Abstract
Mutations in the parkin gene cause autosomal-recessive, juvenile-onset parkinsonism, and parkin dysfunction may also play a role in the pathogenesis of sporadic Parkinson disease (PD). Although its precise function remains largely unknown, parkin seems to play a neuroprotective role. Several studies indicate that changes in parkin solubility induced by post-translational modifications, such as S-nitrosylation or dopamine modification, comprise one mechanism of parkin inactivation associated with disease. Protein phosphorylation events have recently been linked to the molecular mechanism(s) underlying PD, but the role of this post-translational modification for parkin function has remained unclear. Here we report that compound phosphorylation of parkin by both casein kinase I and cyclin-dependent kinase 5 (cdk5) decreases parkin solubility, leading to its aggregation and inactivation. Combined kinase inhibition partially reverses the aggregative properties of several pathogenic point mutants in cultured cells. Enhanced parkin phosphorylation is detected in distinct brain areas of individuals with sporadic PD and correlates with increases in the levels of p25, the activator of cdk5. These findings indicate that casein kinase I and cdk5 may represent novel combinatorial therapeutic targets for treating PD.
INTRODUCTION
Parkinson disease (PD) is a progressive and substantially disabling neurodegenerative disorder (1–3). Its clinical symptoms primarily result from the progressive and rather selective degeneration of dopaminergic neurons of the substantia nigra pars compacta. Besides cell death, a pathological hallmark of PD in surviving neurons comprises Lewy bodies, ubiquitylated intraneuronal inclusions rich in α-synuclein (4).
Even though largely a sporadic disorder, there are several genes associated with inherited forms of PD. One commonly implicated is PARK2, the gene encoding for parkin (5). Indeed, mutations in the parkin gene are responsible for a large percentage of autosomal-recessive, juvenile-onset parkinsonism (6,7). A variety of homozygous and compound heterozygous mutations have been reported, and although mutations that reduce but do not abolish parkin function are accompanied by dopaminergic cell loss in the presence of Lewy bodies (8,9), homozygous loss-of-function parkin mutations seem to be associated with a lack of Lewy bodies (10), raising the possibility that parkin may be involved in Lewy body biogenesis. Furthermore, parkin may also play a role in sporadic PD, given that it is present in Lewy bodies from sporadic PD patients (11,12).
Parkin functions as an E3 ubiquitin ligase (13), and inactivation of its catalytic activity may lead to dopaminergic cell death due to the accumulation of toxic substrate protein(s). Recent studies suggest that changes in parkin solubility comprise a major mechanism of parkin inactivation both in familial and sporadic PD. For example, a wide range of pathogenic parkin point mutations result in decreased parkin solubility and promote its aggregation (14–16). In addition, an array of oxidative stressors (17), as well as direct post-translational parkin modifications, including dopamine modification (18) or S-nitrosylation (19,20), lead to dramatic changes in the solubility of parkin, thereby highlighting a mechanism for parkin dysfunction in the pathogenesis of idiopathic PD.
Protein phosphorylation is another post-translational modification which has recently been linked to mechanism(s) underlying PD (e.g. 21). As protein kinases are tractable drug targets, these findings may help in the design of novel therapeutic strategies. Parkin has been previously described to be subject to phosphorylation by casein kinase I or by cyclin-dependent kinase 5 (cdk5), with modest changes in its enzymatic E3 ubiquitin ligase activity in either case (22,23). Given that compound or hyperphosphorylation of proteins can have profound effects on their aggregative properties, we sought to determine whether compound phosphorylation of parkin may modulate its aggregative properties. We hypothesized that such phosphorylation-induced changes could contribute directly to the inactivation of parkin and concomitantly reduced survival of dopaminergic neurons in PD.
RESULTS
Compound phosphorylation of parkin in vitro and in cells
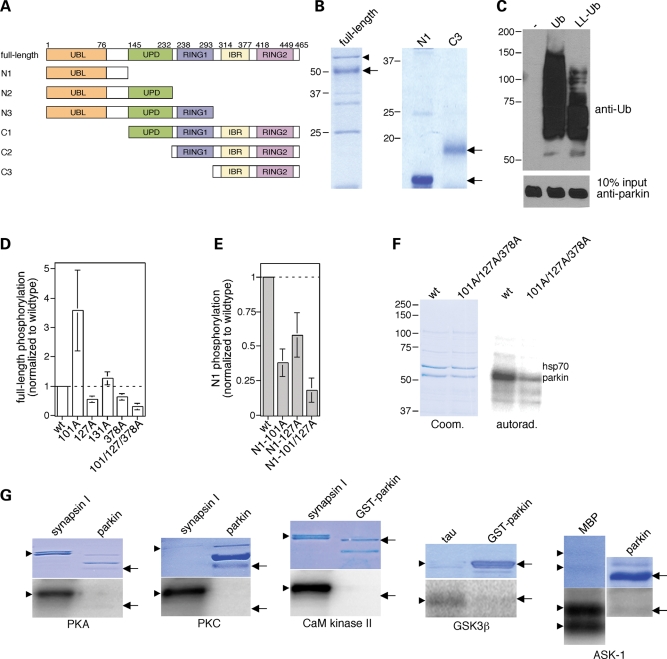
We first carried out in vitro phosphorylation experiments using a set of purified protein kinases and recombinant full-length human parkin protein, or select domains thereof (Fig. 1A and B). Purified full-length parkin displayed both mono- and polyubiquitylation activity in vitro (Fig. 1C) and was phosphorylated by casein kinase I (Fig. 1D–F), as previously reported (22). Site-directed mutagenesis using full-length parkin as well as parkin fragments confirmed that S101 and S378 were phosphorylation sites for casein kinase I (22), and an additional site was identified as S127 (Fig. 1D and E). Indeed, mutations of these three serine residues to alanines almost completely abrogated phosphorylation, indicating that these sites are the major phosphorylation sites for casein kinase I in vitro (Fig. 1D and F). Parkin was not an in vitro substrate for a series of other protein kinases analyzed here (Fig. 1G), indicating that only certain signal transduction cascades may impact upon parkin function in vivo.
Figure 1.
Parkin phosphorylation by casein kinase I in vitro. (A) Schematic representation of parkin domain structure, with domain boundaries shown by amino acid residue numbers above, and of recombinant parkin domain combinations (full-length, N1–N3 and C1–C3) analyzed by in vitro phosphorylation assays. (B) The different recombinant parkin domains were purified as described in Materials and Methods, and analyzed for purity by SDS–PAGE and Coomassie staining. Full-length recombinant parkin as well as N1 and C3 truncated forms are indicated by arrows. Bacterial hsp70 (arrowhead), as determined by mass spectroscopy, co-purified with full-length parkin. (C) Full-length recombinant parkin was catalytically active, as assessed by in vitro autoubiquitylation in the presence of wild-type ubiquitin (Ub) or a lysine-less derivative (LL-Ub) lacking the conjugation sites necessary for polyubiquitylation. When the reaction was performed with LL-Ub, lower levels of ubiquitylation were detected, indicating that recombinant parkin displays both multiple monoubiquitylation as well as polyubiquitylation activity towards itself. (D) Full-length recombinant parkin (wt), or equal amounts of the indicated point-mutated versions (S101A, n = 4; S127A, n = 4; S131A, n = 4; S378A, n = 4; S101A/S127A/S378A, n = 3), were subjected to in vitro phosphorylation reactions using casein kinase I, and phosphate incorporation quantified by using a PhosphorImager (mean±S.E.M.). Note that the S101A mutant displayed increased phosphate incorporation in the context of the full-length protein, possibly reflecting conformational effects. (E) The N1 fragment of recombinant parkin (wt) or equal amounts of the indicated point-mutated versions (S101A, n = 6; S127A, n = 4; S101A/S127A, n = 2) were subjected to in vitro phosphorylation reactions using casein kinase I, and phosphate incorporation quantified by using a PhosphorImager (mean±S.E.M.). (F) Example of a phosphorylation experiment using full-length recombinant parkin (wt) or the triple mutant S101A/S127A/S378A. (G) Parkin phosphorylation by additional protein kinases in vitro. Full-length recombinant parkin or glutathione S-transferase-parkin (arrows) was subjected to in vitro phosphorylation reactions using cAMP-dependent protein kinase (PKA), PKC, Ca2+/calmodulin-dependent protein kinase II (CaM kinase II), GSK3β or ASK-1, as indicated, using various proteins as positive controls (synapsin, tau or MBP, respectively) (arrowheads). None of these kinases significantly phosphorylated parkin in vitro.
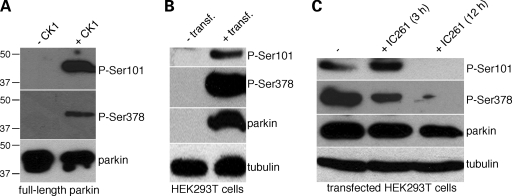
To assess phosphorylation of parkin in cells, we generated phospho-state-specific antibodies against phospho-S101 and phospho-S378. Their phosphorylation-state-specificity was confirmed using recombinant, phosphorylated parkin protein in vitro. The antibodies only detected the protein when previously phosphorylated by casein kinase I, but not its non-phosphorylated form (Fig. 2A). Constitutive phosphorylation of parkin on both S101 and S378 was detected when HEK293T cells were transiently transfected with human parkin (Fig. 2B). Upon treatment of transfected cells with IC261, a selective inhibitor of casein kinase I (24,25), a significant decrease in the phosphorylation state of both S101 and S378 of parkin was detected (Fig. 2C). Similarly, treatment of transfected cells with 35 µm D4476 (26), a distinct, structurally non-related and potent inhibitor of casein kinase I led to a significant decrease in parkin phosphorylation (by 44 ± 10%, mean±S.E.M., n = 3) in the absence of measurable cytotoxicity. Together, these data indicate that S101 and S378 of parkin are phosphorylated by casein kinase I in intact cells as well.
Figure 2.
Parkin phosphorylation by casein kinase I in cells assessed using phospho-state-specific antibodies. (A) Phospho-state-specific antibodies against phospho-S101 (P-Ser101) or against phospho-S378 (P-Ser378) recognize recombinant full-length parkin only upon its in vitro phosphorylation by casein kinase I (+CK1), but not if the protein is not previously subjected to phosphorylation (−CK1). (B) Exogenous human parkin, upon transfection in HEK293T cells (+transf.), is constitutively phosphorylated on both S101 and S378 residues. Cells were treated for 1 h with 500 nm okadaic acid before cell lysis to stabilize the phosphorylation state of parkin. (C) Exogenous human parkin phosphorylation on S101 and S378 decreases upon treatment of HEK293T cells with IC261 (50 µm), a specific inhibitor of casein kinase I.
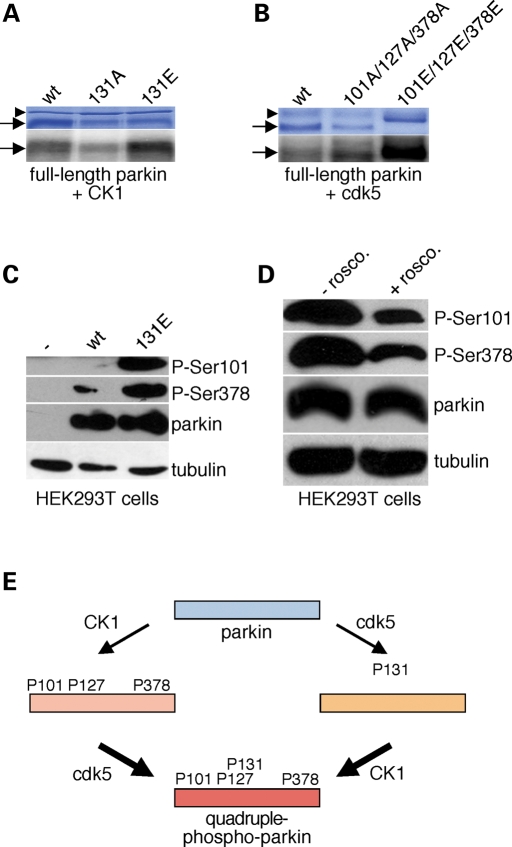
Apart from casein kinase I, parkin is also subject to phosphorylation by cdk5 on S131 (23). A phospho-mimetic mutant on this site displayed enhanced casein kinase I-mediated phosphorylation when compared with wild-type in vitro (307 ± 12%; mean±S.E.M., n = 5) (Fig. 3A). Conversely, a phospho-mimetic mutant on all three casein kinase I sites served as a better substrate for cdk5 when compared with wild-type parkin in vitro (540 ± 240%; mean±S.E.M., n = 3) (Fig. 3B). Similar findings were observed in parkin-transfected HEK293T cells using phospho-state-specific antibodies. On the one hand, an S131E parkin mutant, which mimicks constitutive phosphorylation by cdk5, displayed enhanced phosphorylation by casein kinase I when compared with wild-type parkin (Fig. 3C). On the other hand, blocking the activity of cdk5 by treating transfected HEK293T cells with roscovitine, a highly specific inhibitor for cdk5 (27), decreased phosphorylation on the casein kinase I sites of parkin (to 47 ± 10%; mean±S.E.M., n = 4) (Fig. 3D). Together, these data indicate that phosphorylation of parkin by cdk5 enhances its propensity to serve as a substrate for casein kinase I and vice versa (Fig. 3E).
Figure 3.
Compound phosphorylation of parkin in vitro and in cells. (A) Example of an in vitro phosphorylation experiment using full-length recombinant parkin (wt), or the indicated point-mutated versions (S131A, S131E), and casein kinase I (CK1). (B) Example of an in vitro phosphorylation experiment using full-length recombinant parkin (wt), or the indicated point-mutated versions (S101A/S127A/S378A, S101E/S127E/S378E), and cdk5. Arrows, full-length parkin and mutant versions thereof; arrowhead, bacterial hsp70. (C) HEK293T cells were transfected with either wild-type (wt) or S131E-mutant parkin, and equal amounts of cell extracts analyzed for parkin phosphorylation using phospho-state-specific antibodies. (D) Parkin-transfected HEK293T cells were treated with or without roscovitine (1 µm) for 12 h, and equal amounts of cell extracts analyzed for parkin phosphorylation using phospho-state-specific antibodies. (E) Schematic model depicting how parkin phosphorylation by one kinase may increase its propensity to serve as a substrate for the other kinase, thereby leading to a multiple-phosphorylated state.
Effects of parkin phosphorylation on activity and inclusion formation
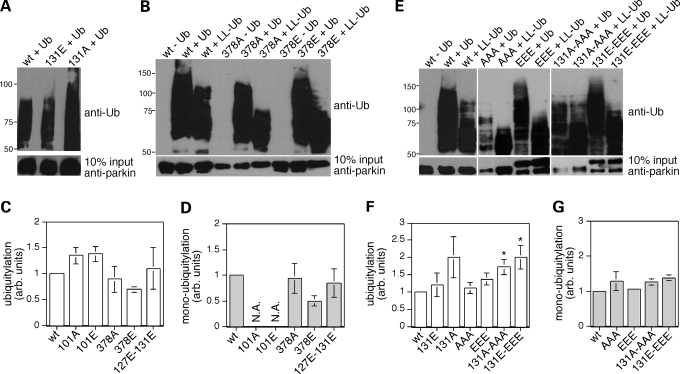
Phosphorylation of parkin may regulate its E3 ubiquitin ligase activity or modulate its insolubility, with downstream effects on dopaminergic cell survival in either case. To determine whether phosphorylation of parkin would affect its catalytic activity, we performed autoubiquitylation assays in vitro using various phospho-mimetic mutants (Fig. 4). A parkin phospho-mimetic mutant for cdk5 phosphorylation (131E) displayed ubiquitylation activity similar to wild-type, whereas its non-phosphorylatable counterpart (131A) was slightly more active, as previously described (23) (Fig. 4A). Parkin phospho-mimetic mutants for the individual casein kinase I sites (101E or 378E) displayed ubiquitylation activity similar to wild-type (Fig. 4B–D). Similarly, a phospho-mimetic mutant for all three casein kinase I sites (101E/127E/378E) did not display significant changes in ubiquitylation activity (Fig. 4E–G), indicating that neither mimicking individual nor combined phosphorylation of the casein kinase I sites affect parkin's ubiquitylation activity. A phospho-mimetic mutant for the adjacent casein kinase I and cdk5 sites (127E/131E) did not display changes in activity (Fig. 4C and D), whereas a phospho-mimetic mutant for compound phosphorylation by both casein kinase I and cdk5 (101E/127E/131E/378E) displayed slightly enhanced autoubiquitylation activity (Fig. 4E–G). However, this modest enhancement was also observed for the respective non-phosphorylatable mutant counterpart (101A/127A/131A/378A), and therefore does not seem to reflect specific effects related to parkin phosphorylation (Fig. 4E–G).
Figure 4.
Phosphomimetic parkin mutants do not display drastic changes in E3 ligase activity. (A) Example of an autoubiquitylation experiment using wild-type, 131A-mutant or 131E-mutant parkin forms, displaying enhanced activity of the 131A-mutant. (B) Example of an autoubiquitylation experiment using wild-type, 378A-mutant or 378E-mutant parkin forms and either using ubiquitin (Ub) or a lysine-less derivative (LL-Ub) to assess polyubiquitylation versus monoubiquitylation activity, respectively. (C) Quantification of experiments of the type depicted in (B), indicating that the individual mutants do not lead to significant changes in the ubiquitylation activity of parkin. The ubiquitin-positive ladder obtained with various parkin-mutant proteins and wild-type ubiquitin was quantified, values were corrected for differences in protein input as analyzed separately using an anti-parkin antibody (844) and normalized to the activity of wild-type parkin. Bars depict mean±S.E.M. (D) Quantification of experiments of the type depicted in (B), indicating that the individual mutants do not lead to significant changes in the monoubiquitylation activity of parkin. The ubiquitin-positive ladder obtained with various parkin-mutant proteins and lysine-less ubiquitin, reflecting multiple mono-ubiquitylation activity, was quantified, corrected for differences in protein input and normalized to the activity of wild-type parkin. Bars depict mean±S.E.M. N.A., not analyzed. (E) Example of an autoubiquitylation experiment using wild-type or the indicated parkin mutants (AAA, 101A/127A/378A; EEE, 101E/127E/378E; 131A-AAA, 101A/127A/131A/378A; 131E-EEE, 101E/127E/131E/378E), and either wild-type ubiquitin (Ub) of a lysine-less derivative (LL-Ub) to assess monoubiquitylation activity. (F) The ubiquitin-positive ladder obtained with various parkin-mutant proteins (131E, n = 3; 131A, n = 2; AAA, n = 3; EEE, n = 3; 131A-AAA, n = 4; 131E-EEE, n = 4) and wild-type ubiquitin was quantified. Values were corrected for differences in protein input as analyzed separately using an anti-parkin antibody (844), and normalized to the activity of wild-type parkin. Bars depict mean±S.E.M. *P < 0.05. (G) The ubiquitin-positive ladder obtained with various parkin mutant proteins (AAA, n = 3; EEE, n = 3; 131A-AAA, n = 4; 131E-EEE, n = 4) and lysine-less ubiquitin, reflecting multiple mono-ubiquitylation activity, was quantified, corrected for differences in protein input and normalized to the activity of wild-type parkin. Bars depict mean±S.E.M. Error bars are only depicted when larger than column lines. The 131A-AAA and 131E-EEE mutants displayed a significant increase in autoubiquitylation activity only as assessed using ubiquitin, but not lysine-less ubiquitin.
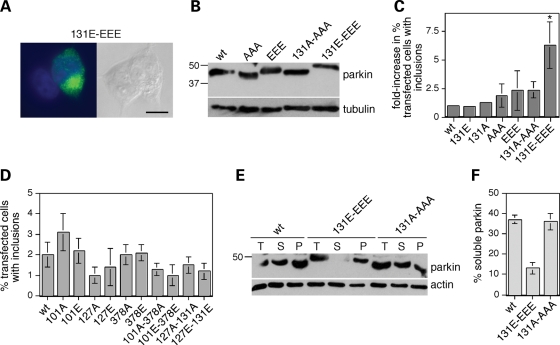
We next addressed whether parkin phosphorylation may modulate its tendency to form inclusions in cell culture and concomitantly alter its solubility in detergent. For this purpose, HEK293T cells were transfected with parkin or various phospho-mimetic or non-phosphorylatable mutants, treated with the proteasome inhibitor MG-132, and the amount of parkin inclusion bodies determined by immunocytochemistry (Fig. 5A). Wild-type and all mutant parkin proteins were overexpressed to similar degrees (Fig. 5B), and the parkin mutant mimicking phosphorylation by only casein kinase I (101E/127E/378E) or by only cdk5 (131E) did not show a significant increase in the number of parkin inclusion bodies when compared with wild-type (Fig. 5C). These results indicate that individually mimicking phosphorylation by either casein kinase I or by cdk5 does not lead to changes in parkin aggregation. In contrast, a pronounced, 6.2 ± 2-fold increase (mean±S.E.M., n = 4) in cells with parkin inclusions was found with the parkin mutant mimicking compound phosphorylation by both protein kinases (101E/127E/131E/378E) (Fig. 5C). Such enhanced aggregation was not observed with a non-phosphorylatable mutant counterpart (101A/127A/131A/378A) (Fig. 5C) and was only observed when mimicking phosphorylation on all four serine residues (Fig. 5D), indicating that it was specific for mimicking compound phosphorylation of parkin by both casein kinase I and cdk5.
Figure 5.
Phospho-mimetic parkin mutants display profound changes in aggregative properties. (A) HEK293T cells were transfected with 101E/127E/131E/378E-mutant parkin, and treated with 5 µm MG-132 for 12 h prior to immunocytochemistry using a polyclonal anti-parkin antibody (844) (green). Nuclei were stained with DAPI. Scale bar, 10 µm. (B) To assure equal levels of overexpression of wild-type and mutant parkin forms, cells were transfected with the various forms as indicated and 50 µg of cell extracts were subjected to western blot analysis 72 h later using an anti-parkin antibody (Abcam) or an anti-tubulin antibody to correct for differences in protein loading. Wild-type and mutant parkin constructs were overexpressed to similar degrees. (C) HEK293T cells were transfected with wild-type parkin or various mutant forms, treated with 5 µm MG-132 for 12 h followed by immunocytochemistry as described above. The percent of transfected cells displaying large perinuclear inclusions was determined by an investigator unaware of treatment groups, and the data plotted as an overall fold-increase in the number of transfected cells displaying large perinuclear inclusions when compared with wild-type parkin-expressing cells (131E; n = 3; 131A; n = 3; AAA, n = 3; EEE, n = 4; 131A-AAA, n = 4; 131E-EEE; n = 4). Bars depict mean±S.E.M. *P < 0.05. Error bars are only depicted when larger than column lines. (D) HEK293T cells were transfected with wild-type parkin or various mutant forms, treated with 5 µm MG-132 for 12 h followed by immunocytochemistry. None of the combinations analyzed led to significant changes in the number of transfected cells with inclusions, indicating that only the quadruple mutant parkin-variant mimicking compound phosphorylation by casein kinase I and cdk5 displays an increased tendency to aggregate. Bars depict mean±S.E.M. (n = 3). (E) Analysis of the distribution of overexpressed wild-type (wt) or mutant parkin variants (treated with 5 µm MG-132 for 12 h before analysis) (total, T) in the Triton X-100-soluble (S) and insoluble pellet (P) fractions of HEK293T extracts upon blotting with an anti-parkin antibody (Abcam) or an anti-actin antibody. (F) Quantification of experiments of the type depicted in (E), where the amount of protein in the supernatant, when compared with that in the pellet, is plotted as % soluble parkin. Bars depict mean±S.E.M. (n = 3).
An increased propensity of parkin to be confined to intracellular aggregates should result in changes in its detergent extractability, as previously described for several pathogenic parkin mutants and stress-induced parkin alterations (14–18). Indeed, although a significant amount of wild-type parkin was found in the Triton-soluble fraction in the presence of MG-132, the quadruple mutant mimicking compound phosphorylation displayed decreased solubility (Fig. 5E and F). In contrast, no change in detergent extractability was observed for the non-phosphorylatable mutant counterpart, which was present in the soluble fraction to a degree similar to wild-type parkin (Fig. 5E and F). Together, these data suggest that simultaneous phosphorylation of parkin on both casein kinase I and cdk5 sites profoundly facilitates its aggregation into inclusion bodies.
Increased phospho-parkin in Parkinson brain
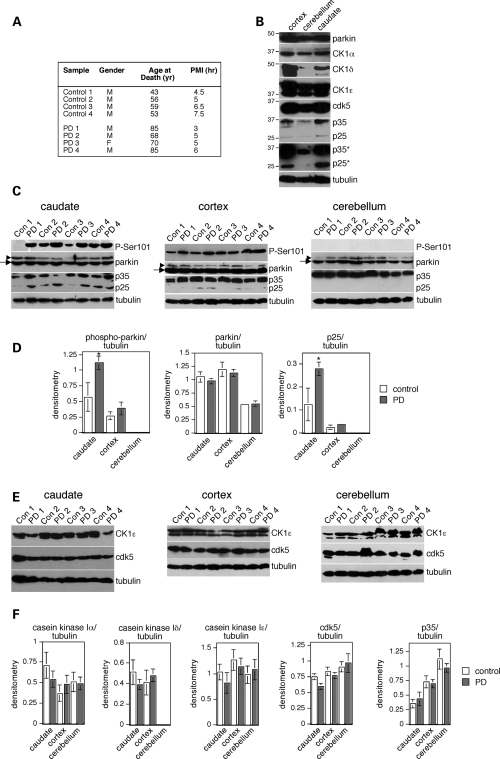
To determine whether parkin undergoes phosphorylation in human brain, and in sporadic PD, we chose to analyze parkin and phospho-parkin levels from distinct brain areas reported to be differentially affected by disease pathology (28). Samples from three distinct brain areas were available from each individual patient, and control and PD cases matched for postmortem interval, tissue-handling and storage conditions as important variables in these comparisons (Fig. 6A). The levels of total parkin were similar in caudate, cortex and cerebellum (Fig. 6B), and there were no differences in the levels of total parkin between control and PD cases in any of the three brain regions analyzed (Fig. 6C and D), as previously described for cortical samples (29). However, we found statistically significant increases in phospho-Ser101 parkin levels in PD versus control cases (Fig. 6C and D). These increases were detected in the caudate, known to be particularly affected by the presence of Lewy bodies and Lewy neurites during relatively early stages of disease development (28). No significant changes in phospho-parkin levels in PD versus control cases were observed in the cortex, and no phospho-parkin could be detected in the cerebellum (Fig. 6C and D), a brain area largely devoid of Lewy bodies and Lewy neurites (27). Together, these data demonstrate a neuroanatomical and disease-specific alteration in the phosphorylation status of parkin.
Figure 6.
Selective increase in phospho-parkin and p25 in distinct areas of idiopathic PD brains. (A) Gender, age at death (years) and postmortem interval (PMI, h) for the eight human brains analyzed here. (B) Relative levels of total parkin, casein kinase I isoforms α, δ and ε, cdk5, p35 and p25 in three distinct brain areas from a control patient, and of tubulin as a protein loading control. *Overexposed blot shows the absence of detectable p25 levels in the cerebellum. (C) Levels of phospho-parkin (P-Ser101), parkin, p35/p25 and tubulin as a protein loading control were analyzed in caudate, cortex and cerebellum from four control and four PD patients which were matched for postmortem interval tissue collection. Arrows indicate parkin band, arrowheads indicate cross-reacting band detected with the anti-parkin antibody (Abcam). (D) Levels of phospho-parkin, parkin and p25 were normalized to tubulin levels and plotted on a histogram (n = 4; mean±S.E.M., *P < 0.05). Error bars are only depicted when larger than column lines. (E) Levels of casein kinase Iε (CK1ε), cdk5 and tubulin as a protein loading control were analyzed in caudate, cortex and cerebellum from four controls and four PD patients matched for postmortem interval tissue collection. (F) Levels of CK1α, δ and ε, cdk5 and p35 were normalized to tubulin levels and plotted on a histogram (n = 4; mean±S.E.M.). Error bars are only depicted when larger than column lines.
As a first step towards identifying the possible underlying mechanism(s) for the differences in phospho-parkin levels in PD when compared with control brains, we analyzed the total levels of casein kinase I α, δ and ε, as well as of cdk5 and its activator p25/p35. Similar levels of the α and ε isoforms of casein kinase I were detected in all three brain areas, whereas the levels of the δ isoform were lower in the cerebellum when compared with caudate and cortex, as previously described (30) (Fig. 6B). Similar levels of cdk5 were detected in all three brain areas, but the levels of p35 were lower in the cerebellum when compared with caudate and cortex, as previously described in rodent brain (31), and no p25 could be detected in the cerebellum (Fig. 6B). No differences were observed in the levels of the three casein kinase isoforms or of cdk5 in PD versus control in any of the three brain areas analyzed (Fig. 6E and F). However, the total levels of p25 were significantly higher in PD when compared with control samples in the caudate, with no changes in the cortex (Fig. 6C and D). Thus, the observed increase in phospho-parkin levels in PD when compared with control may, at least in part, be due to changes in the total levels of p25 in the distinct brain areas analyzed, with concomitant changes in cdk5 activity followed by compound parkin phosphorylation and aggregation.
Modulation of mutant parkin aggregation by a combination of kinase inhibitors
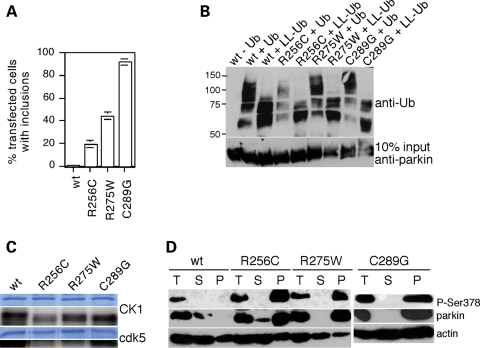
As increased parkin phosphorylation seems to correlate with sporadic PD in vivo and enhances the aggregative properties of parkin in cultured cells, inhibiting casein kinase I and cdk5 activities may have beneficial effects in preventing such protein aggregation. To address this possibility, we quantified the number of aggregates in cells transfected with three pathogenic parkin mutants (R256C, R275W, C289G) previously described to display enhanced aggregative properties when compared with wild-type parkin (14–16). Indeed, all three mutants showed an enhanced propensity to form intracellular aggregates in the absence of proteasome inhibition, with C289G>R275W>R256C (Fig. 7A). These pathogenic mutants did not display significant differences in their autoubiquitylation activity (Fig. 7B) and were subject to phosphorylation by casein kinase I and cdk5 in vitro (Fig. 7C), indicating that their major pathogenic mechanism of action seems to involve enhanced aggregation. Concomitant with their reduced detergent extractability, we observed that they were present in the Triton-insoluble fraction in a heavily phosphorylated manner (Fig. 7D). Therefore, we next studied whether inhibiting casein kinase I and cdk5 activities may modulate the aggregative properties of these mutant proteins.
Figure 7.
Analysis of pathogenic parkin mutants. (A) The percentage of transfected cells displaying parkin aggregates using wild-type or various pathogenic parkin point mutants (wt, n = 5; R256C, n = 6; R275W, n = 11; C289G, n = 10). Bars depict mean±S.E.M. (B) Example of an autoubiquitylation experiment using wild-type or the indicated parkin mutants, and either wild-type ubiquitin (Ub) or a lysine-less derivative (LL-Ub). (C) Example of in vitro phosphorylation experiments using full-length recombinant parkin (wt), or the indicated point-mutated versions, and either casein kinase I (CK1, top) or cdk5 (bottom). (D) Analysis of the distribution of overexpressed wild-type (wt) or the various mutant parkin proteins (total, T) in the Triton X-100-soluble (S) and insoluble pellet (P) fractions of HEK293T extracts upon blotting with a phospho-state-specific parkin antibody (P-Ser378), an anti-parkin antibody (Abcam) or an anti-actin antibody. Note that the phosphorylation status of wild-type parkin in the soluble and insoluble fractions could only be detected in the presence of okadaic acid or upon prolonged exposure of the blot (not shown).
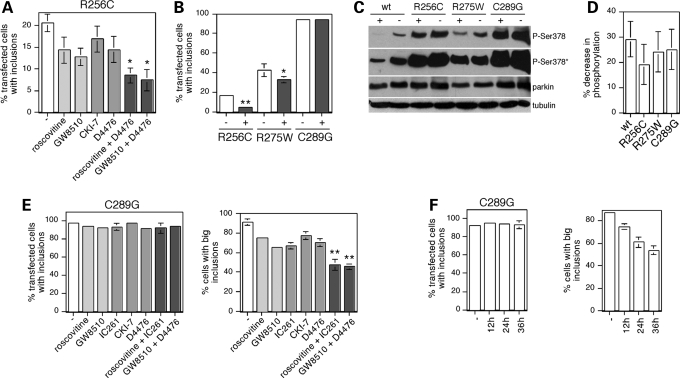
Cells were transfected with the aggregative R256C mutant and analyzed 72 h after transfection. Around 20% of transfected cells displayed large perinuclear inclusions, and treatment of cells during the last 12 h using two structurally dissimilar cdk5-specific inhibitors (roscovitine and GW8510 (32)), or two distinct casein kinase I-specific inhibitors (CKI-7 (24,25)) and D4476 (25,26)) profoundly decreased the number of cells with R256C-mutant parkin aggregates (Fig. 8A). Statistically significant additive effects in decreasing the number of cells with aggregates were observed when using combinations of a cdk5-specific and a casein kinase I-specific inhibitor (Fig. 8A), further indicating that the effects were specific for inhibiting the activity of these two protein kinases. The extent of the additive effect was relatively small, in agreement with the observed crosstalk between casein kinase I and cdk5 phosphorylation of parkin observed in vitro (Fig. 3). Compound inhibition of cdk5 and casein kinase I activities resulted in a roughly 70% decrease in the number of cells displaying aggregates for the R256C mutant, when compared with a 25% decrease for the R275W mutant, with no effects observed for the most aggregative C289G mutant (Fig. 8B). Addition of a casein kinase I-specific and a cdk5-specific inhibitor decreased the phosphorylation status of the three pathogenic parkin mutants analyzed to a similar degree (Fig. 8C and D), indicating that the relative efficiency by which compound kinase inhibition affects aggregation of the respective mutants is likely due to the observed distinct inherent aggregative properties of the mutants (Fig. 7A).
Figure 8.
Inhibition of parkin phosphorylation decreases mutant parkin aggregation. (A) The percentage of transfected cells displaying R256C-mutant parkin aggregates were quantified in the absence (−) or presence of various kinase inhibitors, or combinations thereof, present for 12 h before fixation. Inhibitors used included roscovitine (0.5 µm), GW8510 (0.25 µm), CKI-7 (150 µm) and D4476 (17.5 µm). Bars depict mean±S.E.M. (n = 3). Statistically significant additive effects were observed when using a combination of kinase inhibitors, when compared with individual inhibitor treatments. *P < 0.1. (B) Quantification of the percentage of transfected cells displaying R256C-mutant, R275W-mutant or C289G-mutant parkin aggregates in the absence (−) or presence (+) of 0.25 µm GW8510 and 17.5 µm D4476 (R256C, n = 5; R275W, n = 3; C289G, n = 4). Bars depict mean±S.E.M. *P < 0.1; **P < 0.01. Error bars are only depicted when larger than column lines. (C) Analysis of the phosphorylation status of parkin in HEK293T cells transfected with wild-type (wt) or mutant parkin variants, treated without (−) or with (+) 0.25 µm GW8510 and 17.5 µm D4476 for 12 h before analysis upon blotting with the phospho-Ser378 antibody (*overexposed blot to show phosphorylation of wild-type parkin), an anti-parkin antibody (Abcam) or an anti-tubulin antibody. (D) Quantification of experiments of the type depicted in (C), where the amount of phospho-parkin in the presence of inhibitors, when compared with that in the absence of inhibitors, is plotted as % decrease in phosphorylation. Bars depict mean±S.E.M. (n = 3). (E) The percentage of transfected cells displaying C289G-mutant parkin aggregates (left) and the percentage of those cells displaying (at least) one large perinuclear aggregate (right) were quantified in the absence (−) or presence of various kinase inhibitors, or combinations thereof, present for 12 h before fixation. Inhibitors used included roscovitine (0.5 µm), GW8510 (0.25 µm), IC261 (25 µm), CKI-7 (150 µm) and D4476 (17.5 µm). Bars depict mean±S.E.M. (n = 3). Statistically significant additive effects were observed when using a combination of kinase inhibitors, when compared with individual inhibitor treatments. **P < 0.05. (F) The percentage of transfected cells displaying C289G-mutant parkin aggregates (left) and the percentage of those cells displaying (at least) one large perinuclear aggregate (right) were quantified in the absence (−) or presence of 0.5 µm roscovitine and 150 µm CKI-7. Inhibitors were added 12 h before fixation (12 h), 24 and 12 h before fixation (24 h) or 36, 24 and 12 h before fixation (36 h).
As the C289G-parkin mutant accumulated in perinuclear aggregates in virtually all transfected cells (Fig. 8B), we reasoned that the effects of inhibiting parkin phosphorylation may be best studied by analyzing the size and number of aggregates for this potent aggregative parkin mutant. Indeed, although treatment of transfected cells during the last 12 h using distinct cdk5 inhibitors, casein kinase I inhibitors or a combination thereof did not decrease the number of cells with mutant parkin inclusions, we observed a decrease in the number of cells displaying a large perinuclear aggregate (Fig. 8E). The effects of individual inhibitor treatment were significant on its own, and statistically significant additive effects were observed when using a combination of both inhibitors, with a nearly 50% reduction in the number of cells with large perinuclear aggregates (Fig. 8E). In addition, prolonged and repetitive incubation of cells with a mixture of a cdk5-specific and a casein kinase I-specific inhibitor resulted in additive beneficial effects in decreasing the number of cells with large C289G-mutant parkin aggregates with time (Fig. 8F). Together, these data show that inhibiting compound phosphorylation of parkin can regulate its aggregative properties in culture, indicating that such treatment is useful in maintaining parkin's protective function in cultured cells.
DISCUSSION
Here, we report that parkin, a protein essential for the sustained survival of dopaminergic neurons, is subject to compound phosphorylation in vitro and in cultured cells. Such compound phosphorylation by casein kinase I and cdk5 enhances its insolubility, leading to aggregation and concomitant inactivation. Although phosphorylation does not change parkin's E3 ubiquitin ligase activity, increased aggregation effectively decreases the amount of soluble parkin protein able to exert a neuroprotective role (33). Notably, only simultaneous, but not individual phosphorylation by either casein kinase I or cdk5 seems to alter parkin solubility. Moreover, phosphorylation of parkin by one kinase seems to enhance its susceptibility to serve as a substrate for the other kinase. In this manner, simultaneous activation of both kinases may cooperatively contribute towards parkin inactivation.
To assess whether parkin phosphorylation may occur in humans, we analyzed samples from caudate nucleus, cortex and cerebellum of control and PD brains. We observed enhanced parkin phosphorylation in PD when compared with control in the caudate, with no changes in the cortex. Of note, parkin phosphorylation could not be detected in the cerebellum in either control or PD samples. The neuroanatomical differences in parkin phosphorylation between control and sporadic PD samples correlate with the relative extent to which these distinct brain areas are affected by disease pathology (28), indicating that abnormal parkin phosphorylation and concomitant inactivation may contribute to neuronal degeneration in sporadic PD.
We further examined whether the increase in parkin phosphorylation in PD brains may correlate with increases in the levels of cdk5, its activator p25/p35 or casein kinase I. Although we observed no changes in the levels of cdk5 or the levels of distinct casein kinase isoforms, we did find an increase in the levels of p25 in the caudate in PD when compared with control samples, with no changes in the cortex and the absence of detectable p25 in the cerebellum. In this context, it is interesting to note that p25 is known to lead to prolonged activation of cdk5 (34) and that cdk5 and p35 have been reported to accumulate in Lewy bodies in postmortem PD brains (35,36). Furthermore, an increase in cdk5 levels and activity has been observed in an 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of PD (37), and various neurotoxic insults induce generation of p25 from p35 (38), indicating that the activity of cdk5 may play a key role in the pathogenesis of sporadic PD (39).
Little is known regarding de-regulation of casein kinase I activities during neurodegeneration (40), but most casein kinase I isoforms seem to be basally active (41). Our present data suggest that increased cdk5-mediated parkin phosphorylation would lead to a concomitant increase in casein kinase I-mediated phosphorylation events, as detected here using phospho-state-specific antibodies, thereby generating a compound phosphorylated protein with decreased solubility.
Finally, the generally high basal casein kinase I and cdk5 activities suggest a very active role for protein phosphatases in controlling the steady-state levels of parkin phosphorylation. Indeed, a variety of studies indicate that inhibition of phosphatase activities, with concomitant hyperphosphorylation of proteins, can lead to PD-related neurodegeneration (42,43). Thus, a complex balance between kinase and phosphatase activities, possibly in a cell-type and region-specific manner, may be subject to acute and/or chronic regulation.
To determine whether inhibition of casein kinase I and cdk5 activities would display beneficial effects in decreasing the aggregative properties of pathogenic parkin-mutant protein, we chose to analyze three distinct point mutants whose pathogenic mechanism of action seems to involve enhanced aggregation (14–16). Importantly, using a combination of inhibitors against casein kinase I and cdk5, we could decrease the number of cells displaying parkin aggregates for two out of three mutants tested (R256C and R275W). For the third point mutant (C289G), which displayed the most dramatic aggregative phenotype, combined kinase inhibition led to a decrease in the number of cells displaying large perinuclear aggregates, indicating beneficial effects of such inhibitor treatment in modulating the profound aggregation even of this pathogenic mutant.
In conclusion, our experiments indicate that regulating the phosphorylation status of parkin has beneficial effects in reducing parkin aggregation and concomitant inactivation. Although future studies will be necessary to determine the neuroprotective or beneficial effects of combined kinase inhibition for PD subjects, the present results may have additional implications for other approaches in treating this neurodegenerative disorder, including gene replacement (44,45) or transplantation therapies (46).
MATERIALS AND METHODS
Plasmid construction
Human full-length parkin was amplified by PCR (primer sequences: 5′-TTA TGA ATT CAT ATA GTG TTT GTC AGG TTC AAC-3′ and 5′-TTT AAA GCT TTT ACA CGT CGA ACC AGT GGT CCC-3′) using a full-length piTrex human parkin cDNA construct and cloned into the EcoRI/HindIII restriction sites of the pGEX-KG vector (47). Constructs encoding sequences for the different human parkin domains (N1, N2, N3, C1, C2, C3) were generated by PCR using the piTrex parkin cDNA construct as a template and subcloned into the pGEX-KG vector as described above. For expression of parkin in mammalian tissue culture cells, human full-length parkin was amplified by PCR and subcloned into the EcoRI/XbaI restriction sites of the pCMV5-SV40-hGH vector (48). Point-mutated constructs were generated using the QuickChange Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer's instructions. The entire coding sequences of all constructs used in this study were verified by DNA sequencing. The sequences of all primers used in the present study are available upon author's request.
Protein purification
All recombinant proteins were expressed as N-terminally tagged glutathione S-transferase fusion proteins in Escherichia coli BL21 DE3 cells and purified essentially as described (49) with minor modifications as outlined below. A 5 ml overnight culture of cells was diluted 200-fold and grown at 37°C to an OD600 of around 0.6. Cells were incubated at 16°C for 15 min, followed by induction with 0.1 mm IPTG for 16–20 h at 16°C. Bacteria were pelleted at 10 000g for 10 min at 4°C, and the cell pellet resuspended in 12 ml (per liter culture) of resuspension buffer (PBS containing 1% TX-100, 1 mm PMSF, 1 mm DTT, 50 µg/ml RNAse, 50 µg/ml DNAse and 100 µg/ml lysozyme). The cell resuspension was incubated for 30 min at 4°C on a rotary shaker, followed by three sonication pulses (30 s each, separated by 30 s intervals) on ice. Upon centrifugation at 16 000g for 20 min at 4°C, the soluble fraction was filtered through a 0.22 µm filter and diluted with 6 ml of PBS containing 1% TX-100, 1 mm PMSF and 1 mm DTT (per liter of culture). Proteins were bound to glutathione Sepharose beads (Pharmacia) (750 µl of packed beads per liter of culture) for 2 h at 4°C. Beads were washed two times in PBS/1% TX-100, two times in PBS and two times in thrombin elution buffer (50 mm Tris–HCl, pH 8, 150 mm NaCl, 2.5 mm CaCl2, 0.1% (v/v) β-mercaptoethanol). Pelleted beads were resuspended to a 50% slurry with thrombin elution buffer, and protein eluted with 50 units of thrombin (from bovine plasma; Sigma) per liter of culture for 1 h at 4°C with shaking. Purified proteins were dialyzed into PBS containing 1 mm DTT for 1 h at 4°C and small aliquots of recombinant parkin proteins were frozen at –20°C and only thawed up once. Protein concentration was determined by the BCA assay (Pierce) according to the manufacturer's instructions. Protein purity of all protein preparations was determined by Coomassie Blue staining, and in most cases was around 80–90%. In most cases, enzymatic activity of recombinant full-length parkin was assayed by autoubiquitylation reactions (see below) before the protein was used in other assays.
In vitro phosphorylation assays
Various recombinant wild-type and point-mutated full-length and truncated parkin proteins (1 µg) were subjected to in vitro phosphorylation reactions using buffer and enzyme conditions as outlined below. Phosphorylation by casein kinase I (0.5 units recombinant rat casein kinase Iδ; New England Biolabs) was performed in 50 mm Tris–HCl, 5 mm DTT, 10 mm MgCl2, pH 7.5, using 20 ng casein as a positive control. Phosphorylation by cyclin-dependent protein kinase 5 (cdk5) (20 ng of human full-length cdk5/p25; Upstate) was performed in 8 mm MOPS/NaOH, 0.2 µM EDTA, 10 mm Mg-acetate, pH 7.0, using 500 ng of histone H1 as a positive control. Phosphorylation by casein kinase II (1 µg recombinant human casein kinase II; New England Biolabs) was performed in 20 mm Tris–HCl, 50 mm KCl, 10 mm MgCl2, pH 7.5, using 20 ng of casein as a positive control. Phosphorylation by cAMP-dependent protein kinase A (PKA) (2.5 units of recombinant murine PKA; New England Biolabs) was performed in 50 mm Tris–HCl, 10 mm MgCl2, pH 7.5, using 1 µg of purified bovine synapsin I as a positive control. Phosphorylation by glycogen synthase kinase 3β (GSK3β) (1 µg of recombinant human his-tagged GSK3β; Calbiochem) was performed in 25 mm Tris–HCl, pH 7.5, 5 mm β-glycerolphosphate, 12 mm MgCl2, 2 mm DTT, 100 µM Na3VO4 using 100 ng of recombinant human tau protein (Calbiochem) as a positive control. Phosphorylation by 1 µg of protein kinase C (PKC) was performed in 50 mm HEPES, pH 7.4, 10 mm Mg-acetate, 1 mm EGTA, 1 mm EDTA, 1.5 mm CaCl2, 1 mm DTT, 50 µg/ml phosphatidylserine and 4 µg/ml diacylglycerol using 1 µg of bovine synapsin I as a positive control. For phosphorylation by Ca2+/calmodulin-dependent protein kinase II (CaMKII), 2.5 units of recombinant rat CaMKII (New England Biolabs) were activated for 10 min at 30°C in a buffer containing 50 mm Tris–HCl, pH 7.5, 10 mm MgCl2, 2 mm DTT, 0.1 mm EDTA, 100 µM ATP, 1.2 µM calmodulin and 2 mm CaCl2. One microgram of recombinant parkin was diluted in the same buffer and supplemented with 200 µM ATP, and 1 µg of bovine synapsin I served as a positive control. Phosphorylation by apoptosis signal-regulating kinase 1 (ASK1) (150 ng or recombinant human ASK1; Upstate) was performed in 8 mm MOPS/NaOH, 0.2 µM EDTA, 10 mm Mg-acetate, pH 7.0, using 1 µg of mouse myelin basic protein (MBP) (Sigma) as a positive control.
Unless otherwise stated, all reactions were carried out in a final volume of 40 µl and were initiated by the addition of ATP (final concentration of 100 µm) with trace amounts of [γ−32P]ATP (GEHealthcare, specific activity 150 mCi/ml). After 30 min at 30°C with shaking (450 rpm), reactions were terminated by the addition of 0.2 volumes of 5× sample buffer and boiling for 5 min at 95°C. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) on 10% polyacrylamide gels (or 12.5% gels for assays containing N1-parkin or C3-parkin domains), followed by staining with Coomassie blue dye. Incorporation of 32P was quantified using a PhosphorImager (Molecular Dynamics) and corrected for background values. Differences in protein amounts were quantified on Coomassie-stained gels using QuantityOne (Bio-Rad) and corrected for background values, and radioactivity values were corrected for differences in protein loading.
Autoubiquitylation reactions
Recombinant full-length parkin (1 µg) was incubated with 80–150 ng of E1 enzyme (His-E1, Biomol), 2 µg of E2 enzyme (His-UbcH7, Biomol) and 5 µg of ubiquitin (Sigma), or lysine-less or methylated ubiquitin (BostonBiochem), in a buffer containing 50 mm Tris–HCl, pH 7.4, 2 mm ATP, 1 mm DTT and 5 mm MgCl2. The reactions were conducted at 30°C for 90 min with shaking (450 rpm) and terminated by adding 0.2 volumes of 5× sample buffer and heating for 5 min at 95°C. Samples were resolved in 7.5% SDS–PAGE gels, followed by immunoblotting using a monoclonal anti-ubiquitin antibody (clone 6C1, Sigma) at 1:1000 dilution overnight at 4°C, and equal amount of protein loading was assured by immunoblotting samples independently with an anti-parkin antibody (844) at 1:1000 dilution overnight at 4°C.
Generation of anti-parkin and anti-phospho-parkin antibodies
Antibodies against parkin and phospho-parkin were generated in collaboration with PhosphoSolutions, Aurora, CO, USA. Briefly, antibodies were generated in rabbits against human parkin (around amino acids 400) (844) and against chemically phosphorylated S101 (EREPQS(phospho)LTRVDL) and S378 (GEGECS(phospho)AVFEAS) peptides. Phospho-state-specific antibodies were affinity-purified via sequential chromatography on phospho- and dephospho-peptide affinity columns. The p-Ser101 antibody was generally used to detect phospho-parkin in human brain extracts, whereas the p-Ser378 antibody was preferentially used to detect phospho-parkin in transfected HEK293 cell extracts.
Cell culture, transfection and cell lysis
HEK293T cells were cultured in 100-mm dishes and grown at 37°C in 5% CO2 in growth medium (Dulbecco's modified Eagle's medium) with 4 mm glutamine and non-essential amino acids (Sigma), 10% heat-inactivated fetal bovine serum (Invitrogen), penicillin (100 units/ml) and streptomycin (100 units/ml). Confluent cells were harvested using 0.05% trypsin, 0.02 mm EDTA in PBS and subcultured at a ratio of 1:4 to 1:6. Cells at 50–60% confluency were transfected using 8 µg of plasmid of interest and 80 µl of PolyFect transfection reagent (Qiagen) per 100 mm dish according to the manufacturer's specifications for 4 h in growth medium, followed by addition of a fresh medium.
For preparation of extracts, cells were collected 48 h after transfection. Prior to collection, transfected cells were treated with 1 µm roscovitine (Calbiochem) for 12 h to inhibit endogenous cdk5 activity, with 50 µm IC261 (3-[(2,4,6-trimethoxyphenyl)methylidenyl]indolin-2-one) (Calbiochem) for either 3 or 12 h to inhibit endogenous casein kinase I activity, or with 35 µm D4476 for 12 h to inhibit endogenous casein kinase I activity. Cell viability was assessed by visual inspection as well as by Trypan blue exclusion assays. Although treatment of cells with 50 µm IC261 for 12 h caused a slight decrease in cell viability (by 3.6 ± 1.6%, mean±S.E.M., n = 3), treatment of cells with IC261 for 3 h, or treatment of cells with any other kinase inhibitor analyzed in the present study did not display any effect upon cell viability. Where indicated, cells were further treated with 0.5 µm okadaic acid (Alomone Labs) for 1 h prior to processing to inhibit endogenous phosphatase activity. Cells were rinsed once in ice-cold PBS followed by resuspension in 1–1.5 ml of lysis buffer per 100 mm dish (1% SDS in PBS containing 1 mm PMSF, 1 mm Na3VO4 and 5 mm NaF). Resuspended cells were incubated for 15 min at 4°C in a rotary shaker, sonicated twice (two pulses for 1 s each) and centrifuged at 13 500 rpm for 10 min at 4°C. Protein concentrations of supernatants were estimated using the BCA assay (Pierce), followed by the addition of 0.2 volumes of 5× sample buffer and boiling for 5 min at 95°C. Samples were directly analyzed on western blots, as freeze–thaws were found to abolish detection of phospho-parkin levels. Unless otherwise stated, 80–100 µg of total protein extracts were loaded per lane to ensure sensitive detection of phospho-parkin levels.
For sequential fractionation studies, cells were collected 72 h after transfection. Cells were rinsed once in ice-cold PBS, followed by resuspension in 4 ml ice-cold PBS and the cell suspension equally split into two tubes. After centrifugation for 5 min (1500 rpm, 4°C), pellets were resuspended in 400 µl of buffer (50 mm Tris–HCl, pH 8.0, 300 mm NaCl, 1.5 mm MgCl2, 2 µg/ml chymostatin, 100 units/ml aprotinin, 1 mm PMSF, 1 mm Na3VO4 and 5 mm NaF) either containing 1% SDS (total) or containing 1% Triton X-100. The latter tube was incubated for 10 min at 4°C on a rotary shaker, followed by centrifugation at 82 000g for 20 min at 4°C. The supernatant (Triton-soluble) was removed, and the pellet resuspended in 200 µl of buffer containing 1% SDS, followed by repetitive sonication to obtain complete solubilization (Triton-insoluble). Protein concentration was estimated by the BCA assay (Pierce), and 40 µg of the total and the Triton-soluble fractions and 20 µg of the Triton-insoluble fractions were resolved by SDS–PAGE and analyzed by means of Western blotting. Extracts were independently analyzed using a rabbit anti-actin antibody (Sigma, 1:100) to account for differences in protein loading.
Immunocytochemistry
HEK293T or HEK293T/17 cells were plated onto 6-well plates at 40% confluency and transfected the following day (60% confluency) using 2 µg of DNA and 20 µl of PolyFect transfection reagent according to the manufacturer's instructions. Transfected cells were re-plated the next day at a 1:2 or 1:3 ratio onto coverslips and processed for immunocytochemistry 2 days later. Where indicated, cells were treated with 5 µm MG-132 (carbobenzoxy-l-leucyl-l-leucinal) (Calbiochem) for 12 h to inhibit proteasome activity, which enhances aggresome formation. Cells were then fixed for 30 min at 37°C with 4% (w/v) paraformaldehyde in PBS. Cells were permeabilized in 0.5% Triton X-100 in PBS (3×5 min washes) and preincubated in blocking buffer (10% goat serum; Vector Laboratories) in 0.5% Triton X-100 in PBS for 1 h at room temperature, followed by exposure to primary antibody [1:1000, polyclonal rabbit anti-parkin (844)] diluted in blocking buffer for 1 h at room temperature. Cells were washed in 0.5% Triton X-100 in PBS and incubated with goat anti-rabbit AlexaFluor-488-conjugated secondary antibody (1:1000, Invitrogen), diluted in 0.5% Triton X-100 in PBS, for 1 h at room temperature, followed by washes in 0.5% Triton X-100 in PBS, PBS, H2O and a rinse in 70% ethanol. Fixed cells were mounted using mounting medium containing DAPI (Vector Laboratories) and visualized on a Zeiss microscope using a 40× or 100× oil-immersion objective. Using this technique, transfection levels were usually found to be around 60%.
To determine the percentage of transfected cells containing parkin inclusions, cells overexpressing parkin were scored to display either diffuse cytosolic or aggregate staining. In addition, cells displaying aggregates were scored to either display at least one large perinuclear parkin aggregate or only multiple small aggregates. These values were used to determine the percentage of cells with (at least one) large inclusion. Perinuclear aggregates were independently verified to be aggresomes by double-staining with a monoclonal anti-vimentin antibody (1:200, clone V9, Sigma) and a goat anti-mouse AlexaFluor-594-conjugated secondary antibody (1:1000, Invitrogen). For all determinations, between 250 and 1000 transfected cells were analyzed, and in all cases, additional analysis was performed by an observer blind to conditions. Cells were also examined using a Leica TCS-SP5 confocal microscope and Leica Applied Systems (LAS-AF) image acquisition software. Images were collected using single excitation for each wavelength separately and channels subsequently merged using Adobe Photoshop.
Human cases
All PD cases had suffered from classical parkinsonism and none of them had apparent cognitive impairment or dementia. The postmortem delay between death and tissue processing was between 3 and 7.5 h. The pH of the brain was between 6.6 and 6.9 in all cases. One-half of the brain was immediately cut on coronal sections, 1 cm thick, frozen in dry ice and stored at −80°C until use.
All sporadic PD cases were tested for the G2019S LRRK2 mutation (50) and were also tested for the c.255delA mutation in parkin (51) if their age at onset was before 55 years. None of the samples analyzed here were positive for either mutation.
Variable phenotypes and mixed pathologies can be observed in a significant fraction of postmortem cases, such as the presence of Alzheimer's disease-associated lesions combined with α-synuclein pathology in diagnosed PD cases, implying possible molecular crosstalks in protein aggregation mechanisms (52). In addition, Lewy body pathology has been observed in around 10% of brains from normal elderly subjects (53). To assure that our control subjects do not display Lewy body pathology and that our PD subjects display α-synuclein pathology in the absence of Alzheimer disease (AD)-related lesions, all samples were subjected to an extensive neuropathological study. Analysis was carried out on de-waxed 4-μm-thick paraffin sections of the frontal (area 8), primary motor, primary sensory, parietal, temporal superior, temporal inferior, anterior gyrus cinguli, anterior insular, and primary and associative visual cortices; entorhinal cortex and hippocampus; caudate, putamen and globus pallidus; medial and posterior thalamus; subthalamus; Meynert nucleus; amygdala; midbrain (two levels), pons and bulb; and cerebellar cortex and dentate nucleus. The sections were stained with hematoxylin and eosin, Kluever Barrera and, for immunohistochemistry, to glial fibrillary acidic protein (Dako, 1:250), CD68 (Dako, 1:100), βA-amyloid (Boehringer Mannhein, 1:50), tau AT8 (Innogenetics, 1:500), tau 4R and tau 3R (Upstate, 1:200 and 1:2000, respectively), phosphorylation-specific tau Thr181, Ser202, Ser214, Ser262, Ser396 and Ser422 (all Calbiochem, 1:100, except Thr181, 1:250), αB-crystallin (Abcam, 1:100), ubiquitin (Dako, 1:200), α-synuclein (Chemicon, 1:3000), phosphorylation-specific α-synuclein Ser129 (WAKO, 1:2000) and nitrated/oxidized α-synuclein (Zymed, 1:400). Following incubation with the primary antibody, the sections were incubated with EnVision+system peroxidase for 15 min at room temperature. The peroxidase reaction was visualized with diaminobenzidine and H2O2. In all cases, control of the immunostaining included omission of the primary antibody.
Neuropathological characterization of PD was according to established criteria (54). All PD cases corresponded to stage 4 of Braak and Braak. This implies involvement of selected nuclei of the medulla oblongata, pons and midbrain, amygdala and nucleus basalis of Meynert. All cases showed marked loss of neurons in the substantia nigra pars compacta exceeding 60%, whereas moderate pathology occurred in the caudate and putamen. In no case did Lewy pathology involve the frontal cortex. No cases of dementia with Lewy bodies, according to the guidelines of the specialized international workshop (55,56), were included in the present series. AD-related pathology, including β-amyloid plaques and neurofibrillary tangles, was absent in the present series. Control subjects showed absence of neurological symptoms, metabolic and vascular diseases, and the neuropathological study disclosed no abnormalities including lack of PD- and AD-related pathology.
Human tissues and sample preparation
Freshly frozen brain samples from deceased human subjects were collected at autopsy following informed consent from the next of kin under a protocol approved by the local ethics committee. Brain regions from each control and PD patient analyzed included cortex, cerebellum and caudate. For all samples, patient age, gender, time to postmortem tissue collection and postmortem pathological analysis was known.
Small sections of frozen tissue blocks were added to 700 µl of lysis buffer (1% SDS in PBS, 1 mm PMSF, 1 mm Na3VO4 and 5 mm NaF) and lysed using a 7-ml Dounce homogenizer with a Teflon pestle. Lysis was performed by applying slow strokes during maximally one minute. For cerebellar samples, an additional two pulses of sonication (1 s each) were needed to fully solubilize the tissue. Homogenates were subsequently centrifuged for 10 min at 13'500 rpm at 4°C. Protein concentration of the supernatants was estimated using a BCA assay (Pierce), and 0.2 volumes of 5× sample buffer added to extracts and boiling for 5 min at 95°C. Care was taken to ensure extracts were highly concentrated (>5 mg/ml) such that phospho-parkin levels could be determined using mini-gels. As mentioned above for transfected tissue culture cell extracts, samples were directly analyzed on western blots, as repetitive freeze–thaws were found to abolish detection of phospho-parkin levels and 80–100 µg of total protein extracts were loaded per lane to ensure sensitive detection of phospho-parkin levels.
Western blotting
Proteins were resolved by SDS–PAGE, transferred onto polyvinylidene difluoride membranes (Hybond, GEHealthcare) and probed with primary antibodies overnight at 4°C. The following antibodies were employed: a rabbit polyclonal anti-parkin antibody (1:1000 for cell and brain extracts, ab6177, Abcam), a custom-made anti-parkin antibody 844 (1:1000 for recombinant proteins), an anti-phospho-S101-parkin antibody (1:200 for cell extracts and brain extracts) an anti-phospho-S378-parkin antibody (1:500 for cell extracts; 1:5000 for recombinant proteins), anti-p35 (1:100, C-19, Santa Cruz Biotechnology), anti-cdk5 (1:400, DC17, Santa Cruz Biotechnology), anti-casein kinase Iε (1:250, BD Transduction Laboratories), anti-casein kinase Iα (1:200, C19, Santa Cruz Biotechnology), anti-casein kinase Iδ (1:50, H60, Santa Cruz Biotechnology), anti-α-tubulin (1:5000, clone DM1A, Sigma) and anti-actin (1:100, Sigma). Membranes were washed and incubated with secondary antibodies [anti-rabbit HRP-conjugated antibody (1:2000) or anti-mouse HRP-conjugated antibody (1:2000) (Dako Cytomation)] for 90 min at room temperature, followed by detection using ECL reagents (Roche).
Statistical analysis
Experiments were done the indicated amount of times, and the data were analyzed using a Student's paired t-test.
FUNDING
This work was supported by grants from the Fondo de Investigación Sanitaria (FIS-PI040262), the Fundación Ramón Areces and the Junta de Andalucia. S.H. was supported by a Ramón y Cajal Fellowship. Funding to Pay the Open Access Charge was provided by a grant from the Spanish Ministry of Science and Innovation (BFU2007-63635).
ACKNOWLEDGEMENTS
We thank P. Robinson for providing a construct encoding for human parkin, PhosphoSolutions for generating phosphorylation-state-specific antibodies and C. Suñe for providing HEK293T cells. We thank the patients and their families for tissue donations. We thank A. Delgado for previous technical assistance, and our various colleagues for providing additional reagents and helpful discussions.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Dauer W., Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.Farrer M.J. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat. Rev. Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 3.Thomas B., Beal M.F. Parkinson's disease. Hum. Mol. Genet. 2007;16:R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 4.Shults C.W. Lewy bodies. Proc. Natl Acad. Sci. USA. 2006;103:1661–1668. doi: 10.1073/pnas.0509567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 6.Abbas N., Lücking C.B., Ricard S., Dürr A., Bonifati V., De Michele G., Bouley S., Vaughan J.R., Gasser T., Marconi R., et al. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. Hum. Mol. Genet. 1999;8:567–574. doi: 10.1093/hmg/8.4.567. [DOI] [PubMed] [Google Scholar]
- 7.Lücking C.B., Dürr A., Bonifati V., Vaughan J., De Michele G., Gasser T., Harhangi B.S., Meco G., Denefle P., Wood N.W., et al. Association between early-onset Parkinson's disease and mutations in the parkin gene. N. Engl. J. Med. 2000;342:1560–1567. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- 8.Farrer M., Chan P., Chen R., Tan L., Lincoln S., Hernandez D., Forno L., Gwinn-Hardy K., Petrucelli L., Hussey J., et al. Lewy bodies and parkinsonism in families with parkin mutations. Ann. Neurol. 2001;50:293–300. doi: 10.1002/ana.1132. [DOI] [PubMed] [Google Scholar]
- 9.Pramstaller P.P., Schlossmacher M.G., Jacques T.S., Scaravilli F., Eskelson C., Pepivani I., Hedrich K., Adel S., Gonzales-McNeal M., Hilker R., et al. Lewy body Parkinson's disease in a large pedigree with 77 parkin mutation carriers. Ann. Neurol. 2005;58:411–422. doi: 10.1002/ana.20587. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi S., Wakabayashi K., Ishikawa A., Nagai H., Saito M., Maruyama M., Takahashi T., Ozawa T., Tsuji S., Takahashi H. An autopsy case of autosomal-recessive juvenile parkinsonism with a homozygous exon 4 deletion in the parkin gene. Mov. Disord. 2000;15:884–888. doi: 10.1002/1531-8257(200009)15:5<884::aid-mds1019>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Shimura H., Hattori N., Kubo S., Mizuno Y., Asakawa S., Minoshima S., Shimizu N., Iwai K., Chiba T., Tanaka K., Suzuki T. Immunohistochemical and subcellular localization of parkin protein: absence of protein in autosomal recessive juvenile parkinsonism patients. Ann. Neurol. 1999;45:668–672. doi: 10.1002/1531-8249(199905)45:5<668::aid-ana19>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Schlossmacher M.C., Frosch M.P., Gai W.P., Medina M., Sharma N., Forno L., Ochiishi T., Shimura H., Sharon R., Hattori N., et al. Parkin localizes to the Lewy bodies of Parkinson disease and dementia with Lewy bodies. Am. J. Pathol. 2002;160:1655–1667. doi: 10.1016/S0002-9440(10)61113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimura H., Hattori N., Kubo S., Mizuno Y., Asakawa S., Minoshima S., Shimizu N., Iwai K., Chiba T., Tanaka K., Suzuki T. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nature Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 14.Cookson M.R., Lockhart P.J., McLendon C., O'Farrell C., Schlossmacher M., Farrer M.J. RING finger 1 mutations in parkin produce altered localization of the protein. Hum. Mol. Genet. 2003;12:2957–2965. doi: 10.1093/hmg/ddg328. [DOI] [PubMed] [Google Scholar]
- 15.Sriram S.R., Li X., Ko H.S., Chung K.K., Wong E., Lim K.L., Dawson V.L., Dawson T.M. Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum. Mol. Genet. 2005;14:2571–2586. doi: 10.1093/hmg/ddi292. [DOI] [PubMed] [Google Scholar]
- 16.Hampe C., Ardila-Osorio H., Fournier M., Brice A., Corti O. Biochemical analysis of Parkinson's disease-causing variants of parkin, an E3 ubiquitin-protein ligase with monoubiquitylation capacity. Hum. Mol. Genet. 2006;15:2059–2075. doi: 10.1093/hmg/ddl131. [DOI] [PubMed] [Google Scholar]
- 17.Wang C., Ko H.S., Thomas B., Tsang F., Chew K.C., Tay S.P., Ho M.W., Lim T.M., Soong T.W., Pletnikova O., et al. Stress-induced alterations in parkin solubility promote parkin aggregation and compromise parkin's protective function. Hum. Mol. Genet. 2005;14:3885–3897. doi: 10.1093/hmg/ddi413. [DOI] [PubMed] [Google Scholar]
- 18.LaVoie M.J., Ostaszewski B.L., Weihofen A., Schlossmacher M.G., Selkoe D.J. Dopamine covalently modifies and functionally inactivates parkin. Nat. Med. 2005;11:1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- 19.Chung K.K., Thomas B., Li X., Pletnikova O., Troncoso J.C., Marsh L., Dawson V.L., Dawson T.M. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 20.Yao D., Gu Z., Nakamura T., Shi Z.Q., Ma Y., Gaston B., Palmer L.A., Rockenstein E.M., Zhang Z., Masliah E., et al. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc. Natl Acad. Sci. USA. 2004;101:10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cookson M.R., Dauer W., Dawson T., Fon E.A., Guo M., Shen J. The roles of kinases in familial Parkinson's disease. J. Neurosci. 2007;27:11865–11868. doi: 10.1523/JNEUROSCI.3695-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto A., Friedlein A., Imai Y., Takahashi R., Kahle P.J., Haass C. Parkin phosphorylation and modulation of its E3 ubiquitin ligase activity. J. Biol. Chem. 2005;280:3390–3399. doi: 10.1074/jbc.M407724200. [DOI] [PubMed] [Google Scholar]
- 23.Avraham E., Rott R., Liani E., Szargel R., Engelender S. Phosphorylation of parkin by the cyclin-dependent kinase 5 at the linker region modulates its ubiquitin-ligase activity and aggregation. J. Biol. Chem. 2007;282:12842–12850. doi: 10.1074/jbc.M608243200. [DOI] [PubMed] [Google Scholar]
- 24.Mashhoon N., De Maggio A.J., Tereshko V., Bergmeier S.C., Egli M., Hoekstra M.F., Kuret J. Crystal structure of a conformation-selective casein kinase-1 inhibitor. J. Biol. Chem. 2000;275:20052–20060. doi: 10.1074/jbc.M001713200. [DOI] [PubMed] [Google Scholar]
- 25.Bain J., Plater L., Elliott M., Shpiro N., Hastie C.J., McLauchlan H., Klevernic I., Arthur J.S., Alessi D.R., Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rena G., Bain J., Elliott M., Cohen P. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 2004;5:60–65. doi: 10.1038/sj.embor.7400048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meijer L., Borgne A., Mulner O., Chong J.P., Blow J.J., Inagaki N., Inagaki M., Delcros J.G., Moulinoux J.P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 28.Braak H., Ghebremedhin E., Rub U., Bratzke H., Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 29.Bandopadhyay R., Kingsbury A.E., Cookson M.R., Reid A.R., Evans I.M., Hope A.D., Pittman A.M., Lashley T., Canet-Aviles R., Miller D.W., et al. Synphilin-1 and parkin show overlapping expression patterns in human brain and form aggresomes in response to proteasomal inhibition. Neurobiol. Dis. 2005;20:401–411. doi: 10.1016/j.nbd.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Yasojima K., Kuret J., DeMaggio A.J., McGeer E., McGeer P.L. Casein kinase 1 delta mRNA is upregulated in Alzheimer disease brain. Brain Res. 2000;865:116–120. doi: 10.1016/s0006-8993(00)02200-9. [DOI] [PubMed] [Google Scholar]
- 31.Wu D.C., Yu Y.P., Lee N.T., Yu A.C., Wang J.H., Han Y.F. The expression of cdk5, p35, p39 and cdk5 kinase activity in developing, adult, and aged rat brains. Neurochem. Res. 2000;25:923–939. doi: 10.1023/a:1007544106645. [DOI] [PubMed] [Google Scholar]
- 32.Johnson K., Liu L., Majdzadeh N., Chavez C., Chin P.C., Morrison B., Wang L., Park J., Chugh P., Chen H.M., D'Mello S.R. Inhibition of neuronal apoptosis by the cyclin-dependent kinase inhibitor GW8510: identification of 3′ substituted indolones as a scaffold for the development of neuroprotective drugs. J. Neurochem. 2005;93:538–548. doi: 10.1111/j.1471-4159.2004.03004.x. [DOI] [PubMed] [Google Scholar]
- 33.Feany M.B., Pallanck L.J. Parkin: a multipurpose neuroprotective agent? Neuron. 2003;38:13–16. doi: 10.1016/s0896-6273(03)00201-0. [DOI] [PubMed] [Google Scholar]
- 34.Patrick G.N., Zukerberg L., Nikolic M., de la Monte S., Dikkes P., Tsai L.H. Conversion of p35 to p25 deregulates cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 35.Brion J.P., Couck A.M. Cortical and brainstem-type Lewy bodies are immunoreactive for the cyclin-dependent kinase 5. Am. J. Pathol. 1995;147:1465–1476. [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura S., Kawamoto Y., Nakano S., Akiguchi I., Kimura J. p35nck5a and cyclin-dependent kinase 5 colocalize in Lewy bodies of brains with Parkinson's disease. Acta Neuropathol. 1997;94:153–157. doi: 10.1007/s004010050687. [DOI] [PubMed] [Google Scholar]
- 37.Smith P.D., Crocker S.J., Jackson-Lewis V., Jordan-Sciutto K.L., Hayley S., Mount M.P., O'Hare M.J., Callaghan S., Slack R.S., Przedborski S., et al. Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson's disease. Proc. Natl Acad. Sci. USA. 2003;100:13650–13655. doi: 10.1073/pnas.2232515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee M.S., Kwon Y.T., Li M., Peng J., Friedlander R.M., Tsai L.H. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 39.Smith P.D., O'Hare M.J., Park D.S. CDKs: taking on a role as mediators of dopaminergic loss in Parkinson's disease. Trends Mol. Med. 2004;10:445–451. doi: 10.1016/j.molmed.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Flajolet M., He G., Heiman M., Lin A., Nairn A.C., Greengard P. Regulation of Alzheimer's disease amyloid-β formation by casein kinase I. Proc. Natl Acad. Sci. USA. 2007;104:4159–4164. doi: 10.1073/pnas.0611236104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knippschild U., Gocht A., Wolff S., Huber N., Löher J., Stöter M. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Arias C., Becerra-Garcia F., Arrieta I., Tapia R. The protein phosphatase inhibitor okadaic acid induces heat shock protein expression and neurodegeneration in rat hippocampus in vivo. Exp. Neurol. 1998;153:242–254. doi: 10.1006/exnr.1998.6900. [DOI] [PubMed] [Google Scholar]
- 43.Brown A.M., Baucum A.J., Bass M.A., Colbran R.J. Association of protein phosphatase 1 gamma 1 with spinophilin suppresses phosphatase activity in a Parkinson disease model. J. Biol. Chem. 2008;283:14286–14294. doi: 10.1074/jbc.M801377200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo Bianco C., Schneider B.L., Bauer M., Sajadi A., Brice A., Iwatsubo T., Aebischer P. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson's disease. Proc. Natl Acad. Sci. USA. 2004;101:17510–17515. doi: 10.1073/pnas.0405313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manfredsson F.P., Burger C., Sullivan L.F., Muzyczka N., Lewin A.S., Mandel R.J. rAAV-mediated nigral human parkin over-expression partially ameliorates motor deficits via enhanced dopamine neurotransmission in a rat model of Parkinson's disease. Exp. Neurol. 2007;207:289–301. doi: 10.1016/j.expneurol.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 46.Braak H., Del Tredici K. Assessing fetal nerve grafts in Parkinson's disease. Nat. Med. 2008;14:483–485. doi: 10.1038/nm0508-483. [DOI] [PubMed] [Google Scholar]
- 47.Guan K.L., Dixon J.E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione-S-transferase. Anal. Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 48.Fdez E., Jowitt T.A., Wang M.C., Rajabhosale M., Foster K., Bella J., Baldock C., Woodman P.G., Hilfiker S. A role for SNARE complex dimerization during neurosecretion. Mol. Biol. Cell. 2008;19:3379–3389. doi: 10.1091/mbc.E08-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luzón-Toro B., Rubio de la Torre E., Delgado A., Pérez-Tur J., Hilfiker S. Mechanistic insight into the dominant mode of the Parkinson's disease-associated G2019S LRRK2 mutation. Hum. Mol. Genet. 2007;16:2031–2039. doi: 10.1093/hmg/ddm151. [DOI] [PubMed] [Google Scholar]
- 50.Gilks W.P., Abou-Sleiman P.M., Gandhi S., Jain S., Singleton A., Lees A.J., Shaw K., Bhatia K.P., Bonifati V., Quinn N.P., et al. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- 51.Lücking C.B., Dürr A., Bonifati V., Vaughan J., De Michele G., Gasser T., Harhangi B.S., Meco G., Denefle P., Wood N.W., et al. Association between early-onset Parkinson's disease and mutations in the parkin gene. N. Engl. J. Med. 2000;342:1560–1567. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- 52.Jellinger K.A. Neuropathological aspects of Alzheimer disease, Parkinson disease and frontotemporal dementia. Neurodegener. Dis. 2008;5:118–121. doi: 10.1159/000113679. [DOI] [PubMed] [Google Scholar]
- 53.Mikolaenko I., Pletnikova O., Kawas C.H., O'Brien R., Resnick S.M., Crain B., Troncoso J.C. Alpha-synuclein lesions in normal aging, Parkinson disease, and Alzheimer disease: evidence from the Baltimore Longitudinal Study of Aging (BLSA) J. Neuropathol. Exp. Neurol. 2005;64:156–162. doi: 10.1093/jnen/64.2.156. [DOI] [PubMed] [Google Scholar]
- 54.Jellinger K.A., Mizuno Y. Parkinson's disease. In: Dickson D., editor. Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders. Basel: ISN Neuropath Press; 2003. pp. 159–187. [Google Scholar]
- 55.Ince P.G., McKeith I. Dementia with Lewy bodies. In: Dickson D., editor. Neurodegeneration: The molecular Pathology of Dementia and Movement Disorders. Basel: ISN Neuropath Press; 2003. pp. 188–199. [Google Scholar]
- 56.McKeith I. Dementia with Lewy bodies. Lancet Neurol. 2004;3:19–28. doi: 10.1016/s1474-4422(03)00619-7. [DOI] [PubMed] [Google Scholar]