Abstract
Arteriopathies are the commonest cause of arterial ischaemic stroke (AIS) in children. Repeated vascular imaging in children with AIS demonstrated the existence of a ‘transient cerebral arteriopathy’ (TCA), characterized by lenticulostriate infarction due to non-progressive unilateral arterial disease affecting the supraclinoid internal carotid artery and its proximal branches. To further characterize the course of childhood arteriopathies, and to differentiate TCA from progressive arterial disease, we studied the long-term evolution of unilateral anterior circulation arteriopathy, and explored predictors of stroke outcome and recurrence. From three consecutive cohorts in London, Paris and Utrecht, we reviewed radiological studies and clinical charts of 79 previously healthy children with anterior circulation AIS and unilateral intracranial arteriopathy of the internal carotid bifurcation, who underwent repeated vascular imaging. The long-term evolution of arteriopathy was classified as progressive or TCA. Clinical and imaging characteristics were compared between both groups. Logistic regression modelling was used to determine possible predictors of the course of arteriopathy, functional outcome and recurrence. After a median follow-up of 1.4 years, 5 of 79 children (6%) had progressive arteriopathy, with increasing unilateral disease or bilateral involvement. In the others (94%), the course of arteriopathy was classified as TCA. In 23% of TCA patients, follow-up vascular imaging showed complete normalization, the remaining 77% had residual arterial abnormalities, with improvement in 45% and stabilization in 32%. Stroke was preceded by chickenpox in 44% of TCA patients, and in none of the patients with progressive arteriopathies. Most infarcts were localized in the basal ganglia. In 14 (19%) of TCA patients, transient worsening of the arterial lesion was demonstrated before the arteriopathy stabilized or improved. Thirteen TCA patients (18%) had a recurrent stroke or TIA. Thirty TCA patients (41%) had a good neurological outcome, compared with none of the five patients with progressive arteriopathy. Arterial occlusion, moyamoya vessels and ACA involvement were more frequent in progressive arteriopathies. Cortical infarct localization was significantly associated with poor neurological outcome (OR 6.14, 95% CI 1.29–29.22, P = 0.02), while there was a trend for occlusive arterial disease to predict poor outcome (OR 3.00, 95% CI 0.98–9.23, P = 0.06). Progressive arteriopathy was associated with recurrence (OR 18.77, 95%CI 1.94–181.97, P = 0.01). The majority of childhood unilateral intracranial anterior circulation arteriopathies (94%) have a course that is consistent with TCA, in which transient worsening is common. Although the arterial inflammation probably causing TCA is ‘transient’, most children are left with permanent arterial abnormalities and residual neurological deficits.
Keywords: arterial ischaemic stroke, children, transient cerebral arteriopathy, recurrence, outcome
Introduction
Ischaemic stroke is not uncommon in childhood, with an incidence of at least 3.3/100.000 (Lynch et al., 2002). Causes of and risk factors for arterial ischaemic stroke (AIS) differ from those in adults, and non-atherosclerotic arterial disease is responsible for the majority of childhood AIS (deVeber, 2003; Ganesan et al., 2003; Kirkham and Hogan, 2004). Diagnostic criteria for childhood arteriopathies, including moyamoya, vasculitis, dissection, transient cerebral arteriopathy (TCA) and post-varicella angiopathy (PVA) have recently been proposed (Sébire et al., 2004). TCA was first recognized as an important cause of childhood stroke in 1998. It is characterized by infarction in the lateral lenticulostriate territory due to unilateral intracranial arterial wall disease that affects the distal internal carotid artery (ICA), proximal middle and/or anterior cerebral artery (MCA, ACA). In time, the arteriopathy stabilizes, improves or even completely resolves, sometimes after initial worsening during the first few months (Chabrier et al., 1998; Sébire et al., 2004). When TCA is preceded by Varicella zoster (VZV) infection in the 12 months prior to AIS, the arteriopathy is called PVA (Sébire et al., 1999; Askalan et al., 2001; Sébire et al., 2004; Lanthier et al., 2005). TCA and PVA are considered monophasic inflammatory arteriopathies that remain strictly unilateral and should be differentiated from diffuse cerebral vasculitis and moyamoya disease, both having a different course and prognosis (Fung et al., 2005; Benseler et al., 2006). At presentation, however, the differentiation between progressive arteriopathy and TCA may be difficult in children with unilateral arterial disease; some patients with moyamoya disease present with unilateral arteriopathy that eventually progresses into bilateral steno-occlusive arteriopathy (Kawano et al., 1994; Houkin et al., 1996; Hirotsune et al., 1997), and progressive cerebral vasculitis may also make its debut with unilateral disease (Lanthier et al., 2001; Benseler et al., 2006). There is confusion in the literature about the definition of progressive arteriopathy. Although early worsening is considered part of the TCA/PVA spectrum (Sébire et al., 2004; Sébire, 2006), some authors classify arteriopathies as progressive when second vascular imaging reveals progression of arterial lesions, although the timing of worsening remains uncertain and may only have occurred during the acute stage, with later stabilization or improvement (Danchaivijitr et al., 2006; Miravet et al., 2007).
Early prediction of stroke outcome, recurrence and the course of arteriopathy, could in the future help to guide therapeutic choices, particularly indications for immunosuppressive therapy and/or revascularization surgery. The aims of this study were to describe the evolution of unilateral intracranial anterior circulation arteriopathies, and to explore possible clinical and radiological predictors of the course of arteriopathy, of functional stroke outcome and of recurrence.
Patients and Methods
Patients
Ethical permission to review the medical records and imaging procedures was granted by local institutional ethical committees.
This is a retrospective consecutive cohort study of children between 1 month and 18 years of age at the time of presentation for first AIS in one of three specialized tertiary referral centres in the United Kingdom (Great Ormond Street Hospital for Children, London, 1991–2000, n = 27), France (Hôpital Bicêtre, Paris, 1985–2005, n = 32) and The Netherlands (Wilhelmina Children's Hospital, Utrecht, 1999–2006, n = 20). Children who were previously healthy and presented with neurological symptoms caused by ischaemia in the territory of the anterior cerebral circulation were eligible. Patients were included when:
Parenchymal imaging showed anterior circulation AIS,
Vascular imaging [digital subtraction contrast angiography (DSA) or MR angiography (MRA)] revealed unilateral intracranial large artery disease of the internal carotid bifurcation, i.e. the distal supraclinoid ICA (dICA), proximal ACA and/or MCA,
Vascular imaging was repeated at least once to observe the course of arteriopathy. In most children, the last vascular imaging procedure was performed at least 6 months after stroke but patients could be included if the last angiography performed within 6 months revealed normalization or significant improvement of initial abnormalities, and it was decided by the clinician responsible for the patient, not to repeat vascular imaging thereafter.
Arteriopathy was defined as a focal or segmental stenosis or occlusion, with regular or irregular abnormalities of the arterial wall. Abrupt occlusion of a major cerebral artery at initial angiography without other abnormalities suggestive of arterial wall disease was not sufficient to diagnose arteriopathy, as this may suggest embolic arterial occlusion. Therefore, in all children with arterial occlusion at the time of diagnosis who were included in this study, vascular imaging revealed additional arterial wall abnormalities, consisting of one or more areas of stenosis proximal to but in the same arterial segment as the occlusion, or involvement of stenosed arteries other than the occluded one. Children with bilateral arteriopathy at presentation, extracranial arterial dissection or extracranial ICA fibromuscular dysplasia, according to previously published definitions (Sébire et al., 2004), were not included in the study. Children who were previously diagnosed with a chronic disorder known to predispose to arteriopathic stroke or cerebral arteriopathy (e.g. sickle cell disease, neurofibromatosis, Down syndrome, radiation therapy), or patients who suffered from an acute illness that may cause arteriopathic AIS (e.g. bacterial meningitis) were excluded. A prior diagnosis or precipitating event that may be considered a risk factor contributing to AIS (e.g. anaemia, dehydration) was not a reason for exclusion.
Some of the patients were part of previously described cohorts (Sébire et al., 1999; Braun et al., 2006; Danchaivijitr et al., 2006; Miravet et al., 2007), but were independently reviewed for this study.
Radiological evaluation
Parenchymal imaging
MRI scans generally included axial T1- and T2-weighted MRI, axial or coronal FLAIR, and in some patients diffusion-weighted MRI. Initial and follow-up MR scans were reviewed by three investigators (GS, SC, KB). The main location of the infarct was evaluated. We differentiated between infarcts that were predominantly or exclusively located in the territory of the lenticulostriate perforators (basal ganglia AIS), and those that were mainly located in the cortical and/or subcortical territories of the ACA or MCA (cortical AIS). In basal ganglia AIS, small areas of adjacent white matter or cortical grey matter were often included in the lesion. In mainly cortical AIS, concurrent involvement of basal ganglia was frequently seen. Furthermore, we determined whether the infarct clearly included corona radiata white matter. Follow-up MRI scans were assessed for the occurrence of new infarcts, defined as new ischaemic lesions outside the areas already infarcted on initial imaging.
Vascular imaging
Characterization of arterial lesions
Three-dimensional time-of-flight MRA and four-vessel DSA studies were reviewed for location (dICA, pACA, M1 and/or M2 segments of the MCA, Fig. 1), and type of abnormalities (focal or segmental stenosis, irregular stenosis with beading or occlusion). Beading was defined as alternating short segments of stenosis with normal or dilated segments, similar to a ‘string of pearls’. The presence of abnormal lenticulostriate small collaterals (so-called moyamoya vessels) was recorded, as was arterial occlusion, defined as absence of flow in one or more of the large intracranial arterial branches. Moyamoya vessels and arterial occlusion were noted if they occurred either at initial vascular imaging, or at any time point during follow-up.
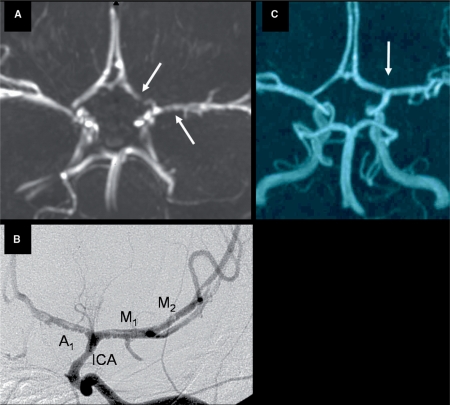
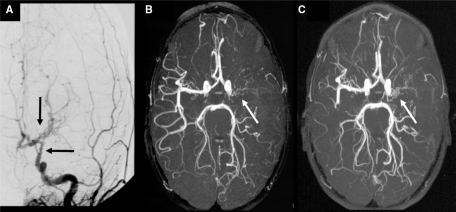
Fig. 1.
Evolution of arterial abnormalities in a 6-year-old girl with typical TCA. (A) MRA at day 1 showing irregular stenosis of the A1 segment of the left ACA (left arrow) and M1 segment of the left MCA (right arrow). (B) Conventional contrast angiography 4 days after stroke illustrating irregular stenoses with string-of-beads sign (beading) involving the distal segment of the ICA, and the A1 and M1 and M2 segments of the ACA and MCA, respectively. (C) Complete resolution of arteriopathy (arrow) on MRA performed 15 months after stroke.
Course of the arteriopathy
To evaluate the eventual course of arteriopathy, the last follow-up vascular imaging was compared with initial and subsequent angiographies. For each patient, vascular imaging procedures were requested at the discretion of the treating physician, not according to a fixed protocol. Angiographical results were visually analysed in a non-quantitative manner, by three investigators (GS, SC, KB). Since MRA may overestimate arterial stenosis compared with DSA (Husson and Lasjaunias, 2004), the course of arteriopathy was assessed in the majority of children by comparison of identical angiography techniques (MRA or DSA) within the same patient. After comparison of all vascular imaging procedures, the course of the arteriopathy was classified as either TCA (according to previously published definitions, (Sébire et al., 2004; Sébire, 2006), or as progressive arteriopathy:
TCA was defined as stabilization, improvement or normalization of arterial anomalies, when comparing the last vascular imaging with the previous angiography procedure(s). For further analysis, patients with TCA were subdivided into a residual arteriopathy group (stabilizing or improving arterial lesions), and a normalizing arteriopathy group (complete resolution of arterial disease). In TCA, the arteriopathy may temporarily progress during the first months after stroke, leading to increased unilateral arterial abnormalities on a second angiography (Sébire et al., 2004; Sébire, 2006). Therefore, when more than two vascular imaging procedures were performed, and the second angiography (independent of its exact timing) showed progression of arterial disease that later stabilized or improved at subsequent angiography, patients were still classified as TCA, and had suffered from transient worsening.
Progressive arteriopathy was characterized by ongoing increase of arterial abnormalities, proven to have occurred beyond 6 months from diagnosis. This could consist of an increase of ipsilateral arterial wall lesions (progressive stenosis, or stenosis evolving into occlusion), occurrence of new ipsilateral lesions (outside the originally affected arterial segment) or progression to bilateral arterial disease.
Clinical evaluation
Medical charts were reviewed in order to collect clinical data regarding gender, age at stroke, preceding chickenpox within 12 months prior to stroke, precipitating events, acute treatment and secondary prevention (antiplatelet and/or anticoagulation therapy), recurrent neurological deficits and overall outcome.
Recurrent stroke or TIA
Recurrent stroke was defined as a sudden deterioration of existing neurological deficits or the sudden occurrence of new neurological deficits lasting >24 h, accompanied by new areas of infarction on cerebral imaging compared with initial MRI. A silent recurrence was defined as new infarction on MRI, without clinical symptoms. All recurrent neurological deficits that lasted <24 h and were not accompanied by new infarction on MRI were defined as probable TIAs because, although definite epilepsy was excluded, retrospective analysis of medical charts did not always categorically differentiate TIAs from seizures. Since the mode of presentation of the index stroke in children is often fluctuating and recurring (Braun et al., 2007), we defined recurrent stroke or TIA only when it occurred >1 week after presentation. For statistical analysis, we grouped recurrent stroke, silent MR infarction and TIAs as ‘any recurrence’.
Functional outcome
Based on the description of the treating physician in the medical charts, overall functional outcome was classified as either ‘good’ (defined as complete recovery, or almost complete recovery with only mild neurological deficits but no loss of function), or ‘poor’ (all others).
Statistical analysis
Clinical and radiological data of patients with progressive arteriopathy and TCA, and of the normalizing and residual arteriopathy subgroups, were compared using Mann–Whitney U-tests for continuous variables, and Pearson chi-square or Fisher's exact tests for categorical variables (when appropriate). To determine possible early predictors of the course of arteriopathy, neurological outcome and stroke recurrence, we used univariate logistic regression modelling. Multivariate regression modelling was performed for predictors with P-values < 0.10. Fisher's exact tests were used to associate possible predictors with outcome when the number of patients in one of four cells was <5. All associations are expressed as odds ratios (OR) with corresponding 95% confidence intervals (CI) and probability values. Statistical significance was considered reached when 95% CI did not include 1, and P < 0.05. All statistical analyses were done with SPSS version 12.
Results
Classification of arteriopathies
The aetiological classification of the total group of 372 patients who were evaluated for AIS in the three participating centres during the specific time intervals is given in Table 1. Seventy-nine previously healthy children fulfilled the inclusion criteria, presenting with anterior circulation stroke and unilateral intracranial arteriopathy. In 28 children, vascular imaging revealed unilateral intracranial arteriopathy, but follow-up vascular imaging was not performed or not available for review. Another 21 patients with documented intracranial unilateral arteriopathy were excluded for previously diagnosed conditions predisposing to arteriopathic stroke [sickle cell disease: n = 10, HIV infection: n = 4, bacterial meningitis: n = 2, possible PHACE syndrome (OMIM 606519): n = 2, Down syndrome, haemolytic uraemic syndrome, neurofibromatosis: each n = 1].
Table 1.
Aetiological classification of 372 children with AIS
| Total AIS | n = 372 |
|---|---|
| Intracranial unilateral anterior circulation arteriopathy | n = 128 |
| Included previously healthy + VI follow up | n = 79 |
| progressive arteriopathy | n = 5 |
| TCA (non-progressive arteriopathy) | n = 74 |
| Excluded no VI follow up or scans not available | n = 28 |
| Excluded prior predisposing disorder | n = 21 |
| Intracranial bilateral arteriopathy at onset | n = 69 |
| Intracranial posterior circulation arteriopathy | n = 12 |
| Extracranial arteriopathy | n = 34 |
| Vascular imaging normal or not performed | n = 85 |
| Cardio-embolic stroke | n = 37 |
| Embolic occlusion, no proof of arteriopathy | n = 4 |
| Other | n = 3 |
VI, vascular imagimg.
Of the included patients, the median age at first stroke was 4.8 (range 0.3–16.3) years. Forty-one patients (52%) were male.
Review of follow-up vascular imaging revealed that five children (6%) had a progressive arteriopathy, while the other 74 patients (94%) fulfilled the criteria for TCA. Normalization of the arteriopathy with no residual abnormalities was seen in 17 of these 74 patients (23%). The remaining 57 children (77%) had residual arterial abnormalities on follow-up vascular imaging, with improvement of the arteriopathy in 33 children (45%) and stabilization in 24 (32%) (Table 2).
Table 2.
Vascular imaging characteristics in 79 children with intracranial arteriopathy
| First VI (median, range) | Second VI (median, range) | Last VI (median, range) | DSA (%) | Average VI pp | Transient worsening (%) | Arterial involvement at any time point |
Angiographical abnormalities |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dICA (%) | pMCA (%) | pACA (%) | Initial occlusion (%) | Occlusion at any time (%) | Beading at any time (%) | Initial moyamoya vessels (%) | Moyamoya vessels at any time (%) | |||||||
| Total unilateral intracranial arteriopathy (n = 79) | 8d (0d to 10m) | 7m (6d to 56m) | 17m (2m to 11y) | 53 (67) | 3 | 14 (17) | 34 (43) | 73 (92) | 24 (30) | 18 (23) | 23 (29) | 26 (33) | 2 (3) | 8 (10) |
| Progressive arteriopathy (n = 5) | 8d (7d to 19d) | 12m (27d to 32m) | 3y (2y to 10y) | 5 (100) | 6 | – | 4 (80) | 5 (100) | 4 (80)a | 2 (40) | 5 (100)a | 2 (40) | 0 (0) | 4 (80)a |
| Transient Cerebral Arteriopathy (n = 74) | 7d (0d to 10m) | 7m (6d to 56m) | 16m (2m to 11y) | 48 (65) | 3 | 14 (19) | 30 (41) | 68 (92) | 20 (27) | 16 (22) | 18 (24) | 24 (32) | 2 (3) | 4 (5) |
| No residual abnormalities (n = 17) | 5d (0d to 4m) | 12m (2m to 30m) | 16m (2m to 8y) | 10 (59) | 3 | 2 (12) | 8 (47) | 14 (82) | 5 (29) | 2 (12) | 2 (12) | 4 (24) | 0 (0) | 0 (0) |
| Residual arteriopathy (n = 57) | 8d (0d to 10m) | 6m (6d to 56m) | 16m (3m to 11y) | 38 (67) | 3 | 12 (21) | 22 (39) | 54 (95) | 15 (26) | 14 (25) | 16 (28) | 20 (35) | 2 (4) | 4 (7) |
| Improving (n = 33) | 6d (0d to 10m) | 5m (6d to 56m) | 16m (3m to 11y) | 21 (64) | 3 | 8 (24) | 13 (39) | 32 (97) | 11 (33) | 6 (18) | 8 (24) | 11 (33) | 1 (3) | 2 (6) |
| Stabilizing (n = 24) | 9d (0d to 8m) | 10m (9d to 27m) | 2y (6m to 7y) | 17 (71) | 3 | 4 (17) | 9 (38) | 22 (92) | 4 (17) | 8 (33) | 8 (33) | 9 (38) | 1 (4) | 2 (8) |
a Significantly different from non-progressive arteriopathy group.
DSA, number of patients in whom conventional angiography was performed; Average VI pp, average number of vascular imaging studies per person; dICA, distal internal carotid artery; pACA, proximal anterior cerebral artery; pMCA, proximal segments of middle cerebral artery; VI, vascular imaging; d = day; y = year; m = month.
In 14 patients with TCA (19%), the second vascular imaging (performed after a median interval of 3.5 months after stroke, range 1–14 months) showed transient worsening of arterial lesions compared with the initial angiography, that later stabilized, improved or normalized (Figs 2 and 3), consistent with TCA. In three of these 14 patients, the second angiogram, at which progression was noted, was performed more than 6 months following stroke (7, 7 and 14 months). Because intermediate scans to determine the exact timing of progression were not performed, and arterial disease improved or stabilized at subsequent angiography, we had no proof of progression beyond 6 months, and therefore classified the course of arteriopathy as TCA in these three patients.
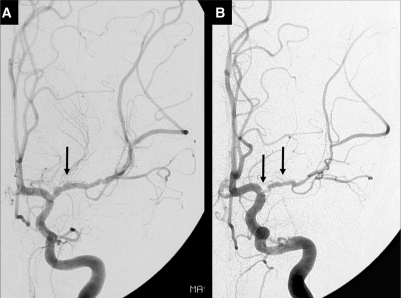
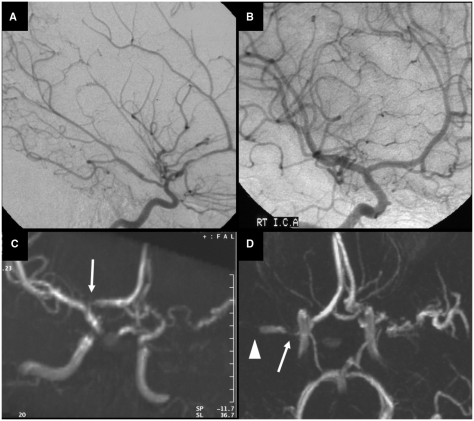
Fig. 2.
Another example of the course of arteriopathy in TCA. Conventional contrast angiography performed at 2 weeks (A) and 7 months (B) after stroke in a 7-year-old boy. (A) There is a long segmental irregular stenosis of the M1 of the MCA with beading at initial angiography. (B) Transient worsening of the disease with severe focal stenosis of the proximal M1 of the MCA (left arrow) and a long segmental stenosis of the M1 (right arrow). The beading, typical for the acute phase of TCA, is less prominent. Repeated angiography 22 months after stroke was identical, proving stabilization of arterial abnormalities (not shown).
Fig. 3.
Example of transient worsening in TCA, leading to complete MCA occlusion but subsequent improvement in an 8-year-old boy. (A) MRA shows a long segmental regular M1 stenosis (arrow) 1 day after stroke and ACA occlusion. (B) Three months later, there is complete occlusion of the origin of the MCA (arrow) and a tapered stenosis of the distal ICA. (C) Twenty months after stroke, MRA has almost normalized, with a residual focal MCA stenosis (arrow) and patent (hypoplastic?) ACA.
In the remaining 60 TCA patients, transient worsening could neither be demonstrated nor excluded, because of the timing and number of angiographical procedures.
Vascular imaging characteristics
Vascular imaging findings are summarized in Table 2 for the total group of 79 patients and for the subgroups of patients with progressive arteriopathy and TCA. The first vascular imaging was performed after a median time of 8 days, the last follow-up angiography procedure was done after a median interval of 1.4 years following stroke. Patients in the progressive arteriopathy group underwent an average number of 6.6 angiography procedures, compared with 3.0 per person in the TCA group. In seven children (9%), vascular imaging consisted only of repeated conventional contrast angiography, in 26 (33%) only of MRA, and in 46 (58%) both MRA and conventional angiography were performed during the course of the disease. In most children the course of the arteriopathy was assessed by comparison of identical angiography techniques (MRA or DSA). In six patients, however, initial vascular imaging consisted of DSA and follow-up imaging of MRA. In none of these children progression of vascular abnormalities over time was noted.
The angiographical abnormalities in Table 2 were recorded to occur either at initial vascular imaging, or at any time point during follow-up. Almost all patients had arterial abnormalities in the proximal MCA. In the five patients with progressive arteriopathy, the ICA and ACA were involved each in four (80%) of these patients at any time point during follow-up [compared with 30 (41%) (P = 0.159) and 20 (27%) (P = 0.028) of 74 patients with TCA, respectively]. Occlusion of an intracranial artery was seen in all patients with a progressive arteriopathy, but in only 18 of 74 patients (24%) at some stage in the course of TCA (P = 0.001). Moyamoya vessels at any time point during angiographical follow-up were seen significantly more often in the progressive group (60% compared with 5%; P = 0.004).
Vascular imaging in TCA
Twenty-four patients revealed arterial beading with a typical irregular stenotic appearance that was most prominent in the acute stage (Figs 1 and 2). Beading was equally frequent in TCA patients with and those without residual artererial abnormalities (35 and 24%, respectively). In three patients, conventional contrast angiography suggested a double lumen or intimal flap (Fig. 1B). In 18 patients, one or more arterial branches appeared occluded at one or several time points after stroke. Two children had a segmental stenosis at onset that progressed at second vascular imaging to complete occlusion (Fig. 3) before showing improvement of arteriopathy. In the other 16 patients with occlusive disease, occlusion was already visible at the first vascular imaging procedure. Of the 18 occlusive arteriopathy patients, two eventually normalized (Fig. 4), eight improved (Fig. 3) and eight showed persistent occlusive arterial disease (Fig. 5). An extensive network of abnormal collateral moyamoya vessels was seen in four patients with occlusive arteriopathy, of whom two later improved and two stabilized (Fig. 5). The two children with persistent unilateral MCA occlusion and moyamoya vessels were followed for ∼3 years and showed no recurrences or progression of arterial disease into bilateral moyamoya.
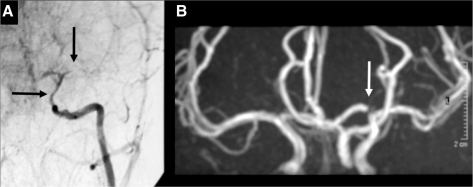
Fig. 4.
Another example of occlusive TCA with subsequent normalization. (A) Conventional angiography 2 weeks after basal ganglia stroke in a 7-year-old girl, showing a long tight distal ICA stenosis (horizontal arrow) and occlusion of the proximal MCA (vertical arrow). (B) Eight years later, there is complete normalization of arteriopathic abnormalities (the arrow points at a flow artefact in the A1 of the ACA).
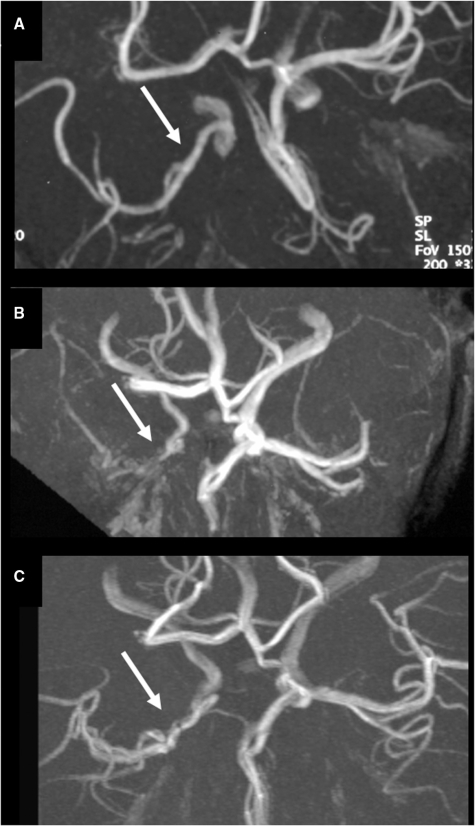
Fig. 5.
Occlusive stabilizing unilateral TCA in a 3-year-old boy who presented with three TIAs and a basal ganglia infarct on MRI. (A) Conventional angiography at 11 days after stroke shows an irregular distal ICA stenosis (horizontal arrow) and a proximal MCA occlusion with the development of some abnormal moyamoya lenticulostriate collaterals (vertical arrow). MRA at 3 months (B) and 3 years (C) after stroke shows a persisting but unchanged MCA occlusion with some abnormal moyamoya collaterals (arrows). There is no contralateral involvement in time.
Vascular imaging in progressive arteriopathy
Although the median time interval until the last follow-up angiography in progressive arteriopathy patients was 3 years, the time at which definite progression of the arteriopathy was established was shorter (1 year, ranging from 6.5 months to 2.7 years), which is comparable to follow-up duration of TCA patients. Four children showed progression from initial unilateral disease to bilateral stenotic or occlusive arteriopathy. One patient showed unilateral progression.
Course of arteriopathy in five children with progressive disease
One 6-year-old girl presented with an ischaemic stroke in the left basal ganglia and loss of flow voids in the left ICA and proximal MCA on acute MRA. Conventional angiography 3 months later confirmed strictly unilateral arteriopathy. After 1 year, about 10 days after discontinuing aspirin, she was re-admitted with neurological deficits caused by ischaemia in the right hemisphere. MRA then suggested involvement of the right ACA, and repeat MRA 3 months later, confirmed by conventional angiography, showed bilateral arteriopathy with severe stenosis of the right MCA and irregular arteriopathy of the left M1 segment (Fig. 6).
Fig. 6.
Progressive arteriopathy in a girl who initially presented with an ischaemic stroke in the left basal ganglia and loss of flow voids in the left ICA and proximal MCA on MRA, 8 days after stroke. She was treated with aspirin and was left with a moderate right hemiparesis and some comprehension and word-finding difficulties. Conventional angiography at 3 months after stroke showed vasculopathy affecting the left side (A) but the right was normal (B). Further MRA 9 months later (C) suggested abnormality of the right proximal ACA (arrow), but the findings were interpreted cautiously in view of the image quality and it was not considered ethical to repeat the conventional arteriography. Three months later, she had a contralateral infarct on MRI, involving the right basal ganglia and insular region (not shown). MRA (D), confirmed by conventional angiography, then showed bilateral arteriopathy with proximal stenosis of the right MCA (arrow) and a more distal occlusion of the right M1 segment (arrow head), as well as irregular stenosis of the left MCA.
A 4-year-old boy had a left sided hemiparesis, and MRI showed cortical high-signal in the right frontal lobe gyri. MRA showed occlusion of the right MCA from the origin to the bifurcation, with apparent pruning of the peripheral temporo-parietal branches. DSA confirmed right MCA stenosis but no definite evidence of vasculitis. Two months later he presented with a further episode of left hemiplegia. MRI revealed an extension of the right MCA territory infarction. Two years and 8 months after the original presentation, he developed a right hemiparesis with expressive aphasia. CT scan showed an infarct in the territory of the left MCA. DSA showed retrograde filling of the ACA and MCA cerebral territories through small moyamoya collaterals from the posterior circulation and vertebral arteries and the left common carotid was markedly stenosed near its origin. The angiographic findings were compatible with a progressive vasculitic process.
An 1-year-old boy showed stenosis of the right pMCA on initial vascular imaging. There was a recurrent TIA of the left hemisphere 2 years later and the MRA showed progression of arteriopathy with bilateral arterial abnormalities.
A 6-year-old girl initially had an irregular stenosis of her left ICA and MCA. Second vascular imaging, 11 months after stroke, revealed identical left MCA stenosis but now also occlusion of her right distal ICA, which remained unchanged at repeated MRA 4 years after stroke.
In a 9-year-old girl, there was ongoing unilateral progression when comparing the acute MRA, performed 8 days after stroke, with follow-up vascular imaging at 26 days, 3 and 6.5 months after stroke. MRI at the latter time point also revealed a silent recurrent infarction. This patient was then diagnosed with neuroborreliosis (Cox et al., 2005).
Clinical and MRI characteristics
Clinical characteristics and MRI results are summarized in Table 3 for the total group of 79 patients and for the subgroups of patients with progressive arteriopathy and TCA. Sixty-four percent of patients had a left-sided infarction, with main localization of the infarct in the basal ganglia in 78%, as illustrated in Fig. 7. VZV infection preceded stroke only in the group of patients with TCA (44%); no patients in the progressive arteriopathy group had chickenpox in the year prior to stroke (P = 0.074).
Table 3.
Clinical and MRI characteristics of 79 children with intracranial arteriopathy
| Age at AIS (median, range) | Male gender (%) | Left hemisphere AIS (%) | Mainly cortical localization (%) | Corona radiata involvement (%) | VZV (%) | Treatment |
Any recurrence (%) | Good outcome (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Only anti-platelet (%) | Anti-coagulant (%) | No Treatment (%) | |||||||||
| Total unilateral intracranial arteriopathy (n = 79) | 4.8y (0.3 to 16.3) | 41 (52) | 50 (64) | 17 (22) | 10 (13) | 32 (41) | 62 (78) | 10 (13) | 5 (6) | 17 (22) | 30 (38) |
| Progressive arteriopathy (n = 5) | 5.9y (1.2 to 9.3) | 2 (40) | 3 (60) | 1 (20) | 0 (0) | 0 (0) | 4 (80) | 0 (0) | 1 (20) | 4 (80)a | 0 (0) |
| Transient Cerebral Arteriopathy (n = 74) | 4.8y (0.3 to 16.3) | 39 (53) | 47 (64) | 16 (22) | 10 (14) | 32 (44) | 58 (78) | 10 (14) | 4 (5) | 13 (18) | 30 (41) |
| No residual abnormalities (n = 17) | 6.0y (1.5 to 16.3) | 5 (29)b | 8 (50) | 3 (18) | 3 (18) | 7 (41) | 14 (82) | 1 (6) | 0 (0) | 2 (12) | 10 (63) |
| Residual arteriopathy (n = 57) | 4.7y (0.3 to 14.4) | 34 (60) | 39 (68) | 13 (23) | 7 (12) | 25 (45) | 44 (77) | 9 (16) | 4 (7) | 11 (19) | 20 (35) |
| Improving (n = 33) | 4.8y (0.6 to 14.4) | 19 (58) | 24 (73) | 8 (24) | 4 (12) | 12 (38) | 25 (76) | 5 (15) | 3 (9) | 6 (18) | 11 (33) |
| Stabilizing (n = 24) | 3.8y (0.3 to 10.8) | 15 (63) | 15 (63) | 5 (22) | 3 (13) | 13 (54) | 19 (79) | 4 (17) | 1 (4) | 5 (21) | 9 (38) |
a Significantly different from non-progressive arteriopathy group.
b Significantly different from residual arteriopathy group.
VZV, Varicella zoster infection preceding stroke within 12 months.
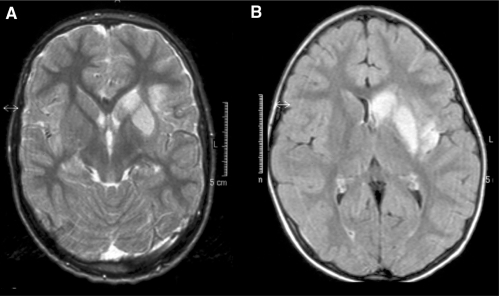
Fig. 7.
Typical examples of infarct localization in TCA, including the head of the caudate nucleus, the lentiform nucleus, some involvement but relative sparing of the internal capsule and some extension in adjacent gray or white matter. (A) T2-weighted MRI in a 7-year-old boy. (B) FLAIR MRI in a 6-year-old girl.
There were no significant differences for age, gender, lateralization and localization of AIS or for treatment, between patients with a progressive arteriopathy and with TCA. Within the group of TCA patients there were no significant differences for clinical and MRI characteristics between the 17 patients with a normalizing arteriopathy, and the remaining 57 with residual arterial lesions, except for gender; there were significantly less boys in the group with normalizing arteriopathy (29% compared with 60%; P = 0.05).
Recurrences
Patients with a progressive arteriopathy had significantly more recurrences than those with TCA (P = 0.007). In the progressive group, four of five patients (80%) had recurrent neurological events during the follow-up period. Two patients had recurrent strokes, one patient had a TIA 2 years after AIS and in one patient a new silent infarction was seen on MRI 6 months after stroke. This patient also had a TIA after 13 months. Thirteen of 74 patients with TCA (18%) had recurrent neurological symptoms in the follow-up period, after a median interval of 3 months (range 1 week to 2.5 years). Of the 17 children with complete normalization of arteriopathy, two patients had a recurrent stroke. In the group of 33 patients with improved arteriopathy at final angiography, three patients had probable TIAs, two patients had a recurrent stroke and one patient had a new lesion on the MRI scan 1 year and 2 months after stroke, without new neurological symptoms (silent recurrence). In the stabilizing group of 24 children, five patients (21%) had a recurrent stroke in the first 8 months after stroke.
There was no significant difference in recurrence rate between TCA patients with normalizing and residual arteriopathy at final angiography (P = 0.499).
Functional outcome
None of the five children with progressive arteriopathy had a good outcome after a median follow-up period of 3 years, compared with 30 of the 74 patients with TCA (41%) who had recovered completely or almost completely with only mild deficits (P = 0.15). Within the TCA group, good functional outcome tended to be more frequent in patients with a normalizing arteriopathy (63%) compared to those with a residual arteriopathy (35%, P = 0.083).
Treatment
Anti-platelet medication was prescribed to four of five (80%) patients with a progressive arteriopathy and to 58 of 74 (78%) patients with TCA. Two children were treated with both antiviral and anti-thrombotic medication. Anti-coagulant treatment was prescribed to ten patients (14%) with TCA and was combined with antiplatelet treatment in four of them. All five children who received no treatment (four TCA and one progressive arteriopathy) had a poor functional outcome, compared with 59% of children who received any form of treatment (P = 0.15).
Predictors of progressive arteriopathy, poor outcome and recurrence
Clinical and radiological parameters, available after presentation, history taking and (vascular) imaging, that could predict poor stroke outcome, recurrent ischaemia or a progressive course of the arteriopathy, are summarized in Table 4.
Table 4.
Possible predictors of poor functional outcome, recurrence and progressive arteriopathy in 79 children with unilateral intracranial arteriopathy
| Possible Predictors (nr) | Poor outcome (n = 48) |
Recurrence (n = 17) |
Progressive arteriopathy (n = 5) |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age at stroke | 0.97 (0.86–1.11) | 0.67 | 0.94 (0.79–1.10) | 0.42 | 1.01 (0.79–1.30) | 0.94 |
| Male gender (41) | 1.18 (0.47–2.95) | 0.72 | 1.06 (0.36–3.09) | 0.92 | 0.60 (0.09–3.79) | 0.59 |
| Preceding VZV (32) | 0.57 (0.22–1.44) | 0.23 | 1.01 (0.34–3.01) | 0.99 | a | 0.07 |
| Left hemisphere infarction (49) | 1.29 (0.50–3.33) | 0.60 | 1.45 (0.45–4.66) | 0.53 | 0.83 (0.13–5.29) | 0.84 |
| Cortical localization (17) | 6.14 (1.29–29.22) | 0.02# | 0.41 (0.08–2.00) | 0.27 | 0.89 (0.09–8.54) | 0.92 |
| Corona radiata involvement (10) | 0.58 (0.15–2.21) | 0.43 | 0.90 (0.17–4.70) | 0.90 | a | 1.00 |
| Initial arterial occlusion (18) | 1.86 (0.59–5.88) | 0.29 | 2.27 (0.70–7.38) | 0.17 | 2.42 (0.37–15.73) | 0.36 |
| Occlusion at any time (23) | 3.00 (0.98–9.23) | 0.06 | 2.79 (0.91–8.50) | 0.07 | a | 0.001# |
| Arterial beading (26) | 1.28 (0.48–3.41) | 0.62 | 0.81 (0.25–2.62) | 0.73 | 1.39 (0.22–8.87) | 0.73 |
| Initial moyamoya vessels (2) | 0.62 (0.04–10.25) | 0.74 | a | 1.00 | a | 1.00 |
| Moyamoya vessels at any time (8) | 4.95 (0.58–42.45) | 0.15 | 2.44 (0.52–11.47) | 0.26 | 70 (6.28–780.83) | 0.001# |
| No anticoagulant treatment (68) | 0.36 (0.07–1.81) | 0.21 | 1.11 (0.21–5.80) | 0.90 | a | 1.00 |
| No treatment (5) | a | 0.15 | 2.62 (0.40–17.13) | 0.31 | 4.38 (0.39–48.80) | 0.23 |
| Transient worsening in TCA (14)b | 0.91 (0.28–2.97) | 0.88 | 2.27 (0.58–8.82) | 0.24 | – | – |
| Progressive arteriopathy (5) | a | 0.15 | 18.77 (1.94–181.97) | 0.01# | – | – |
| Progression at any time (19)c | 1.49 (0.50–4.45) | 0.48 | 4.12 (1.30–13.07) | 0.02# | – | – |
| Any recurrence (17) | 0.64 (0.21–1.88) | 0.41 | – | – | – | – |
a Zero patients in one cell, logistic regression impossible. P-values obtained by Fisher's Exact Tests. # Significant association, P < 0.05.
b Transient progression was studied as a predictor of outcome and recurrence only in the group of TCA patients (n = 74).
c Progression of arterial disease detected at any time during vascular imaging follow-up; includes TCA patients with transient worsening and children with progressive arteriopathies.
Significant association, P < 0.05.
Prediction of progressive arteriopathy
Preceding VZV infection showed a trend towards prediction of non-progressive arteriopathy (P = 0.07). Both arterial occlusion, and the presence of moyamoya vessels at any time during the course of the disease, were significantly related to progressive arteriopathy (P = 0.001). Moyamoya vessels remained a significant predictor after accounting for arterial occlusion (P = 0.035).
Prediction of poor outcome
Cortical infarction was significantly associated with poor functional outcome (OR 6.1, 95% CI 1.3–29.2, P = 0.02). Arterial occlusion at initial vascular imaging did not predict stroke outcome, but the detection of an occluded artery at any time during the course of the disease tended to be associated with poor functional outcome (OR 3.0, 95% CI 1.0–9.2, P = 0.06). Multivariate analysis did not change the level of significance for these two possible predictors (P = 0.03 and 0.08, respectively).
Prediction of recurrence
Arterial occlusion at any time during follow-up tended to be related to recurrence (OR 2.8, 95% CI 0.9–8.5, P = 0.07). Progressive arteriopathy was associated with recurrent stroke or TIA (OR 18.8, 95% CI 1.9–182, P = 0.01). Documented progression at any time during follow-up was also significantly associated with recurrence (OR 4.1, 95% CI 1.3–13.1, P = 0.02). The latter two, however, are inherently dependent, since progression at any time included not only the 14 TCA patients with transient worsening, but also the five children with progressive arteriopathy. Within the subgroup of TCA patients, however, documented transient worsening was not significantly related to recurrence (P = 0.25). Multivariate regression modelling revealed that progression at any time remained significantly associated with recurrence, independent of arterial occlusion (P = 0.03).
Discussion
In this study of a large cohort of children with AIS and unilateral intracranial anterior circulation arteriopathy, we found that the majority of patients (94%) had TCA, which was in 44% of children preceded by chickenpox (PVA). None of the patients with progressive arteriopathy had preceding chickenpox. Around one-fifth of children with TCA showed transient worsening of arterial lesions, later stabilizing or improving. Patients with progressive arteriopathy more often showed arterial occlusion, ACA involvement and abnormal collateral moyamoya vessels. Cortical infarction was predictive of poor functional outcome. Arterial occlusive disease tended to be predictive of poor functional outcome and stroke recurrence. Progression of the arteriopathy was significantly related to stoke recurrence.
Pathophysiology and nomenclature of non-progressive childhood arteriopathies
Developments in vascular imaging techniques have improved our understanding of childhood stroke aetiology. Arteriopathies are found in the majority of children with AIS (Ganesan et al., 2003). Since its first description in 1998 (Chabrier et al., 1998), TCA is increasingly recognized as an important cause of AIS in previously healthy children, with a course similar to that of PVA (Lanthier et al., 2005); (Sébire et al., 2004; Sébire, 2006). Several pathophysiological mechanisms may be responsible for TCA.
Inflammation
Many arguments favour a post-infectious inflammatory mechanism underlying TCA. Firstly, there is strong epidemiological evidence for an association between TCA and VZV infections preceding AIS within 12 months (Chabrier et al., 1998; Sébire et al., 1999; Askalan et al., 2001; Ganesan et al., 2003; Sébire et al., 2004; Lanthier et al., 2005; Danchaivijitr et al., 2006; Sébire, 2006; Miravet et al., 2007). VZV, which resides in the trigeminal ganglion, may migrate through the trigeminal nerves innervating the arterial tree at the level of the distal ICA and proximal MCA (the exact area that is affected in TCA) to cause inflammation of the arterial wall. Secondly, in some patients with TCA/PVA there is direct or circumstantial evidence for VZV virus being the causative agent (Riou et al., 2008); cerebrospinal fluid PCR may be positive for VZV (Moriuchi and Rodriguez, 2000; Nagel et al., 2007), there may be proof of intrathecal production of antibodies directed against VZV, and the VZV antigen has occasionally been demonstrated in smooth muscle cells of affected vessel walls (Hausler et al., 1998; Berger et al., 2000). Thirdly, the angiographical appearance, with beading and irregular multisegmental involvement, is highly suggestive of inflammation (Aviv et al., 2006). Fourthly, the angiographical course of arterial disease, with frequent early progression and subsequent stabilization or improvement (Chabrier et al., 1998; Sébire, 2006), leaving residual arterial abnormality in a substantial proportion, suggests an inflammatory mechanism. Fifthly, the clinical onset of symptoms, which is most frequently non-abrupt (Braun et al., 2007), is in accordance with inflammatory disease, leading to progressive arterial wall involvement and stepwise occlusion of the origin of MCA/ACA perforators. Lastly, TCA is also associated with other infectious agents such as enterovirus, Borrelia burgdorferi and HIV (Ribai et al., 2003; Sébire et al., 2004; Cox et al., 2005; Leeuwis et al., 2007).
Literally, an inflammatory arteriopathy can be denominated ‘vasculitis’ or ‘angiitis’. Some of the patients with TCA described here fulfill the diagnostic criteria of ‘primary angiitis of the central nervous system’ (PACNS), an entity that is particularly well known in the adult population, and is characterized by (i) an acquired neurological deficit unexplained by other causes, (ii) evidence of vasculitis in a CNS biopsy specimen or (iii) a cerebral angiogram with changes characteristic of vasculitis, including areas of smooth-wall segmental narrowing or dilation and occlusions that affect multiple cerebral arteries (Salvarani et al., 2007). Indeed, some authors have previously diagnosed patients with infarct localization, angiographical abnormalities and a course of arterial disease that is typical for TCA/PVA, as childhood PACNS (Aviv et al., 2006; Benseler et al., 2006). Not surprisingly, they found that of 62 patients with PACNS, the majority (68%) had a non-progressive course, with focal deficits and almost exclusively unilateral arterial lesions. The description of these patients is in exact accordance with TCA/PVA. The remaining 32% of these children with possible PACNS, however, had a completely different and progressive course, with frequent bilateral and multifocal MRI abnormalities, distal arterial lesions and a clinical syndrome that was more characterized by diffuse neurological signs and headache (Benseler et al., 2006). In some children with PACNS, cerebral angiography is normal, and a lesional brain biopsy is required to diagnose the disorder (Benseler et al., 2005). Progressive and angio-negative PACNS patients, in contrast to TCA/PVA which apparently is a self-limiting disorder, should be treated with long-term immunosuppressive therapy. Although the angiographic appearance of irregular, beaded or multiple arterial lesions was previously described as ‘aggressive’ (Aviv et al., 2006), we found no correlation between beading and the course of the arteriopathy in our study.
Dissection
Although unlikely in view of the specific course of arterial disease, it cannot be excluded that intracranial arterial dissection is at least partly responsible for the vascular pathology seen in some children with TCA (Chabrier et al., 1998; Sébire, 2006). Diagnostic criteria for extracranial arterial dissection in childhood are well defined (Sébire et al., 2004). However, histopathological examination is not available in these patients with non-progressive disease, and the MR demonstration of an intramural haematoma in relatively small intracranial arteries is difficult. Fat-saturated cross-sectional MRI is recommended in patients with suspected dissection to demonstrate blood within the arterial wall (Caplan, 2008). Possibly, high-resolution MRI in a plane perpendicular to the longitudinal axis of the proximal MCA segment could increase the diagnostic yield of MR for intracranial dissections and improve differentiation from inflammatory arteriopathy. Only three of our patients had angiographical evidence of an intimal flap or double lumen, none had a pseudoaneurysm and none suffered from subarachnoid haemorrhage. Medical charts reported an insignificant trauma or minor head injury 1 h to 2 weeks preceding stroke in 12 of our 74 TCA patients. The frequency of recent trauma in healthy children, however, is unknown. Possibly, an inflamed arterial wall is more vulnerable to the development of dissecting lesions. Therefore, some of the patients described here may have had intracranial dissection, as pathologically these conditions are not mutually exclusive. In a recent review, 60% of anterior circulation childhood dissections were intracranial, typically non-traumatic (Fullerton et al., 2001). Since irregular arterial narrowing was considered a sufficient criterion to diagnose dissection and include children in this review, we suggest that some patients with presumed intracranial dissection could just as well have suffered from inflammatory TCA/PVA. In a recent study applying more strict diagnostic criteria for arterial dissection in children, the MCA was involved in only 22% of anterior circulation dissections (Rafay et al., 2006).
Fibromuscular dysplasia
In the past, few case reports have described young patients with unilateral beading dICA and/or MCA arteriopathy considered diagnostic of isolated intracranial fibromuscular dysplasia (FMD) (DiFazio et al., 2000). These patients may have suffered from TCA, a diagnosis that could only have been made after follow-up vascular imaging, revealing disappearance of the typical string of beads sign and stabilization or improvement of arteriopathy. A definitive diagnosis of FMD requires arterial abnormalities involving the ICA and renal arteries, and can only be confirmed by histopathological examination (Sébire et al., 2004).
‘Unilateral Moyamoya’
The angiographical diagnosis of moyamoya requires bilateral stenosis or occlusion of the terminal ICA or its branches, accompanied by an uni- or bilateral abnormal collateral network of small lenticulostriatal vessels (Sébire et al., 2004). However, several studies have reported unilateral onset of moyamoya (also called ‘possible moyamoya’), with or without later progression into bilateral disease. From a pooled series of 52 children with unilateral angiographical moyamoya, 25 (48%) were shown to develop bilateral moyamoya at follow-up angiography after an interval of months to years (Kawano et al., 1994; Matsushima et al., 1994; Houkin et al., 1996; Hirotsune et al., 1997; Kelly et al., 2006; Seol et al., 2006). Most of these children were surgically treated in the initial unilateral stage of their disease. In our study, the frequency of unilateral moyamoya vessels is probably underestimated, since six of eight patients with persistent occlusive arterial disease underwent MRA as their last imaging procedure, whereas conventional angiography is superior in the detection of these abnormal small collaterals. Nevertheless, it is evident that, first, occlusive arterial disease may very well improve and even normalize; second, even when vascular occlusion is accompanied by the formation of reactive moyamoya collaterals, the arteriopathy may stabilize and even improve in time. Therefore, ‘unilateral moyamoya’ is possibly at one end of the spectrum of non-progressive intracranial arteriopathies, and may therefore be diagnosed as TCA or PVA, provided that long-term angiographical follow-up does not reveal bilateral evolution of disease (Fig. 5). Surgical treatment in children with unilateral distal ICA occlusions should therefore ideally only be performed when the patient suffers from recurrent symptoms and repeated angiography has ruled out improving arterial disease.
Incidence of TCA/PVA
The majority of this consecutive cohort of previously healthy children, selected for unilateral intracranial arteriopathy, have disease which is eventually non-progressive. Little is known about the frequency of TCA/PVA among the entire population of children with AIS. In a Toronto cohort of 98 children with AIS, 30 (31%) were diagnosed with TCA/PVA, which constitutes 43% of the children who were previously healthy (Braun et al., 2007). In a selected British cohort of 50 children who underwent MRA within 3 months after stroke and repeated MRA at least 1 month later, 24 (48%) showed improvement with time and could be diagnosed with TCA (Danchaivijitr et al., 2006). This, however, may be an underestimate; some children with ‘unchanged’ arteriopathy probably also fulfilled the diagnostic criteria for TCA (i.e. no worsening after 6 months), and in several patients with a presumed progressive arteriopathy, worsening of arterial lesions could have occurred during the first months after stroke which is not incompatible with the diagnosis of TCA (Sébire et al., 2004; Sébire, 2006). In our study, the 74 children with proven TCA represented 20% of the total cohort of 372 children with AIS, and 32% of those who were previously healthy. In another 28 children, angiographical findings were suspect of TCA, but vascular imaging follow-up was not performed or unavailable. The true frequency of TCA in our cohort could therefore be as high as 27%. Naturally, referral biases to the above three specialized childhood stroke centres may have influenced the reported frequencies.
Outcome, recurrence rate and treatment
Although the inflammatory phase of TCA is supposedly transient, 59% of children are left with permanent neurological deficits, and 77% have residual arterial lesions at repeated angiography. In this study, the overall recurrence rate of ischaemic symptoms was 22%, with 18% recurrences in the TCA group. This probably is an underestimate, because recurrent events during the first week after presentation were not included in the analysis, since these may be considered part of the index stroke. Three recent publications have in depth addressed recurrence rates in childhood stroke. In the study of Sträter et al. (2002), 6.6% of children with an AIS had a recurrent ischaemic stroke. TIAs were not included in the endpoint. Thirty percent of previously healthy children with AIS in London had a recurrent stroke or TIA (Ganesan et al., 2006). In a Californian population-based cohort study, the 5-year cumulative recurrence rate after childhood ischaemic stroke was 19% (Fullerton et al., 2007). In all three studies, children with vasculopathy were shown to be particularly at risk. It has recently been suggested that children with progressive arteriopathies have a higher risk of stroke recurrence, although this did not reach statistical significance (Danchaivijitr et al., 2006). We confirm that the risk of stroke recurrence in previously healthy children with unilateral intracranial arteriopathy is significantly associated with progression of arterial disease.
There are relatively few clinical and radiological parameters that may, based on this study, help predict the course of childhood unilateral intracranial arteriopathy, outcome and recurrence and crucially, these may not be available at initial presentation. Preceding chickenpox tends to be associated with non-progressive arterial disease. A mainly cortical localization is associated with poor functional outcome. Arterial occlusion at any (initial or follow-up) angiography may predict poor outcome and stroke recurrence. Occlusion and moyamoya vessels are associated with progressive arteriopathy. When the arteriopathy completely normalizes in TCA, functional outcome tends to be better. Finally, documented progression at any time during follow-up increases the chance of recurrent symptoms. We found no relation between outcome or recurrence and arterial beading and antiplatelet or anticoagulant treatment. Obviously, the limited statistical power of our study hampers the ability to identify all independent predictors of outcome, recurrence and course of the arteriopathy. We will in future studies address the predictive value of laboratory investigation in childhood arteriopathies.
Given the large number of patients with residual neurological deficits and the high recurrence rate in the early stages after stroke in this cohort of children, investigation of acute treatment and appropriate secondary prevention strategies seems mandatory. Given the presumed inflammatory nature of disease, a short-term treatment with immunosuppressives, possibly combined with antiviral medication when stroke is preceded by VZV, is the most rational choice of treatment in children presenting with unilateral intracranial arteriopathy (deVeber, 2005; Benseler et al., 2006; Braun et al., 2007; Miravet et al., 2007). Randomized controlled trials are needed to prove efficacy of such a treatment regimen.
In conclusion, the vast majority of children with AIS and anterior circulation intracranial arteriopathy suffer from TCA, presumably of inflammatory origin, that is associated with preceding VZV in 44% of cases. Many of these children have documented transient worsening of arteriopathy, with later stabilization or improvement. When acute vascular imaging demonstrates intracranial arteriopathy in children with AIS, MRA should be repeated 3–6 months after diagnosis, and again at 6–12 months in most patients (Sébire et al., 2004; Sébire, 2006), since prediction of the eventual course of arterial disease is shown to be difficult based on criteria available at stroke onset. ‘Transient cerebral arteriopathy’ is a frequent cause of childhood AIS, specifically in previously healthy children. Because most children have permanent neurological sequelae and residual arterial lesions, rigorous assessement of anti-inflammatory treatment seems rational.
Funding
Wilhelmina Research Fund (to M.B.); Maarten Kappelle Foundation (to M.B.). Inserm (Institut National de la Santé et de la Recherche Médicale to S.C.); R&D funding from the National Health Service executive (to UK work); Wellcome Trust (0353521/B/92/2 to F.J.K.); CIHR, FRSQ and La Fondation des Etoiles pour la Recherche sur les Maladies Infantiles, Canada (to G.S.).
Acknowledgements
We gratefully acknowledge the patients and their parents and all paediatricians, neurologists and paediatric neurologists who referred their stroke patients to our centres.
Glossary
Abbreviations
- AIS
arterial ischaemic stroke
- (d)ICA
(distal) internal carotid artery
- DSA
digital subtraction contrast angiography
- (p)ACA
(proximal) anterior cerebral artery
- (p)MCA
(proximal) middle cerebral artery
- PACNS
primary angiitis of the central nervous system
- PVA
post-varicella angiopathy
- TCA
transient cerebral arteriopathy
- VZV
varicella zoster virus
References
- Askalan R, Laughlin S, Mayank S, Chan A, MacGregor D, Andrew M, et al. Chickenpox and stroke in childhood: a study of frequency and causation. Stroke. 2001;32:1257–62. doi: 10.1161/01.str.32.6.1257. [DOI] [PubMed] [Google Scholar]
- Aviv RI, Benseler SM, Silverman ED, Tyrrell PN, deVeber G, Tsang LM, et al. MR imaging and angiography of primary CNS vasculitis of childhood. AJNR Am J Neuroradiol. 2006;27:192–9. [PMC free article] [PubMed] [Google Scholar]
- Benseler SM, deVeber G, Hawkins C, Schneider R, Tyrrell PN, Aviv RI, et al. Angiography-negative primary central nervous system vasculitis in children: a newly recognized inflammatory central nervous system disease. Arthritis Rheum. 2005;52:2159–67. doi: 10.1002/art.21144. [DOI] [PubMed] [Google Scholar]
- Benseler SM, Silverman E, Aviv RI, Schneider R, Armstrong D, Tyrrell PN, et al. Primary central nervous system vasculitis in children. Arthritis Rheum. 2006;54:1291–7. doi: 10.1002/art.21766. [DOI] [PubMed] [Google Scholar]
- Berger TM, Caduff JH, Gebbers JO. Fatal varicella-zoster virus antigen-positive giant cell arteritis of the central nervous system. Pediatr Infect Dis J. 2000;19:653–6. doi: 10.1097/00006454-200007000-00015. [DOI] [PubMed] [Google Scholar]
- Braun KP, Kappelle LJ, Kirkham FJ, deVeber G. Diagnostic pitfalls in paediatric ischaemic stroke. Dev Med Child Neurol. 2006;48:985–90. doi: 10.1017/S0012162206002167. [DOI] [PubMed] [Google Scholar]
- Braun KP, Rafay MF, Uiterwaal CS, Pontigon AM, deVeber G. Mode of onset predicts etiological diagnosis of arterial ischemic stroke in children. Stroke. 2007;38:298–302. doi: 10.1161/01.STR.0000254484.10680.c6. [DOI] [PubMed] [Google Scholar]
- Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4:34–42. doi: 10.1038/ncpneuro0683. [DOI] [PubMed] [Google Scholar]
- Chabrier S, Rodesch G, Lasjaunias P, Tardieu M, Landrieu P, Sébire G. Transient cerebral arteriopathy: a disorder recognized by serial angiograms in children with stroke. J Child Neurol. 1998;13:27–32. doi: 10.1177/088307389801300105. [DOI] [PubMed] [Google Scholar]
- Cox MG, Wolfs TF, Lo TH, Kappelle LJ, Braun KP. Neuroborreliosis causing focal cerebral arteriopathy in a child. Neuropediatrics. 2005;36:104–7. doi: 10.1055/s-2005-837573. [DOI] [PubMed] [Google Scholar]
- Danchaivijitr N, Cox TC, Saunders DE, Ganesan V. Evolution of cerebral arteriopathies in childhood arterial ischemic stroke. Ann Neurol. 2006;59:620–6. doi: 10.1002/ana.20800. [DOI] [PubMed] [Google Scholar]
- deVeber G. Risk factors for childhood stroke: little folks have different strokes! Ann Neurol. 2003;53:149–50. doi: 10.1002/ana.10461. [DOI] [PubMed] [Google Scholar]
- deVeber G. In pursuit of evidence-based treatments for paediatric stroke: the UK and Chest guidelines. Lancet Neurol. 2005;4:432–6. doi: 10.1016/S1474-4422(05)70120-4. [DOI] [PubMed] [Google Scholar]
- DiFazio M, Hinds SR, Depper M, Tom B, Davis R. Intracranial fibromuscular dysplasia in a six-year-old child: a rare cause of childhood stroke. J Child Neurol. 2000;15:559–62. doi: 10.1177/088307380001500814. [DOI] [PubMed] [Google Scholar]
- Fullerton HJ, Johnston SC, Smith WS. Arterial dissection and stroke in children. Neurology. 2001;57:1155–60. doi: 10.1212/wnl.57.7.1155. [DOI] [PubMed] [Google Scholar]
- Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics. 2007;119:495–501. doi: 10.1542/peds.2006-2791. [DOI] [PubMed] [Google Scholar]
- Fung LW, Thompson D, Ganesan V. Revascularisation surgery for paediatric moyamoya: a review of the literature. Childs Nerv Syst. 2005;21:358–64. doi: 10.1007/s00381-004-1118-9. [DOI] [PubMed] [Google Scholar]
- Ganesan V, Prengler M, McShane MA, Wade AM, Kirkham FJ. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003;53:167–73. doi: 10.1002/ana.10423. [DOI] [PubMed] [Google Scholar]
- Ganesan V, Prengler M, Wade A, Kirkham FJ. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation. 2006;114:2170–7. doi: 10.1161/CIRCULATIONAHA.105.583690. [DOI] [PubMed] [Google Scholar]
- Hausler MG, Ramaekers VT, Reul J, Meilicke R, Heimann G. Early and late onset manifestations of cerebral vasculitis related to varicella zoster. Neuropediatrics. 1998;29:202–7. doi: 10.1055/s-2007-973561. [DOI] [PubMed] [Google Scholar]
- Hirotsune N, Meguro T, Kawada S, Nakashima H, Ohmoto T. Long-term follow-up study of patients with unilateral moyamoya disease. Clin Neurol Neurosurg. 1997;99(Suppl 2):S178–81. doi: 10.1016/s0303-8467(97)00043-7. [DOI] [PubMed] [Google Scholar]
- Houkin K, Abe H, Yoshimoto T, Takahashi A. Is ‘unilateral’ moyamoya disease different from moyamoya disease? J Neurosurg. 1996;85:772–6. doi: 10.3171/jns.1996.85.5.0772. [DOI] [PubMed] [Google Scholar]
- Husson B, Lasjaunias P. Radiological approach to disorders of arterial brain vessels associated with childhood arterial stroke-a comparison between MRA and contrast angiography. Pediatr Radiol. 2004;34:10–15. doi: 10.1007/s00247-003-1109-0. [DOI] [PubMed] [Google Scholar]
- Kawano T, Fukui M, Hashimoto N, Yonekawa Y. Follow-up study of patients with ‘unilateral’ moyamoya disease. Neurol Med Chir (Tokyo) 1994;34:744–7. doi: 10.2176/nmc.34.744. [DOI] [PubMed] [Google Scholar]
- Kelly ME, Bell-Stephens TE, Marks MP, Do HM, Steinberg GK. Progression of unilateral moyamoya disease: a clinical series. Cerebrovasc Dis. 2006;22:109–15. doi: 10.1159/000093238. [DOI] [PubMed] [Google Scholar]
- Kirkham FJ, Hogan AM. Risk factors for arterial ischemic stroke in childhood. CNS Spectr. 2004;9:451–64. [PubMed] [Google Scholar]
- Lanthier S, Armstrong D, Domi T, deVeber G. Post-varicella arteriopathy of childhood: natural history of vascular stenosis. Neurology. 2005;64:660–3. doi: 10.1212/01.WNL.0000151851.66154.27. [DOI] [PubMed] [Google Scholar]
- Lanthier S, Lortie A, Michaud J, Laxer R, Jay V, deVeber G. Isolated angiitis of the CNS in children. Neurology. 2001;56:837–42. doi: 10.1212/wnl.56.7.837. [DOI] [PubMed] [Google Scholar]
- Leeuwis JW, Wolfs TF, Braun KP. A child with HIV-associated transient cerebral arteriopathy. AIDS. 2007;21:1383–4. doi: 10.1097/QAD.0b013e3281053a30. [DOI] [PubMed] [Google Scholar]
- Lynch JK, Hirtz DG, deVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116–23. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- Matsushima T, Inoue T, Natori Y, Fujii K, Fukui M, Hasuo K, et al. Children with unilateral occlusion or stenosis of the ICA associated with surrounding moyamoya vessels—‘unilateral’ moyamoya disease. Acta Neurochir (Wien) 1994;131:196–202. doi: 10.1007/BF01808612. [DOI] [PubMed] [Google Scholar]
- Miravet E, Danchaivijitr N, Basu H, Saunders DE, Ganesan V. Clinical and radiological features of childhood cerebral infarction following varicella zoster virus infection. Dev Med Child Neurol. 2007;49:417–22. doi: 10.1111/j.1469-8749.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- Moriuchi H, Rodriguez W. Role of varicella-zoster virus in stroke syndromes. Pediatr Infect Dis J. 2000;19:648–53. doi: 10.1097/00006454-200007000-00014. [DOI] [PubMed] [Google Scholar]
- Nagel MA, Forghani B, Mahalingam R, Wellish MC, Cohrs RJ, Russman AN, et al. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology. 2007;68:1069–73. doi: 10.1212/01.wnl.0000258549.13334.16. [DOI] [PubMed] [Google Scholar]
- Rafay MF, Armstrong D, Deveber G, Domi T, Chan A, MacGregor DL. Craniocervical arterial dissection in children: clinical and radiographic presentation and outcome. J Child Neurol. 2006;21:8–16. doi: 10.1177/08830738060210010101. [DOI] [PubMed] [Google Scholar]
- Ribai P, Liesnard C, Rodesch G, Giurgea S, Verheulpen D, David P, et al. Transient cerebral arteriopathy in infancy associated with enteroviral infection. Eur J Paediatr Neurol. 2003;7:73–5. doi: 10.1016/s1090-3798(03)00016-3. [DOI] [PubMed] [Google Scholar]
- Riou EM, Amlie-Lefond C, Echenne B, Farmer M, Sébire G. Cerebrospinal fluid analysis in the diagnosis and treatment of arterial ischemic stroke. Pediatr Neurol. 2008;38:1–9. doi: 10.1016/j.pediatrneurol.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Salvarani C, Brown RD Jr, Calamia KT, Christianson TJ, Weigand SD, Miller DV, et al. Primary central nervous system vasculitis: analysis of 101 patients. Ann Neurol. 2007;62:442–51. doi: 10.1002/ana.21226. [DOI] [PubMed] [Google Scholar]
- Sébire G. Transient cerebral arteriopathy in childhood. Lancet. 2006;368:8–10. doi: 10.1016/S0140-6736(06)68944-7. [DOI] [PubMed] [Google Scholar]
- Sébire G, Fullerton H, Riou E, deVeber G. Toward the definition of cerebral arteriopathies of childhood. Curr Opin Pediatr. 2004;16:617–22. doi: 10.1097/01.mop.0000144441.29899.20. [DOI] [PubMed] [Google Scholar]
- Sébire G, Meyer L, Chabrier S. Varicella as a risk factor for cerebral infarction in childhood: a case-control study. Ann Neurol. 1999;45:679–80. doi: 10.1002/1531-8249(199905)45:5<679::aid-ana22>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Seol HJ, Wang KC, Kim SK, Lee CS, Lee DS, Kim IO, et al. Unilateral (probable) moyamoya disease: long-term follow-up of seven cases. Childs Nerv Syst. 2006;22:145–50. doi: 10.1007/s00381-005-1234-1. [DOI] [PubMed] [Google Scholar]
- Sträter R, Becker S, von EA, Heinecke A, Gutsche S, Junker R, et al. Prospective assessment of risk factors for recurrent stroke during childhood – a 5-year follow-up study. Lancet. 2002;360:1540–5. doi: 10.1016/S0140-6736(02)11520-0. [DOI] [PubMed] [Google Scholar]