Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) 38 is a multifunctional anti-inflammatory and anti-apoptotic neuropeptide widely distributed in the nervous system. The objective of this study is to determine whether PACAP38 is neuroprotective against sodium nitroprusside (SNP) and thrombin, two mechanistically distinct neurotoxic agents. Treatment of primary cortical neuronal cultures with 1 mM SNP for 4 h causes neuronal cell death that is significantly reduced by 100 nM PACAP38. PACAP38 down-regulates SNP-induced cell cycle protein (cyclin E) expression and up-regulates p57KIP2, a cyclin-dependent kinase inhibitor as well as the anti-apoptotic protein Bcl-2. Similarly, neuronal death induced by 100 nM thrombin or the thrombin receptor activating peptide (TRAP 6) is reduced by PACAP38 treatment. Thrombin-stimulated cell cycle protein (cdk4) expression is decreased by PACAP38 while PACAP38 inhibits thrombin-mediated reduction of p57KIP2. However, the decrease in Bcl-2 evoked by thrombin is not affected by PACAP38. Finally, both SNP and thrombin (or TRAP) increase caspase 3 activity, an effect that is decreased by PACAP38. These data show that PACAP38 supports neuronal survival in vitro suppressing cell cycle progression and enhancing anti-apoptotic proteins. Our results support the possibility that PACAP could be a useful therapeutic agent for reducing neuronal cell death in neurodegenerative diseases.
Keywords: PACAP, sodium nitroprusside, thrombin, apoptosis, neurons, cell cycle
Introduction
Pituitary adenylate cyclase-activating peptide (PACAP) is a multifunctional neuropeptide widely distributed in the nervous system [1, 2]. PACAP is a member of the vasoactive intestinal peptide family and functions as a neurotransmitter, neuromodulator, and neurotrophic factor (reviewed in [3, 4]). It is synthesized as a 176-amino acid precursor and cleavage of the precursor protein yields to the formation of the 38 amino acid PACAP38, a shortened PACAP27 and a 29 amino acid PACAP related protein (PRP), with PACAP38 as the predominant form in the brain [1, 5–7]. PACAP exerts its biological action via three different receptors belonging to the family 2 of G-protein-coupled receptors: PAC1, VPAC1 and VPAC2 [8, 9]. PACAP and its receptors are expressed in various regions of the CNS [10, 11]. Mice lacking PACAP38 show altered cerebellar neurodevelopment and increased caspase 3 activation [12]. In animal models of cerebral ischemia and Parkinson’s disease PACAP38 has been shown to be neuroprotective [13, 14]. In human neuroblastoma cells PACAP38 stimulates the nonamyloidogenic pathway for processing amyloid precurser protein [15]. Taken together, these data suggest that PACAP has neurotrophic and neuroprotective functions in the brain. In vitro studies involving exposure of neuronal cultures to various neurotoxins including amyloid beta, hydrogen peroxide and glutamate reveal the strong anti-apoptotic effects of PACAP38 [16–18]. Finally, synergy between direct actions of PACAP38 and PACAP-stimulated secretion of interleukin-6 (IL-6) in the hippocampus demonstrates the diversity of mechanisms that contribute to PACAP-mediated neuroprotection [19].
The multi-step process of neuronal cell death is complex and highly regulated. We have previously shown that mechanisms involved in neuronal cell death utilize multiple pathways that are influenced by subtle differences among neuronal cell phenotypes and vary depending on the nature of the neurotoxic insult [20]. For example, in differentiated PC12 cells tumor necrosis factor-α evokes release of LDH with no associated morphologic changes, whereas in response to the nitric oxide (NO) generator, sodium nitroprusside (SNP), cultured cortical cells exhibit LDH release with pronounced morphologic changes. Nitric oxide is a known mediator of inflammatory processes which play an important role in the pathogenesis of several neurodegenerative diseases [21, 22]. High NO levels generated in response to cytokines or the excitatory neurotransmitter glutamate can result in neuronal cell death; as neurons are particularly sensitive to NO toxicity [23–25]. Several mechanisms have been proposed for NO-induced cell death including oxidative stress and mitochondrial alterations [26, 27]. In mixed cortical neuron/glia cultures lipopolysaccharide-induced secretion of NO was diminished by PACAP, implying a role for PACAP38 in the regulation of NO production [28]. A direct interaction between the effects of PACAP on NO-mediated neurotoxicity has not been described.
The inappropriate expression or activation of cell cycle related proteins is associated with neuronal cell death in human neurodegenerative diseases [29, 30]. Experimentally driving the cell cycle in an adult neuron leads to cell death rather than cell division and blocking cell cycle initiation can prevent neuronal cell death evoked by cerebral ischemia or traumatic brain injury [31, 32]. Cell cycle progression is regulated by specific proteins, the cyclins, cyclin-dependent kinases (cdks) and cdk inhibitors. The balance in the expression of these proteins determines cell cycle progression. Cyclin E is essential for progression through the G1 phase of the cell cycle and initiation of DNA replication [33]. The Cip/Kip family of cdk inhibitors shows a broad spectrum of inhibitory effects on cyclin/cdk complexes, with p57KIP2 playing an important regulatory role by opposing the activity of Cyclin D1 and E that both promote cell cycle progression [34, 35]. We have shown that thrombin, a multifunctional serine protease with demonstrated neurotoxic properties, causes neurons to re-enter the cell cycle [36]. Also, in cultured neurons and astrocytes, thrombin induces apoptosis involving tyrosine kinase and RhoA activities [37]. In vivo administration of thrombin into the brain causes memory impairment, neuronal cell loss and reactive gliosis [38]. The ability of PACAP to mitigate thrombin neurotoxicity has not been explored.
The objective of this study is to determine whether PACAP38 is neuroprotective against SNP and thrombin, two mechanistically distinct neurotoxic agents, focusing on the role of cell cycle regulators (cyclin E and p57KIP2) and the anti-apoptotic protein Bcl-2 in this process.
Materials and Methods
Materials
Sodium nitroprusside (SNP), 5-fluoro-2′-deoxyuridine, human thrombin and thrombin receptor activating peptide 6 (TRAP6) were obtained from Sigma-Aldrich (St. Louis, MO, USA) while PACAP38 was obtained from GeneScript (Piscataway, NJ, USA). Cell culture reagents and media were purchased from Invitrogen (Carlsbad, CA, USA) including Dulbecco’s modified Eagle’s medium (DMEM), heat inactivated horse serum (HIHS), Hank’s balanced salt solution (HBSS), Neurobasal medium, B-27 and N-2 supplements. Cell Titer96 AQueous One Cell Cell Proliferation Assay Kit for cell survival assay (Cat. No G3581) was obtained from Promega (Madison, WI, USA). QuantiZyme Assay System for Caspase-3 Cellular Activity Assay Kit Plus was purchased from BioMol International (Cat. No. AK-703, Plymouth Meeting, PA, USA). Antibodies for western analysis were as follows: Cyclin E (M-20 1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), p57Kip2 (MS-897-P0 1:100, Labvision Corp., Freemont, CA, USA), Bcl-2 (ab7973 1:100, AbCam Cambridge, MA, USA) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), (MAB374, Chemicon, Temecula, CA, USA). Secondary antibodies conjugated with peroxidase were obtained from BioRad (Hercules, CA, USA) including the polyvinylidene diflouride (PVDF) membrane and the commercial kit for determining total protein concentration based on the Bradford assay (Cat No. 500-0006).
Methods
Primary Neuronal Cultures
Rat cerebral cortical cultures were prepared as previously described [20, 39]. Cortices were isolated from 18 day gestation rat fetuses, washed 3x with Hank’s balanced salt solution (HBSS), and pipette-triturated in 10 ml Brooks Logan solution. The cells were seeded at a density of 3–5 × 105 cells per ml on 6-well poly-L-lysine coated plates. Neurobasal medium containing B-27 supplement, antibiotic/antimycotic, glutamine (0.5 mM) and 5-fluoro-2′-deoxyruridine (20 μg/ml) was applied on day 2. On day 5, fresh medium without 5-fluoro-2′-deoxyruridine was added. Neuronal cultures were used for experiments after 8–9 days in culture.
Cell Treatments
SNP solution was freshly prepared each time before use. SNP, thrombin, TRAP6 and PACAP were prepared in H2O and then added to the cells at appropriate concentrations. Treatment media consisted of Neurobasal media containing N-2 supplement, 0.5 mM glutamine, and antibiotic/antimycotic. Concentrations of reagents used and duration of treatments are indicated for specific experiments in the figure legends.
MTT Cell Survival Assay
Cell survival was measured using the Cell Titer96 AQueous One Cell Cell Proliferation Assay Kit. The MTT substrate is prepared at a 1:40 dilution in treatment media. The cells were incubated at 37°C for 10–15 min and absorbance read at 490 nm. The assay is based on conversion of the tetrazolium salt MTT to formazan, a purple dye. This cellular reduction reaction involves the pyridine nucleotide cofactors NADH/NADPH and is only catalyzed by living cells. The intensity of product color is proportional to the number of living cells in the culture. Survival of control samples (untreated cells) is standardized to 100 and the amount of survival of each experimental sample is expressed as a percent of control.
Caspase 3 Activity Assay
Neuron cultures were exposed to a lowered dose (0.5 mM) of SNP for an extended 24 h treatment to detect the change in levels of active caspase 3 and caspase 3 activity. Caspase-3 activity was measured using QuantiZyme Assay System for Caspase-3 Cellular Activity Assay Kit Plus following the manufacturer’s protocols with minor modifications. The assay is based on caspase-3 cleavage of the Ac-DEVD-pNA substrate. The substrate cleavage results in a colorimetric reaction monitored by reading absorbance at 405 nm. Total protein in the cytosolic extract was quantified and 20 to 30 μg total protein in 50 μl assay buffer was used for each 100 μl activity reaction assay. The reaction was started with the addition of 50 μl Ac-DEVD-pNA substrate. Caspase activity was monitored by reading the plate continuously at 405 nm at defined time intervals. Data were plotted as absorbance units (OD405 nm) versus time for each sample.
Western Blot Analysis
Neurons were washed with 1x PBS and lysed in buffer (20 mM Tris-HCl pH 7.4, 50 mM NaCl, 0.5 % NP-40, 0.5 % deoxycholate, 0.5 % SDS, 1mM EDTA, 1 μg/ml aprotinin). After homogenization on ice, the cell lysates were clarified by centrifugation at 15,000 × g for 10 mins at 4°C. The supernatant was used for western blot analysis. Total protein concentration was determined using a commercial kit (Bio-Rad, Cat No. 500-0006) based on the Bradford protein assay method. For detection of the various proteins, 20–25 μg total protein was loaded in each lane and separated by SDS-PAGE electrophoresis. Primary antibodies used include Cyclin E (M-20 1:1000), p57Kip2 (MS-897-P0 1:100) and Bcl-2 (ab7973 1:100). Bands were detected using chemiluminescence on X-ray film. Western blot images were scanned and quantified using Quantity One v4.6 software (BioRad, Hercules, CA). Blots were later stripped with stripping buffer at 50°C for 1 h and re-probed with GAPDH (MAB374 1:5000). Densitometric measurements of bands were normalized to corresponding GAPDH levels and control sample was set arbitrarily to 100. For p57KIP2 and cdk4, densitometric measurements were performed only for the band representing the active form of the protein. Treatment values were then expressed as percent of control.
Statistical Analysis
Prism version 4.0 software (GraphPad Inc., San Diego CA) was used for graphical presentation and statistical analysis. Statistical analysis used included student’s t-test and one-way ANOVA followed by Newman-Keul’s post hoc multiple comparison tests to compare the different treatment groups. A significant difference was defined as p < 0.05. Data are presented as means ± SEM of at least 3 independent experiments, performed in triplicate.
Results
PACAP38 protected neurons against SNP-induced cell death
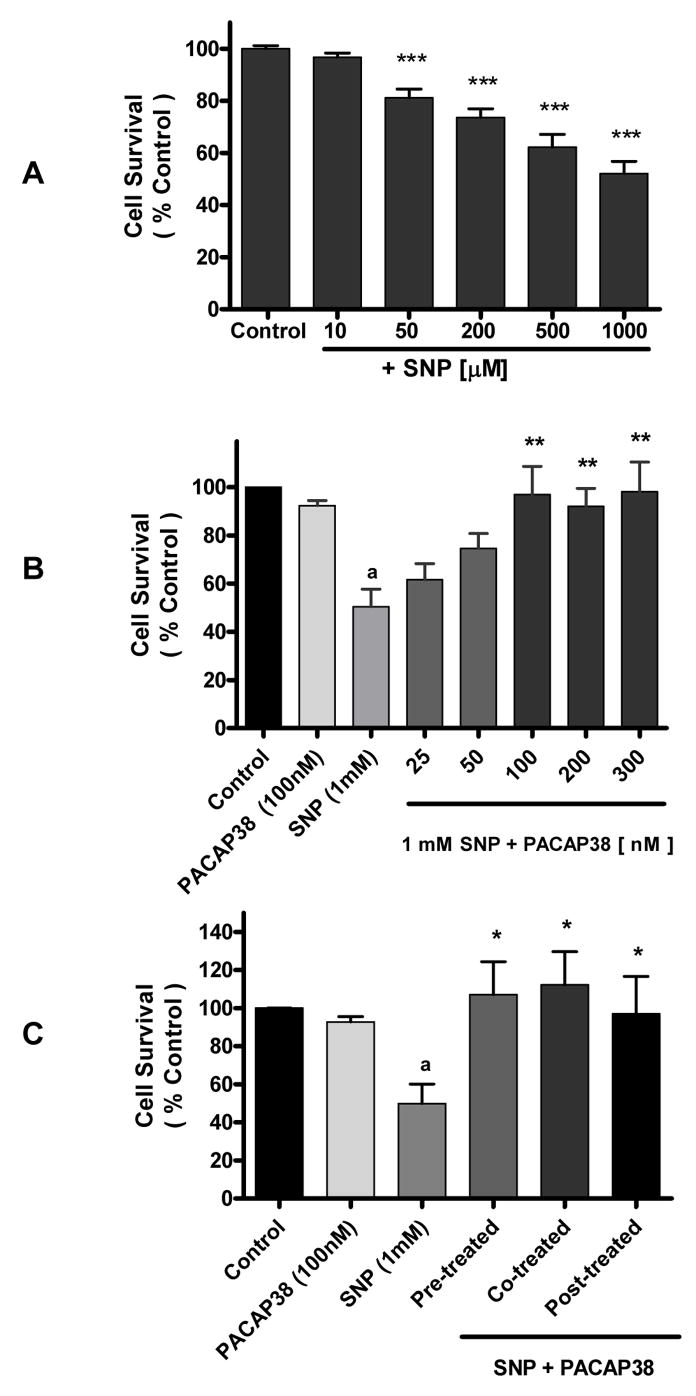
Neurons exposed to SNP over a 10 μM – 1mM range demonstrated a dose-dependent decrease in cell survival (Figure 1A). Based on the observation that at 1 mM SNP neurons showed approximately 50% cell survival, subsequent experiments were performed using that SNP concentration. Cells treated with 1 mM SNP were co-incubated with increasing concentrations (25 – 300 nM) of PACAP38 for 4 h and cell survival evaluated. The data showed that PACAP38 increased cell survival in a dose-dependent manner and that at 100 nM PACAP38 cell survival was comparable to untreated control cultures (Figure 1B). Incubation of neuronal cultures with PACAP38 alone did not affect cell survival. Exposure of neuronal cultures to PACAP38 either 1 h prior to or 1 h after SNP treatment resulted in significant (p<0.05) protection of neuronal neurons against SNP-induced toxicity (Figure 1C).
Figure 1. Pituitary adenylate cyclase activating peptide (PACAP) 38 protects cortical neurons against sodium nitroprusside (SNP) treatment.
Data represents cell survival as measured by MTT assay and expressed as percent of untreated control (100%). Data are means ± SEM of three or 4 experiments performed in triplicate. One-way Analysis of variance was performed followed by Newman-Keuls Multiple comparison test. A. SNP evokes neuronal cell death. Cortical neurons were treated with a range of SNP concentrations (10 μM – 1 mM) for 4 h. *** p <0.001 vs control. B. Cortical neurons were treated with 1 mM SNP and co-incubated with a range of PACAP38 concentrations (25 nM – 300 nM for 4 h). a p<0.01 vs control, ** p<0.01 vs 1 mM SNP. C. Cortical neurons were exposed to PACAP38 at the time of SNP (1 mM, 4 h) treatment (Co-treatment), or 1 h prior (Pre-treated), or 1 h after SNP treatment (Post-treatment).
a p<0.05 vs Control
* p<0.05 vs 1mM SNP.
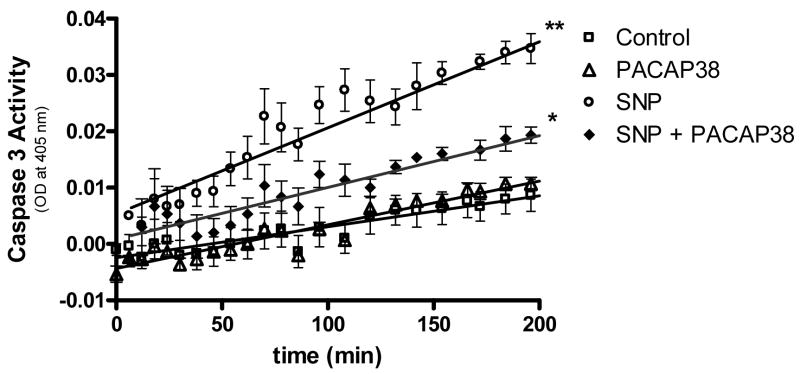
The ability of SNP and SNP plus PACAP38 to affect neuronal cell apoptosis was assessed by measuring caspase 3 activity. To detect activated caspase 3, neuronal cultures were exposed to longer SNP treatment (24 h vs 4 h) at a lower dosage. Treatment of neuronal cultures with SNP (0.5 mM) for 24 h evoked a significant (p<0.01) increase in caspase 3 activity. This increased activity was reduced significantly (p<0.05) by incubating neuronal cultures with SNP plus 100 nM PACAP38 (Figure 2).
Figure 2. PACAP38 decreased SNP-induced increase in caspase-3 activity level.
Cultured neurons were treated with medium (control), PACAP38 (100 nM), SNP (0.5 mM) or with 100 nM PACAP38 plus 0.5 mM SNP for 24 h. Neuronal cultures were rinsed, cells collected and lysates analyzed for caspase activity measured as conversion of substrate (OD at 405 nm). Data are means ± SEM of three performed in triplicate.
* p<0.05 vs SNP
** p<0.01 vs control
PACAP38 inhibited SNP-induced cyclin E upregulation and enhanced p57KIP2 expression
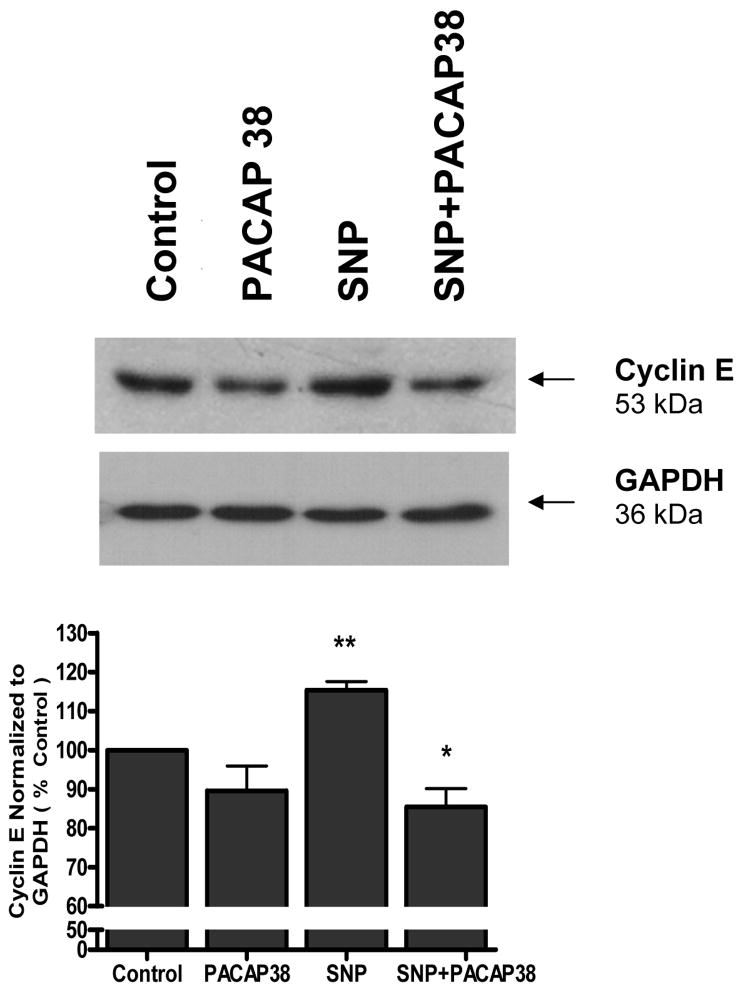
Cortical neurons were exposed to either 1 mM SNP, 100 nM PACAP38, or SNP plus PACAP38 for 4 h, the cell lysates collected and western blot analysis performed for cyclin E expression. A 53 kDa band corresponding to cyclin E was detectable in all samples (Figure 3). PACAP38 alone caused a slight but not significant decrease in cyclin E levels. Exposure of neuronal cultures to SNP evoked a significant (p<0.01) increase in cyclin E compared to untreated control cultures. Incubation of neurons with both PACAP38 and SNP resulted in a significant (p<0.05) reduction in SNP-induced cyclin E expression (Figure 3).
Figure 3. PACAP38 downregulated SNP-induced cell cycle protein (cyclin E) expression.
Cortical neurons were exposed to either medium (control), 1 mM SNP, 100 nM PACAP38, or SNP plus PACAP38 for 4 h, the cell lysates collected and western blot analysis performed for cyclin E expression. Histogram represents densitometric measurements of three western blot experiments normalized to GAPDH levels. Treatments were compared by one-tailed t-tests.
** vs control
* vs SNP
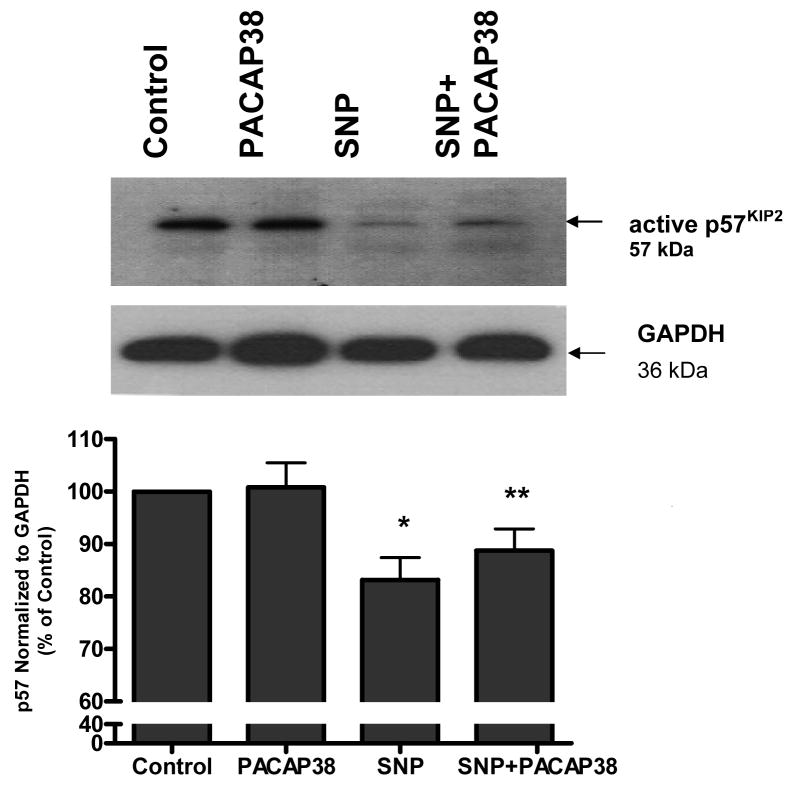
Western blot analysis of neuronal cultures showed the band for the cyclin kinase inhibitor p57KIP2. Densitometric analysis showed that SNP treatment reduced the expression of p57KIP2 (p<0.05) compared to untreated cells (Figure 4). Incubation of cultures with PACAP38 and SNP treatment caused a significant (p<0.01) increase in p57KIP2 level compared to SNP alone (Figure 4).
Figure 4. PACAP38 blocks SNP-induced reduction in p57Kip2.
Cortical neurons were exposed to either medium (control), 100 nM PACAP38, 1 mM SNP, or SNP plus 100 nM PACAP38 (SNP+PACAP38) for 4 h. Cell lysates were collected and western blot analysis performed for p57Kip2 expression. Treatments were compared by one-tailed t-tests.
* p< 0.05 vs control
** p< 0.01 vs SNP
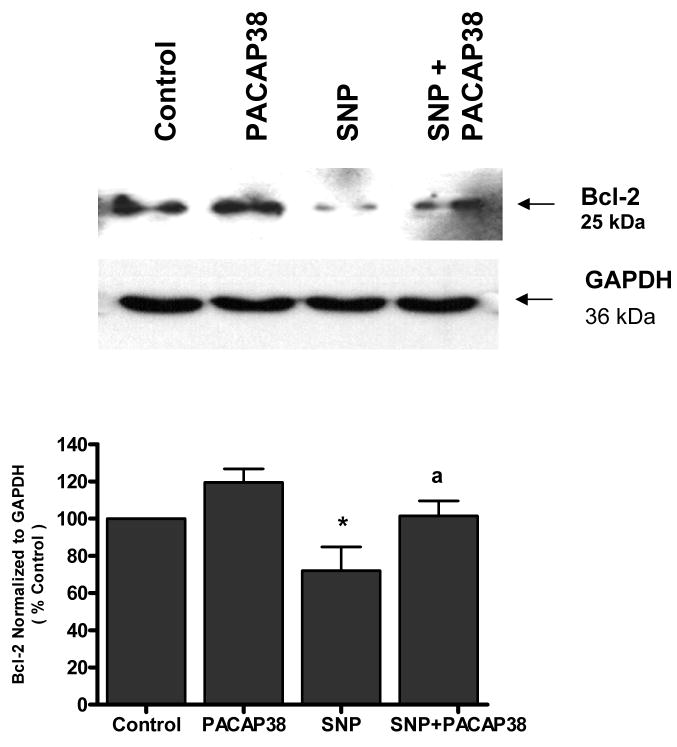
PACAP38 attenuated Bcl-2 down regulation induced by SNP
Neuronal cultures were analyzed by western blot analysis for the anti-apoptotic protein Bcl-2. Untreated cultures express a basal level of protein that was significantly (p<0.05) reduced by exposure to SNP (0.5 mM) for 24 h (Figure 5). Pre-treatment of neurons with 100 nM PACAP38 prior to SNP exposure significantly (p<0.05) blocked SNP-induced Bcl-2 down regulation.
Figure 5. PACAP38 attenuated Bcl-2 down regulation induced by SNP.
Neuronal cultures were treated with PACAP38 (100 nM), SNP (0.5 mM) for 24 h or exposed to PACAP38 for 1 h and then treated with SNP 24 h (SNP + PACAP38) and Bcl-2 expression analyzed by western blot analysis. Histogram represents densitometric measurements of three western blot experiments normalized to GAPDH levels. Treatments were compared by one-tailed t-tests.
* p< 0.05 vs control
a p< 0.05 vs SNP
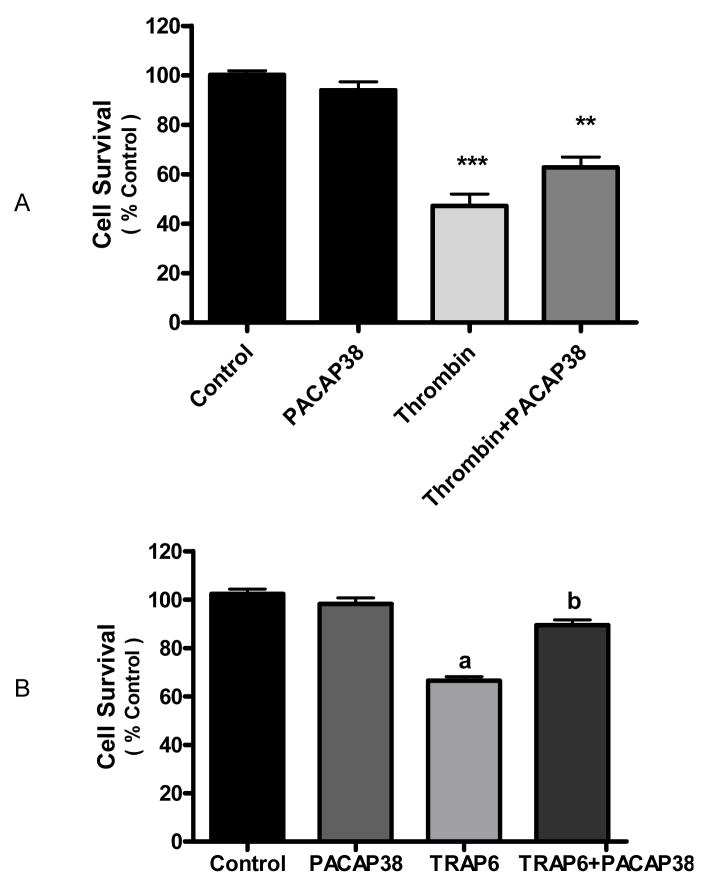
PACAP38 protected neurons against thrombin-induced cell death
We explored the effect of PACAP on neuronal cell death evoked by the neurotoxin thrombin. Addition of thrombin (100 nM) to neuronal cultures for 24 h caused a significant (p<0.001) decrease in cell survival, as assessed by MTT assay. Co-incubation of thrombin-treated neurons with PACAP38 (100 nM) increased cell survival compared to thrombin alone (p<0.01) (Figure 6A).
Figure 6. Thrombin evoked neuronal death was decreased by PACAP38.
A. Cortical neurons were treated with either media (control), PACAP38 (100 nM), thrombin (100 nM) or thrombin plus PACAP38 for 24 h and cell survival measured by MTT assay. Data are means ± SEM of three experiments and expressed as percent of untreated control (100%). One-way analysis of variance was performed followed by Neuman-Keuls’ multiple comparison. *** p<0.001 vs control; ** p<0.01 vs thrombin. B. PACAP38 protected against thrombin neurotoxicity via the thrombin receptor. Cortical neurons were treated with either media (control), PACAP38 (100 nM), TRAP6 (0.6 mM) or TRAP6 plus PACAP38 for 24h and cell survival measured by MTT assay. One-way analysis of variance was performed followed by Neuman-Keuls multiple comparison test. Data are means ± SEM of four experiments.
a p<0.001 vs control
b p<0.001 vs TRAP6.
We also used the thrombin receptor activating peptide (TRAP6) to clarify whether the decrease in thrombin-induced cell death evoked by PACAP38 was directed against the thrombin receptor or against the protease activity of thrombin. Previous dose experiments using TRAP6 indicated that 0.6 mM caused approximately 50% neuronal death (unpublished data). Here treatment of neuronal cultures with 0.6 mM TRAP6 evoked a significant (p<0.001) decrease in cell survival (Figure 6B). Co-incubation of neurons with both TRAP6 and 100 nM PACAP38 significantly (p<0.001) increased cell survival compared to TRAP6 alone.
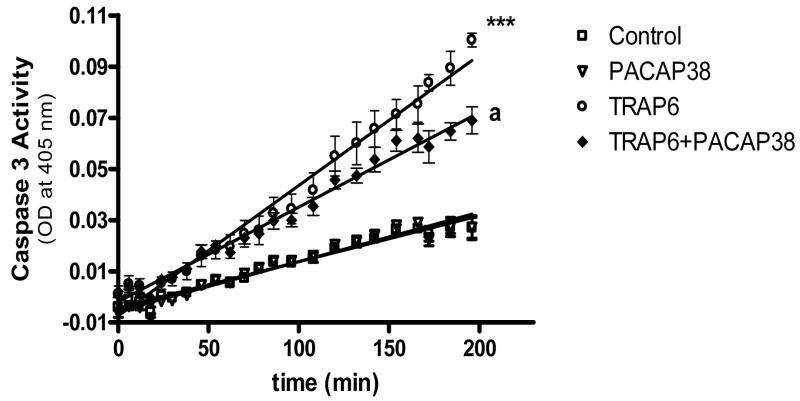
The ability of TRAP6 and TRAP6 plus PACAP38 to affect neuronal cell apoptosis was assessed by measuring caspase 3 activity. Treatment of neuronal cultures with TRAP6 (0.6 mM) for 24 h evoked a significant (p<0.001) increase in caspase 3 activity. This increased activity was significantly (p<0.001) reduced when neuronal cultures were co-incubated with both 100 nM PACAP38 and TRAP6 compared to TRAP6 alone (Figure 7).
Figure 7. PACAP38 decreased TRAP6-induced increase in caspase 3 activity level.
Cultured neurons were treated with medium (control), PACAP38 (100 nM), TRAP6 (0.6 mM) or TRAP6 plus PACAP38 for 24 h. Neuronal cultures were washed, cells collected and lysates analyzed for caspase 3 activity measured as conversion of substrate (OD at 405 nm). Data are means ± SEM of three experiments performed in triplicate.
*** p< 0.001 vs control
a p<0.001 vs TRAP6
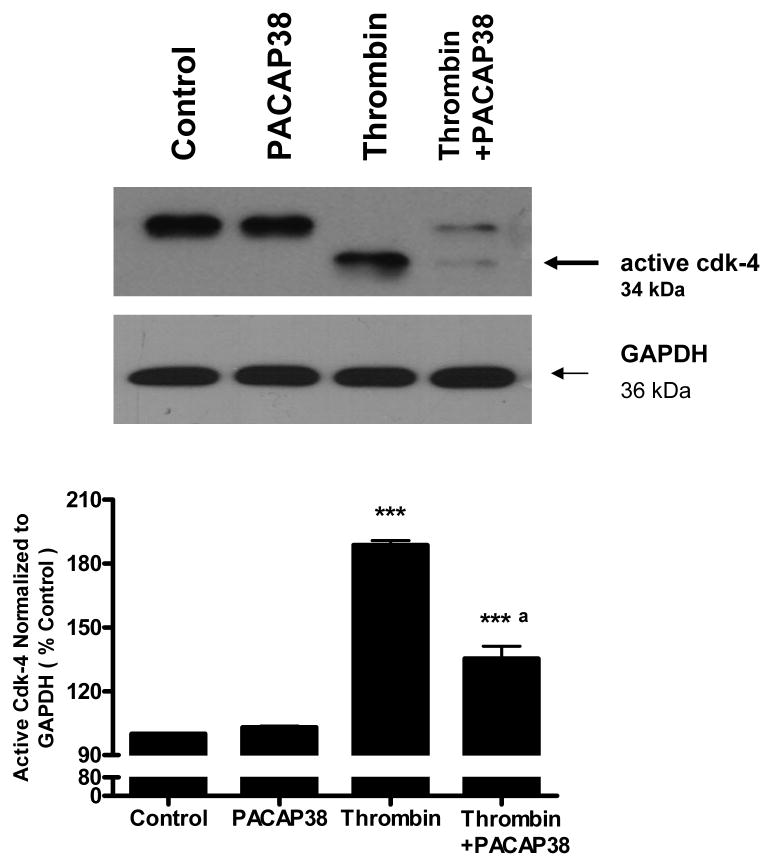
PACAP blunted the effects of thrombin on cell cycle proteins cdk4 and p57KIP2
Western blot analysis of untreated neuronal cultures as well as neurons treated with PACAP38 (100 nM) showed expression of the 34 kDa band which corresponds to the inactive, constitutively expressed form of the cell cycle kinase cdk4 (Figure 8). Exposure of neurons to 100 nM thrombin for 24 h resulted in the appearance of a faster migrating band corresponding to the active, smaller form of cdk4 [40]. Incubation of neuronal cultures with PACAP38 1 h after thrombin exposure significantly (p<0.001) decreased the level of active cdk4 (Figure 8).
Figure 8. PACAP38 decreased formation of active cdk4 in thrombin treated neurons.
Cultured neurons were incubated with either medium (control), PACAP38 (100 nM), thrombin (100 nM), or treated with thrombin and later exposed to PACAP38 (thrombin + PACAP38) for 1 h. Cultures were incubated for 24 h, washed, cells collected and lysates analyzed for cdk4 expression by western blot analysis. Histogram represents densitometric measurements of the active, faster migrating band from three werstern blot experiments normalized to GAPDH levels. Treatments were compaired by one-tailed t-test.
*** p<0.001 vs control
a p<0.001 vs thrombin
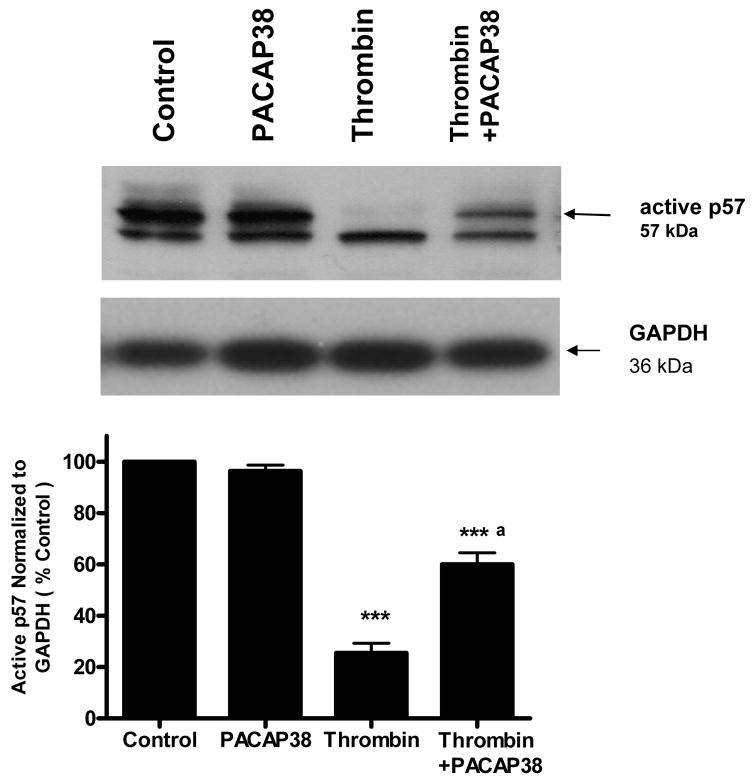
We also investigated the effect of PACAP38 on p57KIP2 expression in thrombin-treated cells. Untreated neurons and neuronal cultures treated with 100 nM PACAP38 showed a high level of active p57KIP2. Exposure of neurons to thrombin caused a significant decrease (p<0.001) in the level of active p57 KIP2. However, incubation of neuronal cultures with PACAP38 1 h after thrombin exposure significantly (p<0.001) increased the level of active p57KIP2 detectable (Figure 9).
Figure 9. PACAP38 increased formation of active p57Kip2 in thrombin treated neurons.
Cultured neurons were treated with either medium (control), PACAP38 (100 nM), thrombin (100 nM) or treated with thrombin and later exposed to PACAP38 for 1 h. Cultures were incubated for 24 h washed, cells collected and lysates analyzed for p57Kip2 expression by western blot analysis. Histogram represents densitometric measurements of three western blot experiments normalized to GAPDH levels. Treatments were compared by one-tailed t-test.
***p<0.001 vs control
a p<0.001 vs thrombin
PACAP38 did not affect Bcl-2 down-regulation induced by thrombin
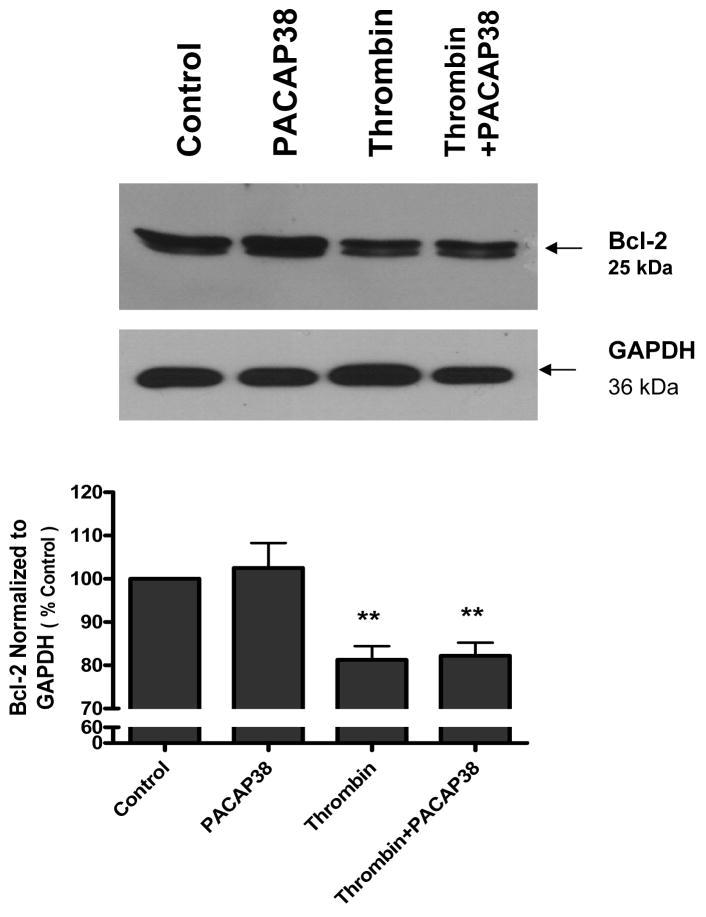
Western blot analysis indicated that untreated neurons as well as PACAP38 treated cells expressed a basal level of the Bcl-2 protein (Figure 10). Incubation of neurons with thrombin for 24 h resulted in a significant (p<0.01) reduction in expression of Bcl-2. Post-treatment of cultures with PACAP38 had no effect on thrombin-induced decrease in Bcl-2 level (Figure 10).
Figure 10. PACAP38 did not affect Bcl-2 down regulation induced by thrombin.
Cultured neurons were treated with either medium (control), PACAP38 (100 nM), thrombin (100 nM) or treated with thrombin and later exposed to PACAP38 (thrombin + PACAP38). Cultures were incubated for 24 h, washed, cells collected and lysates analyzed for Bcl-2 expression by western blot analysis. Histogram represents densitometric measurements of three western blot experiments normalized to GAPDH levels. Treatments were compared by one-tailed t-test.
** p< 0.01 versus control
DISCUSSION
Neuronal cell death is a tightly controlled process involving multiple checkpoints that regulate apoptosis in neurons [41]. It is likely that diverse mechanisms contribute to the ability of neurotrophic factors to support neuronal survival in the face of toxic injury. Indeed, activation of cell surface receptors and signaling pathways in neurons as well as release of secondary trophic factors may all contribute to neuroprotection. For example, in pyramidal neurons PACAP activation of the G-protein coupled PACAP receptor reduces apoptosis via inhibition of the mitogen-activated protein kinase family [19]. In addition, PACAP has been shown to protect against gp120-induced neurotoxicity by release of the chemokines RANTES and macrophage inhibitory protein -1α [42]. Neurotoxins, such as nitric oxide and thrombin, also utilize multiple pathways to bring about neuronal cell death.
The neurotoxic properties of NO in vitro and in vivo have been extensively documented [21, 43, 44]. The ability of nitric oxide to cause oxidant stress and oxidative injury has been thought to account for its neurotoxicity. Nitric oxide can be scavenged in a rapid reaction with superoxide to generate peroxynitrite [44]. Peroxynitrite is a potent oxidant and the primary component of nitroxidative stress. High nitroxidative stress can initiate a cascade of redox reactions which trigger apoptosis and evoke cytotoxic effects in neurons [45]. Despite a wealth of data on NO-mediated neurotoxicity, possible effects of NO on cell cycle activation, an important mechanism of neuronal cell death, have not been previously defined.
Re-initiation of the cell cycle in post-mitotic neurons is increasingly implicated in neuronal cell death and degenerative diseases of the CNS [46–48]. Expression of cell cycle proteins has been detected in neurons from Alzheimer’s disease and stroke patients [49, 50]. Cell cycle proteins are induced by traumatic brain injury and prevention of this induction has been shown to limit subsequent neuronal cell death [31]. Control of the cell cycle involves the dynamic interplay between proteins that favor vs. those that oppose cell cycle progression. The cell cycle protein cyclin E is essential for progression through the G1 phase of the cell cycle and has a major role in control of G1 to S phase transitions. The CIP/KIP family (p21CIP1, p27KIP1, p57KIP2) inhibits the actions of cyclins including cyclin E as well as several cyclin-dependent kinases [34, 35]. The effect of NO on cell cycle induction has not been previously defined. Here we show that treatment with the NO-releasing compound SNP causes neuronal death that is associated with an increase in cyclin E expression and a decrease in expression of the cell cycle inhibitor p57KIP2. Attenuation of NO-mediated death by PACAP is accompanied by a corresponding decrease in cyclin E levels and an increase in p57 expression. Cell cycle progression is tightly controlled by the cyclins, cyclin-dependent kinases and their corresponding inhibitors. Thus, increased cyclin E expression with SNP treatment is indicative of cell cycle re-entry and PACAP attenuation of cyclin E expression demonstrates anti-mitogenic effects of the neuroprotective protein. The protective action of PACAP is likely also mediated via its effect on p57KIP2 expression. In this regard, an anti-mitogenic action of PACAP on p57KIP2 expression has been documented in neural progenitor cells [51]. Our data here indicate that in differentiated, quiescent neurons, PACAP38 also evokes neuroprotection, in part, through effects on p57KIP2 expression.
In contrast to a relative dearth of information on NO and cell cycle activation, there are considerable data on the mitogenic effects of thrombin [52] and its action on cell cycle progression in neuronal cells [36, 53] and other cell types [54–57]. We have previously shown that thrombin activates the cell cycle cascade by sequentially inducing cyclin D, cyclin E and cdk4 and that thrombin-induced death of primary cortical neurons is decreased by inhibition of cdk4 activity [36]. In the present study we demonstrate that exposure of cultured neurons to PACAP38 protects neurons from thrombin-induced death. PACAP decreases the expression of the active form of cdk4. We show constitutive expression of inactive cdk4 in control and PACAP-treated cultured neurons. With thrombin exposure the inactive form is converted to active cdk4 and in cells exposed to both thrombin and PACAP there is a significant decrease in active cdk4. Furthermore, PACAP helps increase the level of a cell cycle inhibitor (p57KIP2) that is down regulated by thrombin. These data demonstrate that there are multiple and specific cell cycle targets that are likely to contribute to neuroprotection of PACAP.
Although both thrombin and SNP evoke neuronal cell death, their mechanisms of action are distinct. NO-induced effects on cell viability are rapid and immediate at the doses studied, where as the actions of thrombin require a longer time frame [36, 58]. Also, in a previous study examining mechanisms of neuronal cell death we show that multiple mechanisms are involved in neuronal cell death that vary depending on the nature of the neurotoxic insult and are influenced by subtle differences among neuronal cell phenotypes [20]. Caspase inhibition is efficient in reducing nucleosome accumulation in primary cortical cultures stimulated by TNFα and thrombin. In contrast, the same effect is not observed in differentiated PC12 cells. In PC12 cells TNFα-induced LDH release is reduced by caspase inhibition. Because TNFα treatment induces both LDH release and nucleosome accumulation in PC12 cells, caspase inhibition may enhance cell survival under conditions that induce a mixed apoptotic/necrotic response. Pytlowany and colleagues show that In PC12 cells NO released from SNP decreases cell viability in a time and concentration dependent manner, with a higher concentration of NO causing immediate and sustained decrease in cell survival without evoking a corresponding immediate activation of caspase 3 [59]. In the current study we find that NO generated by 0.5 mM SNP activates caspase 3 in a longer time frame (24 h). Similarly, although treatment of neuronal cultures with PACAP improves survival to both thrombin and SNP the biochemical response is not identical. For example, PACAP is more effective at restoring p57KIP2 expression in thrombin-treated compared to NO-exposed cells. Our results suggest that PACAP protects neurons from cell death through multiple mechanisms and that its protective effects are evident even if administered post-stress [60]. It has been shown that cell cycle proteins in apoptotic neurons can activate pro-apoptotic protein expression, which leads to completion of the apoptotic process. However, whether there is a regulation of pro-survival mediators such as Bcl-2 in this process is not clear. Apoptosis depends on the balance between pro-apoptotic and pro-survival proteins being tipped in favor of the former. The protective effect of PACAP has been associated with increased expression of Bcl-2 in rat cerebellar granule cells [61] and other members of the Bcl family in cardiomyocytes [62, 63]. In this study, we sought to determine whether the protective effect of PACAP38 proceeded via restoring this balance by increasing expression of Bcl-2, a pro-survival mediator. The protective effect of PACAP38 on the regulation of Bcl-2 expression is also agonist-variable. Untreated neuronal cultures express a basal level of the anti-apoptotic protein Bcl-2 that is reduced by exposure to either SNP or thrombin. The SNP-induced reduction of Bcl-2 is reversible by PACAP38 while thrombin-induced reduction of Bcl-2 is unaffected by PACAP38. The restoration of Bcl-2 by PACAP38 in SNP treated neurons whereas not in thrombin treated neurons demonstrates that there are at least two mechanisms involved in altering the apoptosis survival balance mediated by PACAP38. We have earlier shown that in thrombin-mediated neuronal apoptosis, there is an upregulation of pro-apoptotic protein Bim [36]. Therefore a decrease in Bcl-2 may not be necessary for favoring apoptosis, as an increase in Bim would naturally do so. However, in the case of NO-mediated neurotoxicity, a decrease in Bcl-2 may be essential to favor apoptosis. Studies using simvastatin to prevent NO-mediated neurotoxicity have also shown that Bcl-2 upregulation is an important step in the protection mechanism [64, 65]. Such upregulation of Bcl-2 has not been described with respect to protection against thrombin-induced neurotoxicity.
It has been suggested that in neurodegenerative diseases neurons function in a dynamic steady state, balancing chronic apoptotic stressors and compensatory survival pathways [41]. The two neurotoxins understudy here, NO and thrombin have both been implicated in the pathogenesis of neurodegenerative disease. The current data indicate that in terminally differentiated neurons in culture, PACAP38 can be neuroprotective by at least via two mechanisms; suppression of entry into the cell cycle and by its action on pro-survival proteins in the apoptotic pathways. In neurodegenerative diseases of the brain multiple neurotoxic agents could be produced/secreted at the same time. Thus, neuroprotective agents that can protect against multiple toxic cascades would be especially useful therapeutics. PACAP38 shows promise as one such agent capable of protecting neurons from diverse types of stress, extracellular (e.g. proteases such as thrombin) as well as intracellular (e.g. oxidative stress from NO). These results support the possibility that PACAP could be a useful therapeutic agent for reducing neuronal cell death in neurodegenerative diseases.
Acknowledgments
Sources of support: This work was supported in part by grants from the National Institutes of Health (AG15964, AG020569 and AG028367). Dr. Grammas is the recipient of the Shirley and Mildred Garrison Chair in Aging. The authors gratefully acknowledge the secretarial assistance of Terri Stahl.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–9. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- 2.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–74. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 3.Vaudry D, Gonzalez BJ, Basille M, Pamantung TF, Fournier A, Vaudry H. PACAP acts as a neurotrophic factor during histogenesis of the rat cerebellar cortex. Ann N Y Acad Sci. 2000;921:293–9. doi: 10.1111/j.1749-6632.2000.tb06980.x. [DOI] [PubMed] [Google Scholar]
- 4.Somogyvari-Vigh A, Reglodi D. Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Curr Pharm Des. 2004;10:2861–89. doi: 10.2174/1381612043383548. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki K, Kimura C, Kosaka T, Watanabe T, Ohkubo S, Ogi K, Kitada C, Onda H, Fujino M. Expression of human pituitary adenylate cyclase activating polypeptide (PACAP) cDNA in CHO cells and characterization of the products. FEBS Lett. 1992;298:49–56. doi: 10.1016/0014-5793(92)80020-h. [DOI] [PubMed] [Google Scholar]
- 6.Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N, Arimura A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38) Biochem Biophys Res Commun. 1990;170:643–8. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- 7.Piggins HD, Stamp JA, Burns J, Rusak B, Semba K. Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdala of the rat. J Comp Neurol. 1996;376:278–94. doi: 10.1002/(SICI)1096-9861(19961209)376:2<278::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Laburthe M, Couvineau A, Tan V. Class II G protein-coupled receptors for VIP and PACAP: structure, models of activation and pharmacology. Peptides. 2007;28:1631–9. doi: 10.1016/j.peptides.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–70. [PMC free article] [PubMed] [Google Scholar]
- 10.Cauvin A, Robberecht P, De Neef P, Gourlet P, Vandermeers A, Vandermeers-Piret MC, Christophe J. Properties and distribution of receptors for pituitary adenylate cyclase activating peptide (PACAP) in rat brain and spinal cord. Regul Pept. 1991;35:161–73. doi: 10.1016/0167-0115(91)90478-y. [DOI] [PubMed] [Google Scholar]
- 11.Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol. 2004;476:388–413. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- 12.Allais A, Burel D, Isaac ER, Gray SL, Basille M, Ravni A, Sherwood NM, Vaudry H, Gonzalez BJ. Altered cerebellar development in mice lacking pituitary adenylate cyclase-activating polypeptide. Eur J Neurosci. 2007;25:2604–18. doi: 10.1111/j.1460-9568.2007.05535.x. [DOI] [PubMed] [Google Scholar]
- 13.Reglodi D, Tamas A, Somogyvari-Vigh A, Szanto Z, Kertes E, Lenard L, Arimura A, Lengvari I. Effects of pretreatment with PACAP on the infarct size and functional outcome in rat permanent focal cerebral ischemia. Peptides. 2002;23:2227–34. doi: 10.1016/s0196-9781(02)00262-0. [DOI] [PubMed] [Google Scholar]
- 14.Reglodi D, Lubics A, Tamas A, Szalontay L, Lengvari I. Pituitary adenylate cyclase activating polypeptide protects dopaminergic neurons and improves behavioral deficits in a rat model of Parkinson’s disease. Behav Brain Res. 2004;151:303–12. doi: 10.1016/j.bbr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Kojro E, Postina R, Buro C, Meiringer C, Gehrig-Burger K, Fahenholz F. The neuropeptide PACAP promotes the alpha-secretase pathway for processing the Alzheimer amyloid precursor protein. FASEB J. 2006;3:512–4. doi: 10.1096/fj.05-4812fje. [DOI] [PubMed] [Google Scholar]
- 16.Onoue S, Endo K, Ohshima K, Yajima T, Kashimoto K. The neuropeptide PACAP attenuates beta-amyloid (1–42)-induced toxicity in PC12 cells. Peptides. 2002;23:1471–8. doi: 10.1016/s0196-9781(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 17.Vaudry D, Pamantung TF, Basille M, Rousselle C, Fournier A, Vaudry H, Beauvillain JC, Gonzalez BJ. PACAP protects cerebellar granule neurons against oxidative stress-induced apoptosis. Eur J Neurosci. 2002;15:1451–60. doi: 10.1046/j.1460-9568.2002.01981.x. [DOI] [PubMed] [Google Scholar]
- 18.Shintani N, Suetake S, Hashimoto H, Koga K, Kasai A, Kawaguchi C, Morita Y, Hirose M, Sakai Y, Tomimoto S, Matsuda T, Baba A. Neuroprotective action of endogenous PACAP in cultured rat cortical neurons. Regul Pept. 2005;126:123–8. doi: 10.1016/j.regpep.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Shioda S, Ohtaki H, Nakamachi T, Dohi K, Watanabe J, Nakajo S, Arata S, Kitamura S, Okuda H, Takenoya F, Kitamura Y. Pleiotropic functions of PACAP in the CNS: neuroprotection and neurodevelopment. Ann N Y Acad Sci. 2006;1070:550–60. doi: 10.1196/annals.1317.080. [DOI] [PubMed] [Google Scholar]
- 20.Reimann-Philipp U, Ovase R, Weigel PH, Grammas P. Mechanisms of cell death in primary cortical neurons and PC12 cells. J Neurosci Res. 2001;64:654–60. doi: 10.1002/jnr.1119. [DOI] [PubMed] [Google Scholar]
- 21.Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans. 2007;35:1119–21. doi: 10.1042/BST0351119. [DOI] [PubMed] [Google Scholar]
- 22.Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8:766–75. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 23.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 24.Bolanos JP, Almeida A, Stewart V, Peuchen S, Land JM, Clark JB, Heales SJ. Nitric oxide-mediated mitochondrial damage in the brain: mechanisms and implications for neurodegenerative diseases. J Neurochem. 1997;68:2227–40. doi: 10.1046/j.1471-4159.1997.68062227.x. [DOI] [PubMed] [Google Scholar]
- 25.Figueroa S, Oset-Gasque MJ, Arce C, Martinez-Honduvilla CJ, Gonzalez MP. Mitochondrial involvement in nitric oxide-induced cellular death in cortical neurons in culture. J Neurosci Res. 2006;83:441–9. doi: 10.1002/jnr.20739. [DOI] [PubMed] [Google Scholar]
- 26.Gross SS, Wolin MS. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–69. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- 27.Almeida A, Bolanos JP. A transient inhibition of mitochondrial ATP synthesis by nitric oxide synthase activation triggered apoptosis in primary cortical neurons. J Neurochem. 2001;77:676–90. doi: 10.1046/j.1471-4159.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 28.Kong LY, Maderdrut JL, Jeohn GH, Hong JS. Reduction of lipopolysaccharide-induced neurotoxicity in mixed cortical neuron/glia cultures by femtomolar concentrations of pituitary adenylate cyclase-activating polypeptide. Neuroscience. 1999;91:493–500. doi: 10.1016/s0306-4522(98)00606-x. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Varvel NH, Lamb BT, Herrup K. Ectopic cell cycle events link human Alzheimer’s disease and amyloid precursor protein transgenic mouse models. J Neurosci. 2006;26:775–84. doi: 10.1523/JNEUROSCI.3707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woods J, Snape M, Smith MA. The cell cycle hypothesis of Alzheimer’s disease: suggestions for drug development. Biochim Biophys Acta. 2007;1772:503–8. doi: 10.1016/j.bbadis.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Di Giovanni S, Movsesyan V, Ahmed F, Cernak I, Schinelli S, Stoica B, Faden AI. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci U S A. 2005;102:8333–8. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen Y, Yang S, Liu R, Simpkins JW. Cell-cycle regulators are involved in transient cerebral ischemia induced neuronal apoptosis in female rats. FEBS Lett. 2005;579:4591–9. doi: 10.1016/j.febslet.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Burke K, Cheng Y, Li B, Petrov A, Joshi P, Berman RF, Reuhl KR, DiCicco-Bloom E. Methylmercury elicits rapid inhibition of cell proliferation in the developing brain and decreases cell cycle regulator, cyclin E. Neurotoxicology. 2006;27:970–81. doi: 10.1016/j.neuro.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elledge SJ, Harper JW. Cdk inhibitors: on the threshold of checkpoints and development. Curr Opin Cell Biol. 1994;6:847–52. doi: 10.1016/0955-0674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 35.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 36.Rao HV, Thirumangalakudi L, Desmond P, Grammas P. Cyclin D1, cdk4, and Bim are involved in thrombin-induced apoptosis in cultured cortical neurons. J Neurochem. 2007;101:498–505. doi: 10.1111/j.1471-4159.2006.04389.x. [DOI] [PubMed] [Google Scholar]
- 37.Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. J Neurosci. 1997;17:5316–26. doi: 10.1523/JNEUROSCI.17-14-05316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mhatre M, Nguyen A, Kashani S, Pham T, Adesina A, Grammas P. Thrombin, a mediator of neurotoxicity and memory impairment. Neurobiol Aging. 2004;25:783–93. doi: 10.1016/j.neurobiolaging.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Grammas P, Moore P, Weigel PH. Microvessels from Alzheimer’s disease brains kill neurons in vitro. Am J Pathol. 1999;154:337–42. doi: 10.1016/S0002-9440(10)65280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobashi Y, Kudoh T, Toyoshima K, Akiyama T. Persistent activation of CDK4 during neuronal differentiation of rat pheochromocytoma PC12 cells. Biochem Biophys Res Commun. 1996;221:351–5. doi: 10.1006/bbrc.1996.0599. [DOI] [PubMed] [Google Scholar]
- 41.Cotman CW. Apoptosis decision cascades and neuronal degeneration in Alzheimer’s disease. Neurobiol Aging. 1998;19:S29–32. doi: 10.1016/s0197-4580(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 42.Brenneman DE, Hauser JM, Spong C, Phillips TM. Chemokine release is associated with the protective action of PACAP-38 against HIV envelope protein neurotoxicity. Neuropeptides. 2002;36:271–80. doi: 10.1016/s0143-4179(02)00045-8. [DOI] [PubMed] [Google Scholar]
- 43.Araujo IM, Carvalho CM. Role of nitric oxide and calpain activation in neuronal death and survival. Curr Drug Targets CNS Neurol Disord. 2005;4:319–24. doi: 10.2174/1568007054546126. [DOI] [PubMed] [Google Scholar]
- 44.Chung KK. Say NO to neurodegeneration: role of S-nitrosylation in neurodegenerative disorders. Neurosignals. 2006;15:307–13. doi: 10.1159/000109071. [DOI] [PubMed] [Google Scholar]
- 45.Malinski T. Nitric oxide and nitroxidative stress in Alzheimer’s disease. J Alzheimers Dis. 2007;11:207–18. doi: 10.3233/jad-2007-11208. [DOI] [PubMed] [Google Scholar]
- 46.Copani A, Condorelli F, Caruso A, Vancheri C, Sala A, Giuffrida Stella AM, Canonico PL, Nicoletti F, Sortino MA. Mitotic signaling by beta-amyloid causes neuronal death. FASEB J. 1999;13:2225–34. [PubMed] [Google Scholar]
- 47.Copani A, Uberti D, Sortino MA, Bruno V, Nicoletti F, Memo M. Activation of cell-cycle-associated proteins in neuronal death: a mandatory or dispensable path? Trends Neurosci. 2001;24:25–31. doi: 10.1016/s0166-2236(00)01663-5. [DOI] [PubMed] [Google Scholar]
- 48.Liu DX, Greene LA. Neuronal apoptosis at the G1/S cell cycle checkpoint. Cell Tissue Res. 2001;305:217–28. doi: 10.1007/s004410100396. [DOI] [PubMed] [Google Scholar]
- 49.Smith MZ, Nagy Z, Esiri MM. Cell cycle-related protein expression in vascular dementia and Alzheimer’s disease. Neurosci Lett. 1999;271:45–8. doi: 10.1016/s0304-3940(99)00509-1. [DOI] [PubMed] [Google Scholar]
- 50.Hayashi T, Sakai K, Sasaki C, Zhang WR, Abe K. Phosphorylation of retinoblastoma protein in rat brain after transient middle cerebral artery occlusion. Neuropathol Appl Neurobiol. 2000;26:390–7. doi: 10.1046/j.1365-2990.2000.00264.x. [DOI] [PubMed] [Google Scholar]
- 51.Carey RG, Li B, DiCicco-Bloom E. Pituitary adenylate cyclase activating polypeptide anti-mitogenic signaling in cerebral cortical progenitors is regulated by p57Kip2-dependent CDK2 activity. J Neurosci. 2002;22:1583–91. doi: 10.1523/JNEUROSCI.22-05-01583.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Reiser G. Thrombin signaling in the brain: the role of protease-activated receptors. Biol Chem. 2003;384:193–202. doi: 10.1515/BC.2003.021. [DOI] [PubMed] [Google Scholar]
- 53.Liu DZ, Cheng XY, Ander BP, Xu H, Davis RR, Gregg JP, Sharp FR. Src kinase inhibition decreases thrombin-induced injury and cell cycle re-entry in striatal neurons. Neurobiol Dis. 2008;30:201–11. doi: 10.1016/j.nbd.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aragay AM, Collins LR, Post GR, Watson AJ, Feramisco JR, Brown JH, Simon MI. G12 requirement for thrombin-stimulated gene expression and DNA synthesis in 1321N1 astrocytoma cells. J Biol Chem. 1995;270:20073–7. doi: 10.1074/jbc.270.34.20073. [DOI] [PubMed] [Google Scholar]
- 55.Grabham P, Cunningham DD. Thrombin receptor activation stimulates astrocyte proliferation and reversal of stellation by distinct pathways: involvement of tyrosine phosphorylation. J Neurochem. 1995;64:583–91. doi: 10.1046/j.1471-4159.1995.64020583.x. [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Ubl JJ, Stricker R, Reiser G. Thrombin (PAR-1)-induced proliferation in astrocytes via MAPK involves multiple signaling pathways. Am J Physiol Cell Physiol. 2002;283:C1351–64. doi: 10.1152/ajpcell.00001.2002. [DOI] [PubMed] [Google Scholar]
- 57.Nicole O, Goldshmidt A, Hamill CE, Sorensen SD, Sastre A, Lyuboslavsky P, Hepler JR, McKeon RJ, Traynelis SF. Activation of protease-activated receptor-1 triggers astrogliosis after brain injury. J Neurosci. 2005;25:4319–29. doi: 10.1523/JNEUROSCI.5200-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smirnova IV, Zhang SX, Citron BA, Arnold PM, Festoff BW. Thrombin is an extracellul;ar signal that activates intracellular death protease pathways inducing apopotosis in model motor neuron. J Neurobiol. 1998;36:64–80. [PubMed] [Google Scholar]
- 59.Pytlowany M, Strisznaider JB, Jesko H, Cakala M, Stroszajdar RP. Molecular mechcanism of PC12 cell death evoked by sodium nitroprusside, a nitric oxide donor. Acta Biochem Pol. 2008 In Press. [PubMed] [Google Scholar]
- 60.Vaudry D, Rousselle C, Basille M, Falluel-Morel A, Pamantung TF, Fonatine M, Fournier A, Vaudry H, Gonzalez BJ. Pituitary adenylate cyclase activating polypeptide protects rat cerebellar granule neurons against ethanol-induced apoptotic cell death. Proc Natl Acad Sci U S A. 2002;99:6398–403. doi: 10.1073/pnas.082112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aubert N, Falluel-Morel A, Vaudry D, Xifro X, Rodriguez-Alvarez J, Fisch C, de Jouffrey S, Lebigot JF, Fournier A, Vaudry H, Gonzalez BJ. PACAP and C2-ceramide generate different AP-1 complexes through a MAP-kinase-C2-dependent pathway: involvement of c-Fos in PACAP-induced Bcl-2 expression. J Neurochem. 2006;99:1237–50. doi: 10.1111/j.1471-4159.2006.04148.x. [DOI] [PubMed] [Google Scholar]
- 62.Gasz B, Racz B, Roth E, Borsiczky B, Ferencz A, Tamas A, Cserepes B, Lubics A, Gallyas F, Jr, Toth G, Lengvari I, Reglodi D. Pituitary adenylate cyclase activating polypeptide protects cardiomyocytes against oxidative stress-induced apoptosis. Peptides. 2006;27:87–94. doi: 10.1016/j.peptides.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 63.Racz B, Gasz B, Gallyas F, Jr, Kiss P, Tamas A, Szanto Z, Lubics A, Lengvari I, Toth G, Hegyi O, Roth E, Reglodi D. PKA-Bad-14-3-3 and Akt-Bad-14-3-3 signaling pathways are involved in the protective effects of PACAP against ischemia/reperfusion-induced cardiomyocyte apoptosis. Regul Pept. 2008;145:105–15. doi: 10.1016/j.regpep.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 64.Johnson-Anuna LN, Eckert GP, Franke C, Igbavboa U, Muller WE, Wood WG. Simvastatin protects neurons from cytotoxicity by up-regulating Bcl-2 mRNA and protein. J Neurochem. 2007;101:77–86. doi: 10.1111/j.1471-4159.2006.04375.x. [DOI] [PubMed] [Google Scholar]
- 65.Franke C, Noldner M, Abdel-Kader R, Johnson-Anuna LN, Gibson Wood W, Muller WE, Eckert GP. Bcl-2 upregulation and neuroprotection in guinea pig brain following chronic simvastatin treatment. Neurobiol Dis. 2007;25:438–45. doi: 10.1016/j.nbd.2006.10.004. [DOI] [PubMed] [Google Scholar]