Abstract
Objectives
The effects of the antileishmanial chalcone 2′,6′-dihydroxy-4′-methoxychalcone (DMC) on Leishmania amazonensis sterol composition and biosynthesis were investigated to obtain information about the mechanism of growth inhibition by DMC on this parasite.
Methods
The interference of sterol biosynthesis by DMC was studied in drug-treated promastigotes by two different methods. (i) Newly synthesized sterols from parasites grown in the presence of [3H]mevalonate were analysed by thin layer chromatography (TLC)/fluorography. (ii) Total sterols extracted from the parasites grown with or without DMC were characterized by gas chromatography coupled to mass spectroscopy (GC/MS).
Results
TLC and GC/MS analyses of sterols extracted from DMC-treated promastigotes revealed the accumulation of early precursors and a reduction in the levels of C-14 demethylated and C-24 alkylated sterols, as well as a reduction in exogenous cholesterol uptake.
Conclusions
This study demonstrates that the natural chalcone DMC alters the sterol composition of L. amazonensis and suggests that the parasite target is different from other known sterol inhibitors.
Keywords: lipid metabolism, cholesterol, ketoconazole, Trypanosoma
Introduction
Leishmaniases are a complex of clinically well-distinguished diseases ranging from localized cutaneous to the fatal visceral leishmaniasis. They are distributed in as many as 88 countries, where 400 million people are at risk of infection. The conventional treatment still relies on parenteral antimonials or amphotericin B that can be ineffective, toxic and/or costly. Oral hexadecylphosphocholine (miltefosine) is currently used in India for the treatment of visceral leishmaniasis in antimony-resistant cases, but teratogenicity and a narrow therapeutic window pose limitations.1
Although many natural products have shown activity in in vitro models of leishmaniasis, chalcones are among the few that have succeeded in animal models. Licochalcone A and derivatives have shown efficacy in experimental models of visceral and cutaneous leishmaniasis,2 and the activity of 2′,6′-dihydroxy-4′-methoxychalcone (DMC; see Figure 1) against Leishmania amazonensis was demonstrated in vitro and in vivo.3,4 Although DMC has an antileishmanial activity similar to that of licochalcone A, it is structurally simpler, an advantage in terms of chemical synthesis. Both licochalcone A and DMC were shown to induce mitochondrial swelling in Leishmania major and L. amazonensis, respectively.3,5 However, these compounds appear to have different mechanisms of action: while licochalcone A changes the parasite glycolic pathway due to direct inhibition of parasite fumarate reductase,6 a relatively high concentration of DMC (100 µM) failed to interfere with that enzyme (Professor J. F. Turrens, University of South Alabama, USA, personal communication). Further attempts to identify the DMC target in L. amazonensis using the trypanothione reductase inhibition assay also proved unsuccessful (Professor A. H. Fairlamb, University of Dundee, UK, personal communication).
Figure 1.
Structure of DMC.
Like fungi, the Leishmania and Trypanosoma sterol pathway is considered to be a selective drug target due to the biosynthesis of 24-substituted sterols such as ergosterol and its derivatives, while mammalians use cholesterol instead. Inhibitors of that pathway (statins, allylamines, azoles and 22,26-azasterol) have been shown to cause a depletion of normal sterols and an accumulation of abnormal precursors in Leishmania spp.7 Since sterol inhibitors such as ketoconazole, terbinafine and azasterol induce similar ultrastructural alterations in the mitochondrion of L. amazonensis promastigotes as reported for DMC,3,8,9 we proposed here to evaluate the effect of DMC on parasite sterol biosynthesis.
Materials and methods
Preparation of chalcone DMC
DMC was extracted from the inflorescences of the plant Piper aduncum with dichloromethane, purified and analysed, as described previously.3
Parasite
L. amazonensis (isolate MHOM/BR/75/Josefa) was maintained as promastigotes at 26°C in medium 199 (M199; Sigma-Aldrich) supplemented with 5% heat-inactivated fetal calf serum, 100 µg/mL streptomycin and 100 U/mL penicillin for a maximum of four subcultures.
Analysis of L. amazonensis sterols by thin layer chromatography (TLC)
Promastigotes of L. amazonensis (106 cells/mL) were treated for 24 h with DMC (10 and 25 µM), pentamidine (100 µg/mL, Rhone-Poulenc) or ketoconazole (150 nM, Sigma-Aldrich). Both RS[5-3H]mevalonolactone (100 µCi, specific activity of 60 mCi/mol; American Radiolabel Chemicals) and saponified simvastatin (100 µM; a gift of Dr A. Corsini, University of Milan, Italy) were present in all cultures. After incubation, 107 parasites from each culture were extensively washed and their neutral lipids extracted with chloroform–methanol (2:1, v/v), followed by partition with petroleum ether. The extracts were then spotted on silica gel 60 TLC plates (Merck) and developed with toluene–diethyl ether (9:1, v/v). 3H-labelled sterols were visualized by autoradiography using EN3HANCE spray (PerkinElmer).
Analysis of total sterol pattern by gas chromatography coupled to mass spectrometry (GC/MS)
L. amazonensis promastigotes were grown with 2.5 and 5.0 µM DMC or medium alone for 72 h. Then, 5 × 107 parasites from each culture were extensively washed, and their neutral lipids were extracted as for TLC. The samples were injected onto a Hewlett-Packard 6890 series II gas chromatographer coupled to an HP5973A mass spectrometer. Upon injection, the column temperature was kept at 50°C for 1 min and then increased to 270°C at a rate of 10°C/min and finally to 300°C at a rate of 1°C/min. The carrier gas (He) flow was kept constant at 1.1 mL/min. The injector and the detector temperatures were 250 and 280°C, respectively.
Results
DMC alters sterol biosynthesis by L. amazonensis in a manner different from ketoconazole
To study the sterol biosynthesis through the mevalonate pathway, L. amazonensis promastigotes were cultured with DMC in the presence of radiolabelled mevalonolactone. Ketoconazole and pentamidine were used as positive and negative controls of sterol synthesis inhibitors, respectively. A short 24 h study was conducted to allow for residual sterol metabolism while preventing incorporated mevalonate release due to subsequent parasite death. As shown in Figure 2, the sterols produced by both untreated (lane 1) and pentamidine-treated (lane 5) parasites migrated as one major band likely to be ergosterol plus other 24-alkylated C-14 demethylated compounds. Treatment with 25 and 10 µM DMC (lanes 2 and 3, respectively) rendered the two upper bands more pronounced according to increasing drug concentration. The band positions suggest that the lowest one contains lanosterol and/or other C-14 methylated sterols, similar to treatment with C14-demethylase-blocking ketoconazole (lane 4). The second top band indicates that DMC interferes with sterol biosynthesis in a manner different from ketoconazole, as it was much fainter in the latter.
Figure 2.
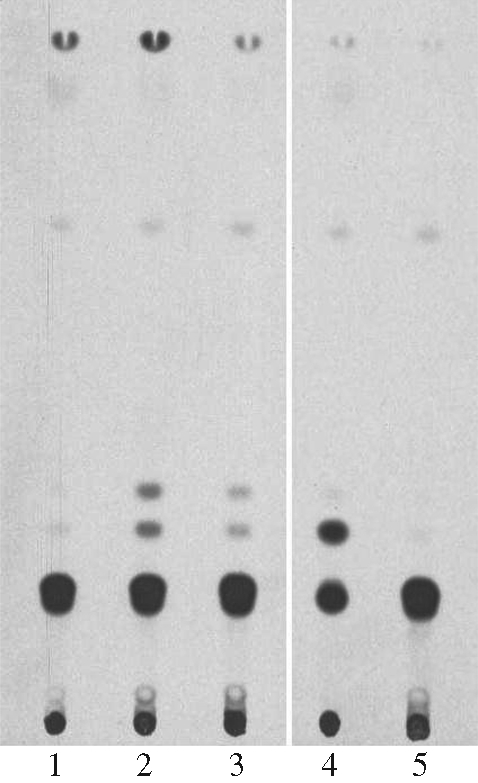
Effect of DMC treatment on the biosynthesis of [3H]sterols in L. amazonensis labelled with [3H]mevalonate. Promastigotes were grown for 24 h with no drug (lane 1), 25 µM DMC (lane 2), 10 µM DMC (lane 3), 150 nM ketoconazole (lane 4) or 100 µg/mL pentamidine (lane 5) together with 100 µM [3H]mevalolactone. Cellular lipids were extracted and resolved by TLC. Each lane contained materials from 107 cells.
Sterol composition of DMC-treated L. amazonensis promastigotes
GC/MS analysis was carried out on neutral lipids extracted from parasites treated with DMC (2.5 and 5 µM). To eliminate pre-existing sterols, the promastigotes were grown with sub-IC50 concentrations of DMC for a prolonged time (72 h). The data in Table 1 revealed several alterations in the sterol profile after DMC treatment, like a reduced uptake of cholesterol (1) from the culture medium. A reduction of dehydroepisterol (6) and episterol (8), which are the most abundant sterols of L. amazonensis,9 was found with increasing DMC concentration. Ergosterol (2), ergosta-5,8-dien-3β-ol (7, an alternative product of the pathway leading to 5-dehydroepisterol), 4,14-dimethylzymosterol (5, a substrate of C24 methyl transferase) and lanosterol (10, an early precursor before the C-14 and C-4 demethylations) accumulated in DMC-treated parasites. Together, these findings indicate an accumulation of early precursors and a reduction in the levels of C-14 demethylated and C-24 alkylated sterols caused by DMC treatment.
Table 1.
Composition of sterols from DMC-treated and non-treated L. amazonensis promastigotes
| Relative amount (%) after treatment with DMC at |
||||
|---|---|---|---|---|
| Compound | MW | 0 µM | 2.5 µM | 5.0 µM |
| 1—Cholesterol | 386 | 23.20 | 11.06 | 2.94 |
| 2—Ergosterol | 396 | 13.20 | 22.33 | 30.76 |
| 3—Unknown | 396 | 6.14 | 2.59 | 2.79 |
| 4—Ergosta-7,22-dien-3β-ol | 398 | 2.12 | 2.98 | 3.46 |
| 5—4,14-Dimethylzymosterol | 412 | — | 4.56 | 4.49 |
| 6—5-Dehydroepisterol | 396 | 13.58 | 10.45 | 4.47 |
| 7—Ergosta-5,8-dien-3β-ol | 398 | 4.34 | 14.58 | 13.09 |
| 8—Episterol | 398 | 20.92 | 16.29 | 18.64 |
| 9—Unknown | 426 | 2.49 | 1.47 | 3.74 |
| 10—Lanosterol | 426 | — | 3.59 | 1.06 |
| 11—Cholesta-5,7-dien-3β-ol | 384 | — | — | 4.96 |
| 12—Stigmasta-5,7,24(241)-trien-3β-ol | 410 | 6.37 | 4.88 | 4.34 |
| 13—Cholesta-7,24-dien-3β-ol | 384 | 5.65 | 5.17 | 5.19 |
MW, molecular weight (Da); —, non-detectable.
Discussion
Chalcones have arisen as a promising class of new antileishmanial agents,2–6 but little is known about their mechanism of action. The TLC studies shown in Figure 2 provide the first evidence that DMC modifies Leishmania sterol composition, compatible with a selective effect of DMC, once no alteration in the TLC lipid profile was observed with pentamidine-treated parasites. The GC/MS analysis further helped to identify the altered sterols, showing the accumulation of ergosta-5,8-dien-3β-ol, 4,14-dimethylzymosterol and lanosterol (4,4,14-trimethylcholesta-8,24-dien-3β-ol), suggestive of joint inhibition of the enzymes C-14 demethylase, C-24 methyl transferase and Δ8-Δ7 isomerase. Whenever those recombinant enzymes become available, enzyme inhibition assays will expectedly confirm this inference.
In Leishmania spp., the predominant sterols are 5-dehydro-episterol and episterol, although ergosterol is present in smaller amounts.7,9 Accumulation of ergosterol in L. amazonensis exposed to DMC was an unexpected finding, as this is formed in the late stages of the biosynthetic cascade. Nevertheless, in one study reported by Rakotomanga et al.,10 in miltefosine-treated Leishmania donovani promastigotes, both intermediate sterols and the total content of C-24 alkylated sterols were significantly reduced, but the ergosterol content remained unchanged, indicating that reduction in ergosterol is not a general rule in sterol biosynthesis inhibition.
In addition to the C-28- and C-29-sterols, Leishmania spp. contain variable amounts of cholesterol, often >10% of the total sterol.7 A pronounced decrease in the cholesterol content was found in DMC-exposed parasites. Lower cholesterol content, which may be a compensatory response to enhanced ergosterol accumulation, might lead to an increased membrane fluidity due to the reduced cholesterol ordering effect and contribute to the toxic effect of DMC.
Leishmania and Trypanosoma parasites produce ergosterol-related sterols by a biosynthetic pathway similar to that operating in pathogenic fungi, but may divert to some extent in the later steps. We have found that DMC is also toxic for Trypanosoma cruzi epimastigotes (IC50 = 5.5 µM), but not for Candida albicans and Candida sake yeasts at concentrations of DMC as high as 3400 µM (our unpublished observation). This is indicative of a higher DMC specificity to trypanosomatid parasites than the known sterol inhibitors ketoconazole, terbinafine or simvastatin that strongly inhibit fungi.
This study indicates that DMC promotes the accumulation of intermediate sterols without inhibiting the biosynthesis of ergosterol, by a still unknown mechanism. This may result in altered membrane fluidity and structure as seen previously by electron microscopy in DMC-treated parasites.3 It also demonstrates that DMC alters the sterol composition of L. amazonensis in a manner different from other known sterol inhibitors, an advantage for combination therapy against cutaneous leishmaniasis.
Funding
E. C. T.-S. received a PhD grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq—Brazil). This work was funded by the National Institutes of Health (AI 70218) and CNPq.
Transparency declarations
None to declare.
References
- 1.Croft SL, Seifert K, Yardley V. Current scenario of drug development for leishmaniasis. Indian J Med Res. 2006;123:399–410. [PubMed] [Google Scholar]
- 2.Zhai L, Chen M, Blom J, et al. The antileishmanial activity of novel oxygenated chalcones and their mechanism of action. J Antimicrob Chemother. 1999;43:793–803. doi: 10.1093/jac/43.6.793. [DOI] [PubMed] [Google Scholar]
- 3.Torres-Santos EC, Moreira DL, Kaplan MAC, et al. Selective effect of 2′,6′-dihydroxy-4′-methoxychalcone isolated from Piper aduncum on Leishmania amazonensis. Antimicrob Agents Chemother. 1999;43:1234–41. doi: 10.1128/aac.43.5.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres-Santos EC, Rodrigues JM, Moreira DL, et al. Improvement of in vitro and in vivo antileishmanial activities of 2′,6′-dihydroxy-4′-methoxychalcone by entrapment in poly(d,l-lactide) nanoparticles. Antimicrob Agents Chemother. 1999;43:1776–8. doi: 10.1128/aac.43.7.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhai L, Blom J, Chen M, et al. The antileishmanial agent licochalcone A interferes with the function of parasite mitochondria. Antimicrob Agents Chemother. 1995;39:2742–8. doi: 10.1128/aac.39.12.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen M, Zhai L, Christensen SB, et al. Inhibition of fumarate reductase in Leishmania major and L. donovani by chalcones. Antimicrob Agents Chemother. 2001;45:2023–9. doi: 10.1128/AAC.45.7.2023-2029.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magaraci F, Jimenez CJ, Rodrigues C, et al. Azasterols as inhibitors of sterol 24-methyltransferase in Leishmania species and Trypanosoma cruzi. J Med Chem. 2003;46:4714–27. doi: 10.1021/jm021114j. [DOI] [PubMed] [Google Scholar]
- 8.Vanniersantos MA, Urbina JA, Martiny A, et al. Alterations induced by the antifungal compounds ketoconazole and terbinafine in Leishmania. J Eukaryot Microbiol. 1995;42:337–46. doi: 10.1111/j.1550-7408.1995.tb01591.x. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues JCF, Attias M, Rodriguez C, et al. Ultrastructural and biochemical alterations induced by 22,26-azasterol, a Δ24(25)-sterol methyltransferase inhibitor, on promastigote and amastigote forms of Leishmania amazonensis. Antimicrob Agents Chemother. 2002;46:487–99. doi: 10.1128/AAC.46.2.487-499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakotomanga M, Blanc S, Gaudin K, et al. Miltefosine affects lipid metabolism in Leishmania donovani promastigotes. Antimicrob Agents Chemother. 2007;51:1425–30. doi: 10.1128/AAC.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]