:886-7. [DOI] [PubMed] [Google Scholar]
- 133.Ellies M, Laskawi R, Rohrbach-Volland S, Rodel R, Beuche W. Blocking of secretion in exocrine glands of the head and neck area with botulinum toxin A: therapeutic option in the treatment of rare diseases. HNO 2001;49:807-13. [DOI] [PubMed] [Google Scholar]
- 134.Marchese Ragona R, Marioni G, Restivo AD, Staffieri A. The role of botulinum toxin in postparotidectomy fistula treatment. A technical note. Am J Otolaryngol 2006;27:221-4. [DOI] [PubMed] [Google Scholar]
- 135.Whelchel DD, Brehmer TM, Brooks PM, Darragh N, Coffield JA. Molecular targets of botulinum toxin in the mammalian neuromuscular junction. Mov Disord 2004;19:S7-S16. [DOI] [PubMed] [Google Scholar]
- 136.Marchese Ragona R, Blotta P, Pastore A, Tugnoli V, Eleopra R, De Grandis D. Management of parotid sialocele with botulinum toxin. Laryngoscope 1999;109:1344-6. [DOI] [PubMed] [Google Scholar]
- 137.Capaccio P, Paglia M, Minorati D, Manzo R, Ottaviani F. Diagnosis and therapeutic management of iatrogenic parotid sialocele. Ann Otol Rhinol Laryngol 2004;113:562-4. [DOI] [PubMed] [Google Scholar]
- 138.Capaccio P, Cuccarini V, Benicchio V, Minorati D, Spadari F, Ottaviani F. Treatment of iatrogenic submandibular sialocele with botulinum toxin. Br J Oral Maxillofac Surg 2006:1-3. [DOI] [PubMed] [Google Scholar]





