Abstract
Neural networks for processing language often are reorganized in patients with epilepsy. However, the extent and location of within and between hemisphere re-organization are not established. We studied 45 patients, all with a left hemisphere seizure focus (mean age 22.8, seizure onset 13.3), and 19 normal controls (mean age 24.8) with an fMRI word definition language paradigm to assess the location of language processing regions. Individual patient SPM maps were compared to the normal group in a voxel-wise comparison; a voxel was considered to be significant if its z-value exceeded ∣2∣. Subsequently, we used principal component analysis with hierarchical clustering of variance patterns from individual difference maps to identify four patient sub-groups. One did not differ from normal controls; one had increased left temporal activation on the margin of regions activated in controls; two others had recruitment in right inferior frontal gyrus, middle frontal gyrus and temporal cortex. Right hemisphere activation in these two groups occurred in homologues of left hemisphere regions that sustained task activation. Our study used novel data driven methods to find evidence for constraints on inter-hemispheric reorganization of language in recruitment of right homologues, and, in a subpopulation of patients, evidence for intra-hemispheric reorganization of language limited to the margins of typical left temporal regional activation. These methods may be applied to investigate both normal and pathological variance in other developmental disorders and cognitive domains.
Keywords: fMRI, language, epilepsy
Introduction
One-third of patients considered for epilepsy surgery exhibit either bilateral or right hemispheric dominance (‘atypical language dominance’) for language assessed by the intra-carotid amobarbital test (IAT) or fMRI (Rasmussen and Milner, 1977; Gaillard et al., 2007) compared with 5% of the normal right-handed population (Pujol et al., 1999; Springer et al., 1999; Szaflarski et al., 2002). These differences are thought to represent either re-organization, where the primary region of language processing has moved, or compensation, where additional areas are recruited to assist in language processing. Inter-hemispheric shifts in language processing are thought to occur in right-sided homologues of Broca's and Wernicke's areas based on visual and region of interest (ROI) studies (Staudt et al., 2001, 2002; Gaillard et al., 2002, 2004, 2007). Intra-hemispheric reorganization/compensation, however, is more difficult to assess. Electro-cortical stimulation (ECS) studies suggest identification of language cortex in areas adjacent to classical language processing areas but normative data are not available for comparison (Ojemann et al., 1989; Devinsky et al., 1993; Hamberger et al., 2007a, b; Sanai et al., 2008). The two standard methods of MRI analysis both suffer from inherent weaknesses. ROI-based approaches are constrained by a priori assumptions and analyses restricted to preselected regions. In contrast, a limitation of traditional fMRI group analysis techniques (e.g. random effects) is that individual heterogeneity is lost (Berl et al., 2005; Price et al., 2006).
In order to overcome these limitations, we used adaptations of voxel based approaches applied to data driven sorting algorithms (Eriksson et al., 2001; Liu et al., 2001; Rugg-Gunn et al., 2001, 2003; Kim et al., 2002, 2003; Turkeltaub et al., 2004; Fair et al., 2006). We tested the hypothesis that patients with left hemisphere onset epileptic foci would exhibit evidence of:
Inter-hemispheric reorganization restricted to right homologues of Broca's and Wernicke's areas; and
Intra-hemispheric reorganization in brain regions adjacent to those areas that typically sustain language.
Further analysis was conducted to test whether identified sub-groups showed differences according to demographic or clinical variables including age of epilepsy onset, duration of seizures, pathology or indicator of early brain insult.
Methods
Subjects
We studied 45 English-speaking patients with a left hemisphere seizure focus determined by clinical characteristics, video EEG and structural MRI. Their mean age was 22.8 years (range 9–57 years), with mean age seizure onset 13.3 years (range 1–38 years) and mean duration of seizures for 9.9 years (range 0–55 years) (Table 1). Eleven patients were younger than 18 years (range 9–15 years). Thirty-one (69%) patients had a temporal focus and 14 (31%) had an extra-temporal focus. Twenty-five (56%) patients had a normal MRI while the remaining patients had an abnormal MRI: 10 (22%) tumour/focal cortical dysplasia; 6 (13%) stroke and 4 (9%) mesial temporal sclerosis (MTS). Eight patients (18%) had atypical handedness determined by clinical assessment. Indication of an early insult was defined as positive if at least one of the following was true: onset was before age 6 years, developmental tumour, focal cortical dysplasia, age at stroke or atypical handedness. Twenty-seven (60%) of the patients had some indication of an early insult. Twenty-three patients were included in a previous study (Gaillard et al., 2007).
Table 1.
Demographic data
| Patients | Normals | |
|---|---|---|
| Number | 45 | 19 |
| Male (%) | 47 | 53 |
| Mean age (years) | 22.8 | 24.8 |
| Age range (years) | 9–57 | 21–56 |
| Right handed (%) | 82 | 100 |
| Mean age of seizure onset (range) | 13.3 years (1–38) | – |
| Mean duration of seizures (range) | 9.9 years (0–55 | – |
| Temporal focus (%) | 69 | – |
| Indication of early insult (%) | 60 | – |
| Normal MRI (%) | 56 | – |
Nineteen right handed, native English speaking, healthy volunteers, mean age 24.8 years (range 21–56), with typical language laterality determined by bootstrap ROI methods constituted the normal control group (Wilke and Lidzba, 2007). We excluded healthy controls with atypical dominance as their inclusion might unduly influence the analysis using the VBM methods described below. Previous investigations have demonstrated that a minimum of 15 healthy subjects is needed to detect reliably left hemisphere language activated regions (Seghier et al., 2008).
Image acquisition
The participants were scanned using whole-brain BOLD functional MRI at 3 T (General Electric Medical Systems, Milwaukee, WI). Gradient echo-planar images were collected using TE (echo time) = 30 ms, field of view (FOV) = 22 × 22 cm, acquisition matrix = 64 × 64, and inter-scan interval (TR) = 2000 ms. Brain volumes consisted of 28 × 4 mm thick axial thick slices. Anatomical images were collected using a 3D fast SPGR sequence and brain volumes consisting of 28 × 4 mm thick axial slices. Images were collected parallel to the anterior commissure–posterior commissure plane (Gaillard et al., 2007).
Experimental paradigm
The fMRI task, an auditory description decision paradigm, is designed to engage and identify the broad language processing network on an individual basis necessary for effective presurgical evaluation. Tasks that require phonological and semantic verbal fluency activate dominant inferior frontal cortex (Broca's area); tasks that incorporate semantic decisions also preferentially activate BA 47 (Poldrack et al., 1999; Bookheimer, 2002). These tasks have also been associated with activation in the left dorsolateral prefrontal cortex (MFG BA 9,46), postulated to be implicated in working memory aspects of task, and also anterior cingulate and mesial frontal cortex that are postulated to reflect attentional demands and motor planning (Binder et al., 1995; Wood et al., 2001). Paradigms that have been used successfully to identify Broca's area for planning epilepsy surgery include verbal fluency (Hertz-Pannier et al., 1997; Yetkin et al., 1998; Lehericy et al., 2000; Fernandez et al., 2001; Ramsey et al., 2001; Adcock et al., 2003; Woermann et al., 2003; Gaillard et al., 2004) and semantic decision (Binder et al., 1996; Frost et al., 1999; Carpentier et al., 2001). Tasks that stress comprehension of sentences and phrases yield stronger activation in Wernicke's area than do fluency and single word semantic decision tasks (Schlosser et al., 1999; Lehericy et al., 2000; Gaillard et al., 2001, 2002, 2004; Staudt et al., 2001; Wise et al., 2001; Thivard et al., 2005); tasks that combine aspects of comprehension and semantic decision of fluency provide both (Bookheimer et al., 1995; Carpentier et al., 2001). Findings from these latter studies have been confirmed by invasive assessments of language systems lateralization by IAT (Carpentier et al., 2001; Gaillard et al., 2002) and localization by ECS (Malow et al., 1996; Bookheimer et al., 1997; Hamberger et al., 2005, 2007a) and thus were adapted for this study. The active condition requires a semantic decision based on a word definition (A long yellow fruit is a banana) identified with a button press while the control condition consisted of reverse speech with a button press upon hearing an after-going tone. The use of sentences rather than single words is designed to activate left temporal ‘receptive’ language cortex, the semantic retrieval and decision aspect of the task is targeted to activate left inferior frontal ‘expressive’ cortex (Gaillard et al., 2007). The task likely draws upon verbal working memory (left mid frontal cortex) and areas implicated in attention and planning in mesial left frontal cortex. Seventy percent of items were correct targets, 30% foils. The task was presented in a block design with five 30 s blocks of alternating active and control conditions for a total time of 5 min. The control and experimental stimuli were presented using the Windows-based program E-prime. Auditory stimuli were digitized and presented via pneumatic earphones. Patients were instructed to remain silent and motionless. Responses were performed via fibre-optic push button response recorded by PC in E-prime version 1.1 (Psychology Software tools, Inc., Pittsburgh, PA).
Data analysis
All image data processing was performed using Statistical Parametric Mapping software (SPM2) (University College London, London) and the Statistical Analysis Toolbox through Matlab (The MathWorks, Inc; Natick, MA). Rendered volumes were created using MRIcroN (Chris Rorden, University of South Carolina, Columbia, SC). Initial preprocessing of the data was performed in SPM2. The volumes were realigned to correct for motion, normalized to a standard brain volume (MNI) and then smoothed to 8 mm FWHM. Individual t maps (contrast images) were then generated by comparing the active and control conditions on a voxel-wise basis using a fixed effects model. A group map for the normal control group was also generated using a random effects analysis. Three further analyses were performed on the individual data sets in order to identify how each individual patient differed from the normal group on a whole brain basis and then to identify common patterns among the individual patients that define subgroups and characterize differing forms of re-organization or compensation.
Individual z-score maps
A voxel wise z-score map for each patient was generated. To do this, a mean and a standard deviation map of the contrast images of the normals were computed. Individual z-score images were thus obtained for each patient by applying the formula (Valuepatient – Meannormals)/SDnormals at each voxel (Fair et al., 2006). The result of this process was a three dimensional brain volume—an activation difference map—for each patient where the value of each voxel indicated the number of standard deviations by which signal intensity in the patient voxel differed from the normal group mean (z-score). A voxel was considered to be significant if its |z|-value exceeded 2 following Fair et al. (2006). To validate this method, the process was repeated on the controls using a ‘leave one out’ method: a z-score map of each control subject compared to the other 18 controls was computed. We performed an additional, more conservative analysis on difference maps |z| > 3.
Collective penetrance maps
To assess the range and location of patient population variability compared to the control group, a masked image of each z-score map was created. For each patient, any voxel exceeding the statistical threshold of 2 SDs was given a value of 1 and any voxel within 2 SDs was given a value of 0. All of the patients’ masked images were then used to create an average image which was multiplied by 100. The resulting value of each voxel indicated the percentage of the patient population who had a significant value for that voxel. This process, and the resulting maps, is similar to percentage of overlap and penetrance methods (Xiong et al., 2000; Seghier et al., 2008). A collective map was generated for all 45 patients. Collective penetrance maps were also generated for subsets of patients according to groups defined by a data driven classification system described in the following section. To validate our use of collective penetrance maps, this process was also performed on the controls’ z-score maps that had been created using the leave one out method. The data from the individual z-score maps and collective maps were overlaid on a group map of activation of the control group. The group map was generated through a random effects analysis using the contrast images from the first level analysis.
Classification using z-score maps
In addition to assessing the consistency of areas of significant variability across patients, the individual z-score values were used to separate the patient population into groups using classification techniques. All voxels exceeding the threshold were included in the analysis to account for both peak values and cluster sizes. The volume of data was first reduced through decimation and then classified using principal components analysis (PCA) and k-means clustering. PCA is often used to explore relationships between variables in large data sets through strategic data reduction. It has been demonstrated to be useful in multidimensional data reduction for classification of fMRI data (Dunteman, 1989; Zhang et al., 2005). K-means analysis is used to divide a sample of points into clusters that minimize intra-cluster variance represented by the sum of squared distances between all points and the cluster centre (Pollard, 1981; Ray, 1999). We used k-means clustering to separate the patients into groups based on the results of the PCA of their z-score maps. The number of groups to generate through the k-means cluster analysis was estimated from a dendrogram of the Euclidian distance matrix of the PCA data.
To determine whether groups identified through k-means clustering also showed relevant differences along demographic or clinical variables, group differences were tested using chi square analysis for categorical data, and t-test and analysis of variance (ANOVA) for continuous variables.
Results
The normal group map activation results are shown as grey voxels in the axial slice Figs 1 and 2 (FDR P < 0.05 corrected). Table 2 provides the coordinates for the activation maxima for the task. Task activation occurred predominantly along the left superior temporal sulcus/mid temporal gyrus (STS/MTG), the left inferior frontal gyrus, left middle frontal gyrus, mesial superior frontal cortex and caudate. There were less pronounced areas of activation in right IFG and MTG (lower z-score, smaller extent). The individual z-score difference maps showed areas in each patient where their patterns of activation differed from the control group (z-score > 2). Z-score maps from a representative patient with typical language laterality and a representative patient with atypical language laterality are displayed in Fig. 1A and B.
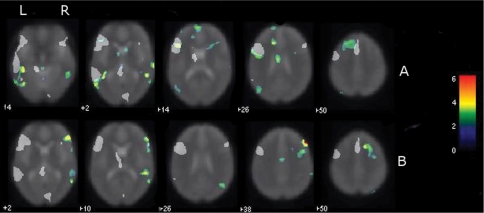
Fig. 1.
Individual difference maps. Grey areas that represent regions ‘activated’ in the control group map at FDR P < 0.05 have been overlaid on the difference maps. Left image is left brain. Colour scale represents voxel z-score, only those voxels with |z| > 2 are shown. Thus only voxels where the patient differed from the control group are shown. (A) z-Score difference map from representative patient with typical language laterality. Note the ‘difference activation’ in left inferior frontal cortex (IFG) demonstrating activation beyond that seen in normal populations in an area typically associated with the task in the normal population. Difference activation is also seen posterior and superior to areas typically activated in controls in the left temporal lobe. (B) z-Score difference map from patient with atypical language laterality. This patient shows difference activation beyond that seen in the normal left language dominant control group in right IFG, middle temporal gyrus and middle frontal gyrus and left cerebellum.
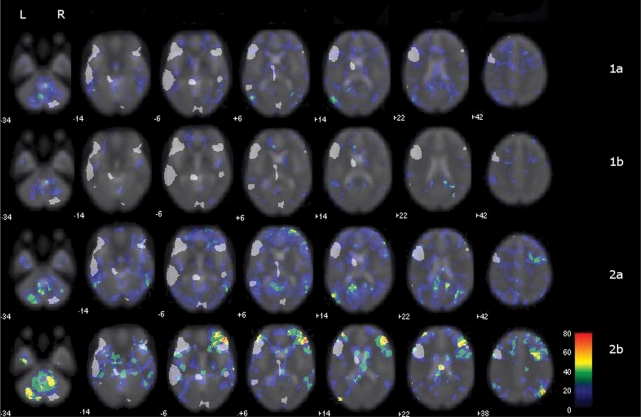
Fig. 2.
Collective group difference penetrance maps. Grey areas that represent regions ‘activated’ in the control group map at FDR P < 0.05 have been overlaid on the group difference penetrance maps. Left image is left brain. The colour bar represents the percent of patients with activation beyond 2 SD |z| for each voxel. Group 1a with difference activation clusters in left temporal lobe. Group 1b exhibits little difference from the normal population. Group 2a, clusters of difference activation in right temporal and right inferior frontal areas. Group 2b clusters of difference activation primarily in right inferior and middle frontal gyri and a right angular gyrus.
Table 2.
Activation location, cluster size and peak values for control group
| Cluster size | z-score | x, y, z values | Region (BA) |
|---|---|---|---|
| 3794 | 5.75 | −42, 32, 18 | LIFG (44/45) |
| 1818 | 4.87 | −52, −32, −8 | LMTG (21) |
| 594 | 4.42 | 10, −84, −32 | R Cerebellum |
| 531 | 4.16 | 40, 26, −6 | RIFG (47) |
| 930 | 3.98 | −10, −8, 6 | L Thalamus |
| 472 | 3.95 | −2, 12, 56 | L Mesial SFG (6) |
| 240 | 3.89 | −50, 2, 54 | LMFG (6) |
| 322 | 3.67 | −20, −4, −12 | L Hippocampus |
| 127 | 3.39 | 46, −30, −6 | RMTG (21) |
| 45 | 3.28 | 58, 22, 10 | RIFG (44/45) |
| 18 | 3.16 | −14, 10, −4 | L Putamen |
| 21 | 3.02 | −6, 18, 6 | L Caudate |
(FDR corrected, P < 0.05).
L = left; R = right; IFG = inferior frontal gyrus; MFG = middle frontal gyrus; SFG = superior frontal gyrus.
The collective ‘penetrance’ map for all patients showed uniformly low values across the collective map when all patients were grouped together, reflecting the variability among the patients. However, subsets of patients were identified based upon patterns of difference map activation identified through the PCA and k-means clustering analysis. K-means cluster analysis identified two broad groups, one with difference maps involving left hemisphere activation [group 1, n = 25 (56%)] and one with right-sided activation [group 2, n = 20 (44%)]. Within each of these two broad groups, two further subsets were identified (Table 3). Group 1a featured a remarkable cluster of difference voxels in the posterior left posterior superior temporal sulcus [Table 4, Fig. 2(1a)]. Group 1b did not have any voxel clusters exceeding mean normal group voxel signal intensity; these patients showed activation patterns comparable with controls [Fig. 2(1b)]. Group 2a had clusters within the right inferior frontal gyrus, middle frontal gyrus, superior frontal gyrus, middle temporal gyrus, right cingulate and left cerebellum [Table 4, Fig. 2(2a)]. Group 2b had clusters primarily in the right inferior gyrus, and middle frontal gyrus, in addition to superior frontal gyrus, right angular gyrus and left cerebellum [Table 4, Fig. 2(2b)].
Table 3.
Groups of patients as divided through k-means clustering
| Group | N | Mean age | Age range | Mean age of onset | Age range onset |
|---|---|---|---|---|---|
| 1a | 16 | 18.8 | 9–30 | 14.1 | 5–30 |
| 1b | 9 | 30.5 | 16–57 | 12.3 | 2–29 |
| 2a | 14 | 24.8 | 9–50 | 14.1 | 1–38 |
| 2b | 6 | 17.4 | 10–27 | 11.0 | 3–16 |
| 1 (combined a and b) | 25 | 23.2 | 9–57 | 13 | 2–30 |
| 2 (combined a and b) | 20 | 22.6 | 9–50 | 13 | 1–38 |
Table 4.
Clusters of voxels with relatively high values for patient groups as determined by k-means clustering for difference maps with z > 2
| Group | Peak coordinates | Peak value (%) | Cluster size | Region |
|---|---|---|---|---|
| 1a | −52, −72, 10 | 50 | 18 | Left Posterior STS (39) |
| 26, −70, −44 | 56 | 79 | R Cerebellum | |
| 1b | NA | NA | NA | NA |
| 2a | 54, 40, 10 | 57 | 12 | R IFG (45) |
| 30, 12, 60 | 50 | 23 | R SFG (6) | |
| 24, 16, 46 | 50 | 12 | R MFG (6/8) | |
| 54, −50, 0 | 43 | 19 | R MTG (21) | |
| 30, −72, 22 | 64 | 97 | RMTG/OG (19/39) | |
| −4, −58, −50 | 50 | 26 | L Cerebellum | |
| 14, −48, 22 | 64 | 41 | R posterior cingulate | |
| 2b | 40, 34, 2 | 67 | 19 | R IFG (44/45) |
| 34, 48, −4 | 83 | 877 | R MFG (10) | |
| 40, 30, 18 | 67 | 108 | R MFG/IFG (45/46) | |
| 14, 20, 48 | 50 | 17 | R SFG (8) | |
| 64, −22, −6 | 67 | 14 | RMTG (21) | |
| 48, −74, 40 | 83 | 111 | R Angular Gyrus (40) | |
| −32, −72, −38 | 83 | 264 | L Cerebellum |
Coordinates relate to the point in each cluster with the highest value. L = left; R = right; IFG = inferior frontal gyrus; MFG = middle frontal gyrus; SFG = superior frontal gyrus; MTG = middle temporal gyrus; STS = superior temporal sulcus; OG = occipital gyrus; NA = no activation differences.
Demographic/clinical variables by patient subsets
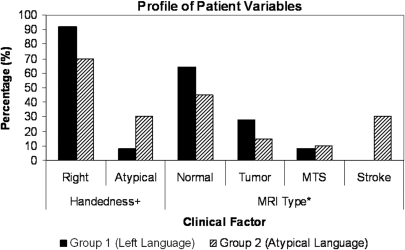
Data were first compared between the two broad groups. The distribution of handedness [χ2 (1, n = 45) = 3.68, P = 0.05] and pathology [χ2 (3, n = 45) = 9.12, P < 0.05] between the two groups was different (Fig. 3). The largely left language lateralized group (Group 1) had a small proportion of left-handed individuals (8%) while the atypical language group (Group 2) had a larger proportion of left-handed individuals (30%). MRI findings for Group 1 were most frequently normal (64%), followed by tumour/dysplasia (28%) and MTS (8%). MRI findings for Group 2 showed that 45% were normal, 30% had stroke, 15% had tumour and 10% had MTS. The distribution of the other factors including age, gender, age of onset, indicators of early insult, seizure duration and location of seizure focus were not different between the two groups.
Fig. 3.
Profile of patient variables among groups. Percent of patients in groups one and two based on handedness and MRI findings. +P = 0.05; *P < 0.05.
Further analysis of the subgroups within the two broad groups was performed, but must be interpreted with caution as some cell counts fall below five. Groups 1a and 1b were significantly different for age [t (23, n = 25) = –2.786, P < 0.05] and seizure duration [t (23, n = 25) = –2.979, P < 0.01] and showed a trend for gender [χ2 (1, n = 25) = 2.93, P = 0.09]: Group 1a, which showed greater differences in posterior left middle temporal activation, had a higher proportion of females (69%) and was younger (mean 19 years) with subsequently shorter duration (mean 5 years). In comparison, Group 1b with activation comparable to the control group, had a higher percentage of males (67%), was older (mean 30 years) with subsequently longer duration (mean 18 years). Groups 2a and b showed a trend for pathology [χ2 (3, n = 20) = 5.45, P = 0.14] and location of seizure focus differences [χ2 (1, n = 20) = 2.54, P = 0.11]. Group 2a, which showed greater right sided activation in both frontal and temporal areas, was largely a temporal focus group (71%) with the following distribution of MRI findings: 8 (57%) normal, 3 (21%) stroke, 2 (14%) MTS and 1 (7%) tumour/dysplasia. Group 2b, which showed little difference from normal temporal activation but profound differences in right frontal activation, was largely an extratemporal focus group (67%) with the following distribution of MRI findings: 3 (50%) stroke, 2 (33%) tumour/dysplasia and 1 (17%) normal.
Using a more stringent cut-off of z > 3 to determine the individual difference maps, the k means clustering analysis identifies one subgroup (n = 6) that differs from the other patients. This group overlaps with Group 2 in the original analysis (two from 2a, four from 2b) and is similar in cluster coordinates to Group 2 subgroups (Table 5).
Table 5.
k-means clustering for difference maps with z > 3
| Group | Peak coordinates | Peak value (%) | Cluster size | Region |
|---|---|---|---|---|
| 2(n = 6) | 52, 38, 8 | 100 | 84 | R IFG (45/46) |
| 50, 36, 4 | 83 | 63 | R IFG (45) | |
| −34, −76, −38 | 67 | 192 | L Cerebellum | |
| 54, 14, 8 | 67 | 169 | IFG (44) | |
| 46, −66, 34 | 67 | 20 | BA 39 | |
| 60, −58, 2 | 67 | 12 | R MTG (21/37) | |
| 34, 40, 42 | 67 | 21 | R MFG (9) | |
| 34, 58, 4 | 67 | 38 | R MFG (46) |
One subgroup, n = six patients is identified. Coordinates are comparable to group 2b in Table 4.
L = left; R = right; IFG = inferior frontal gyrus; MFG = middle frontal gyrus; SFG = superior frontal gyrus; STS = superior temporal sulcus; NA = no activation differences.
Discussion
We found evidence for intra and inter-hemispheric language reorganization in epilepsy patients using a novel quantitative data driven method for comparing individual patient fMRI data to controls. Patients clustered into several groups based on difference activation patterns. One did not differ from the normal population; another showed increased activation in left temporal regions, supporting the notion of intra-hemispheric alteration in language processing; the two other groups had increased right homologue activation suggesting inter-hemispheric shift in language processing.
Several fMRI studies demonstrate a higher incidence of bilateral or right language dominance in epilepsy populations (Binder et al., 1996; Yetkin et al., 1998; Gaillard et al., 2002, 2004, 2007; Adcock et al., 2003; Woermann et al., 2003; Thivard et al., 2005; Weber et al., 2006). Visual-analysis studies describe activation in right hemispheric homologues, and observe rare activation outside these areas (Gaillard et al., 2004, 2007). Quantitative studies relying on ROI methods also find activation in these right homologues in epilepsy (Gaillard et al., 2002; Woermann et al., 2003; Thivard et al., 2005; Weber et al., 2006) and perinatal stroke populations (Staudt et al., 2001, 2002). However ROI methods restrict examination to predefined anatomic or functional areas, and cannot assess activation outside these areas. We provide further quantitative evidence that activation in right hemisphere occurs predominantly in right homologues to Broca's and Wernicke's areas and the distributed language processing network that includes the middle frontal gyrus implicated the working memory demands of language tasks (Berl et al., 2005), and angular gyrus implicated in semantic processing (Chou et al., 2006b; Humphries et al., 2007).
Identifying atypical cognitive activation patterns using functional imaging in patient populations is problematic because they are heterogeneous. Our method uses a voxel wise approach applied on an individual basis that takes into consideration normal control variability (Rugg-Gunn et al., 2001; Muller et al., 2003; Turkeltaub et al., 2004; Fair et al., 2006; Price et al., 2006; Seghier et al., 2008) to identify how an individual patient differs from the normal population. The second level classification, using a principal components and cluster analysis of the resulting z-score difference maps, is then employed to identify subgroups within the patient population. An additional advantage of this approach is the ability to reveal common patterns of activation in regions outside those usually considered in language studies and that may otherwise be overlooked including changes in the dominant hemisphere adjacent to language processing areas. It considers both the extent and degree of activation and is less susceptible to threshold effects than laterality indexes (Gaillard et al., 2002; Berl et al., 2006).
We used a difference map threshold of z > 2 for the primary step of our cluster analysis following Fair et al. (2006). Two standard deviations is also a commonly accepted threshold for many clinical and research investigations with a 5% chance of Type I Error. Spurious and random differences deriving from Type 1 Error in the primary step are unlikely to cluster in the second-level analysis using cluster and penetrance analyses. Other investigators have used z approximating 3, for structural VBM individual difference maps (Eriksson et al., 2001; Rugg-Gunn et al., 2001, 2003). However, the context of these studies differs in two meaningful respects. First, they did not conduct any further analyses. Second, they seek small areas of focal abnormality to determine the bounds of surgical resection, which requires a much more strict threshold for Type 1 Error and acceptance of greater Type 2 Error. At this more conservative threshold, the ‘within hemisphere differences’ among the group 1 subgroups are not sustained—that is, they match the control population. The right hemispheric differences hold for a smaller subpopulation where difference activations have comparable coordinates to Group 2 subgroups. The evidence for intra-hemispheric differences is modest, but appears present in a small population.
With the methods used in this study we cannot distinguish between re-organization and compensation. For example, activation in right regions may indicate the right hemisphere has assumed language processing functions that normally reside in the left hemisphere. However, activation in the right may also reflect recruitment of these areas by some patients to achieve adequate task performance. Unlike ROI analysis employed to generate an asymmetry index, this method does not provide determination of language dominance. Increased task difficulty or linguistic complexity is associated with increased magnitude and extent of right activation (Just et al., 1996; Gaillard et al., 2001). Patients with left seizure focus have reduced laterality indices in temporal and frontal regions even though they may remain left language dominant (Berl et al., 2005). Transcranial magnetic stimulation studies of verbal fluency using 15O water PET demonstrate increased activation in right Broca's homologues when left Broca's is suppressed (Thiel et al., 2006). The degree of fMRI asymmetry may be related to interictal activity in the left hemisphere, and case reports describe normalization of fMRI laterality when left epileptogenic tissue is resected (Helmstaedter et al., 2006; Janszky et al., 2006). These observations provide support for theories that the left hemisphere modulates recruitment in right homologues (Boatman et al., 1999). While activation in angular gyrus is not seen in our word definition decision task, other investigators describe recruitment of angular gyrus in tasks that place emphasis on semantics (Chou et al., 2006a; Humphries et al., 2007) and imagery (Just et al., 2004). Recruitment of angular gyrus may reflect a different strategy for task processing in this sub-population.
Our study also provides evidence for intra-hemispheric reorganization—or compensation—predominantly in the temporal lobe. ECS studies show widespread disruption, including anterior temporal lobe and middle frontal gyrus, of object naming and auditory response naming (the overt version of our task) in patients with epilepsy and gliomas (Ojemann et al., 1989; Devinsky et al., 1993; Hamberger et al., 2007a, b; Sanai et al., 2008). However, these findings may not reflect function in normal subjects. Differences between ECS and fMRI may due to tasks used (Malow et al., 1996), or differences between brain disruption induced by stimulation versus activation elicited by BOLD methods (Bookheimer et al., 1997; Pouratian et al., 2002). Only one study directly compares the same paradigm, an auditory response naming task, between functional imaging and electorcortical stimulation; this study finds good but not complete agreement on an individual basis (Bookheimer et al., 1997). Our normal group maps did show activation extending along the superior temporal sulcus, including anterior extensions in addition to dorsolateral prefrontal cortex, and overlaps with cortex disrupted in ECS studies, but not intra-hemispheric variance. One fMRI study examined the point of maximal fMRI activation in the right frontal lobe in a small number of patients with left hemisphere epilepsy using a verbal fluency task and found the maxima displaced posteriorly and inferiorly towards the insula (Voets et al., 2006). We do not find evidence for activation beyond that seen in normal volunteers in a substantial number of patients in left anterior temporal lobe or right insular regions. Rather we find recruitment of regions adjacent to classical language processing areas, particularly in posterior superior temporal sulcus. It is unclear whether these areas are critical for language processing, or represent alternative strategies for receptive language processing.
These data also suggest an effect of the seizure focus on language network organization. Inter and intra hemispheric difference clusters involving temporal neocortex were associated with a temporal focus. In contrast, extra-temporal foci were associated with alteration in anterior language networks only. As temporal foci may also be associated with changes in IFG activation then frontal functions may be more sensitive to perturbations regardless of location of seizure focus than posterior functions such as receptive speech cortex. ROI analysis has found evidence for similar remote effects of temporal lobe epilepsy (Billingsley et al., 2001; Berl et al., 2005) and have also been observed in structural MR and FDG-PET studies of cerebral metabolism (Theodore, 1988; Henry et al., 1992; DeCarli et al., 1995; Bernasconi et al., 2001, 2003).
Younger age, shorter epilepsy duration and female gender were associated with the group showing temporal lobe intra-hemispheric reorganization. Findings in the literature are equivocal regarding gender differences in language activation due possibly to the complexity of the many variables that might influence activation (Shaywitz et al., 1995; Frost et al., 1999; Plante et al., 2006). Step-wise analysis, however, may facilitate separation of the contribution of such factors to varying activation patterns. Our findings hint that females may more readily be able to compensate or reorganize language intra-hemispherically in temporal regions (Cao et al., 1999; Heiss et al., 1999; Warburton, 1999; Saur et al., 2006; Meinzer et al., 2008; Raboyeau et al., 2008).
Brain injury or epilepsy onset before the age of six is associated with the inter-hemispheric transfer of language capacity identified by IAT or fMRI (Rasmussen and Milner, 1977; Springer et al., 1999; Gaillard et al., 2007) which is supported by behavioural studies of children with early brain injury (Bates and Roe, 2001). ECS data suggests the intra-hemispheric reorganization they identify is associated with later onset epilepsy or brain injury (Devinsky et al., 1993). In this study, we find no overall difference in age of epilepsy onset or brain injury between our left language and atypical language groups. These data suggest other factors in addition to age of injury may play a role in determining within and between hemispheric response to brain insult.
The developmental maturation of language systems may provide a clue to observations regarding the functional expression of atypical language processing networks. Children younger than 7 years old are less strongly lateralized for language dominance than older children and young adults (Gaillard, 2000; Holland et al., 2001). Maturation of transcallosal connections may contribute to the establishment of language dominance (Boatman et al., 1999). If injury were to occur in the children who exhibit greater bilateral speech activation, and before dominance is firmly established, then right homologues may ultimately sustain language function. In contrast, children who do not have more bilateral activation would be constrained to adapt by within hemisphere compensation. This hypothesis and the observation that altered language processing networks with preserved language functions are associated with epilepsy onset, or brain injury, before age 6 years supports the notion of developmental reserve as the source of ‘reorganization’ (Gaillard et al., 2007).
There are limitations to the extent and location of brain areas recruited to perform receptive and expressive aspects of language processing. This process may be dictated by age and developmental factors, in addition to microscopic brain structure that distinguish Brodmann areas that restrict the range for brain compensation. Knowledge of possible patterns of reorganization/compensation, as well as clinical variables that correlate with them, may have implications for predicting which epilepsy patients fare better in terms of developmental and post-surgical outcomes. Our methods are data driven, are not based upon a priori assumptions, and provide a means of identifying heterogeneity among patient subgroups that may vary from normal populations. These methods should also have application to investigating developmental disorders and the effect of brain based diseases on the expression of cognitive functions.
Funding
The Society for Pediatric Research (HD007446 to J.M.); National Institutes of Health, NINDS (R01 NS44280); Partnership for Pediatric Research Epilepsy Foundation (to M.M.B.); Children's Research Institute Avery Award (to M.M.B.); the NINDS Clinical Epilepsy Section Division of Intramural Research.
Acknowledgements
We thank Dr Frank Ritter from the Minnesota Epilepsy Group for his patient referrals, and Miss Sarah Stephens for assistance in preparing the manuscript and, to the patients and families who have participated in our studies.
Glossary
Abbreviations
- ECS
Electro-cortical stimulation
- MTG
mid temporal gyrus
- MTS
mesial temporal sclerosis
- PCA
principal components analysis
References
- Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18:423–38. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- Bates E, Roe K. Language development in children with unilateral brain injury. In: Nelson CA, Luciano M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- Berl MM, Balsamo LM, Xu B, Moore EN, Weinstein SL, Conry JA, et al. Seizure focus affects regional language networks assessed by fMRI. Neurology. 2005;65:1604–11. doi: 10.1212/01.wnl.0000184502.06647.28. [DOI] [PubMed] [Google Scholar]
- Berl MM, Vaidya CJ, Gaillard WD. Functional imaging of developmental and adaptive changes in neurocognition. Neuroimage. 2006;30:679–91. doi: 10.1016/j.neuroimage.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Bernasconi N, Bernasconi A, Caramanos Z, Antel SB, Andermann F, Arnold DL. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126:462–9. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- Bernasconi N, Bernasconi A, Caramanos Z, Dubeau F, Richardson J, Andermann F, et al. Entorhinal cortex atrophy in epilepsy patients exhibiting normal hippocampal volumes. Neurology. 2001;56:1335–9. doi: 10.1212/wnl.56.10.1335. [DOI] [PubMed] [Google Scholar]
- Billingsley RL, McAndrews MP, Crawley AP, Mikulis DJ. Functional MRI of phonological and semantic processing in temporal lobe epilepsy. Brain. 2001;124:1218–27. doi: 10.1093/brain/124.6.1218. [DOI] [PubMed] [Google Scholar]
- Binder J, Rao S, Hammeke T, Frost JA, Bandettini P, Jesmanowicz A, et al. Lateralized human brain language systems demonstrated by task subtraction functional magnetic resonance imaging. Arch Neurol. 1995;52:593–601. doi: 10.1001/archneur.1995.00540300067015. [DOI] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978–84. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- Boatman D, Freeman J, Vining E, Pulsifer M, Miglioretti D, Minahan R, et al. Language recovery after left hemispherectomy in children with late-onset seizures. Ann Neurol. 1999;46:579–86. doi: 10.1002/1531-8249(199910)46:4<579::aid-ana5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–88. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Bookheimer S, Zeffiro TA, Blaxton TA, Gaillard WD, Theodore W. Regional cerebral blood flow during object naming and word reading. Hum Brain Mapp. 1995;3:93–106. [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton T, Malow BA, Gaillard WD, Sato S, et al. A direct comparison of PET activation and electrocortical stimulation mapping for language localization. Neurology. 1997;48:1056–65. doi: 10.1212/wnl.48.4.1056. [DOI] [PubMed] [Google Scholar]
- Cao Y, Vikingstad EM, George KP, Johnson AF, Welch KM. Cortical language activation in stroke patients recovering from aphasia with functional MRI. Stroke. 1999;30:2331–40. doi: 10.1161/01.str.30.11.2331. [DOI] [PubMed] [Google Scholar]
- Carpentier A, Pugh KR, Westerveld M, Studholme C, Skrinjar O, Thompson JL, et al. Functional MRI of language processing: dependence on input modality and temporal lobe epilepsy. Epilepsia. 2001;42:1241–54. doi: 10.1046/j.1528-1157.2001.35500.x. [DOI] [PubMed] [Google Scholar]
- Chou TL, Booth JR, Burman DD, Bitan T, Bigio JD, Lu D, et al. Developmental changes in the neural correlates of semantic processing. Neuroimage. 2006b;29:1141–9. doi: 10.1016/j.neuroimage.2005.09.064. [DOI] [PubMed] [Google Scholar]
- Chou TL, Booth JR, Bitan T, Burman DD, Bigio JD, Cone NE, et al. Developmental and skill effects on the neural correlates of semantic processing to visually presented words. Hum Brain Mapp. 2006a;27:915–24. doi: 10.1002/hbm.20231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, McIntosh AR, Blaxton TA. Use of positron emission tomography for the evaluation of epilepsy. Neuroimaging Clin N Am. 1995;5:623–45. [PubMed] [Google Scholar]
- Devinsky O, Perrine K, Llinas R, Luciano DJ, Dogali M. Anterior temporal language areas in patients with early onset of temporal lobe epilepsy. Ann Neurol. 1993;34:727–32. doi: 10.1002/ana.410340517. [DOI] [PubMed] [Google Scholar]
- Dunteman GH. Principal Components Analysis. Newbury Park, CA: Sage Publications; 1989. [Google Scholar]
- Eriksson SH, Rugg-Gunn FJ, Symms MR, Barker GJ, Duncan JS. Diffusion tensor imaging in patients with epilepsy and malformations of cortical development. Brain. 2001;124:617–26. doi: 10.1093/brain/124.3.617. [DOI] [PubMed] [Google Scholar]
- Fair DA, Brown TT, Petersen SE, Schlaggar BL. fMRI reveals novel functional neuroanatomy in a child with perinatal stroke. Neurology. 2006;67:2246–9. doi: 10.1212/01.wnl.0000249348.84045.0e. [DOI] [PubMed] [Google Scholar]
- Fernandez G, de Greiff A, von Oertzen J, Reuber M, Lun S, Klaver P, et al. Language mapping in less than 15 minutes: real-time functional MRI during routine clinical investigation. Neuroimage. 2001;14:585–94. doi: 10.1006/nimg.2001.0854. [DOI] [PubMed] [Google Scholar]
- Frost JA, Binder JR, Springer JA, Hammeke TA, Bellgowan PSF, Rao SM, et al. Language processing is strongly left lateralized in both sexes: Evidence from function MRI. Brain. 1999;122:199–208. doi: 10.1093/brain/122.2.199. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, Grandin CB, Braniecki SH, Papero PH, et al. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59:256–65. doi: 10.1212/wnl.59.2.256. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, et al. FMRI language task panel improves determination of language dominance. Neurology. 2004;63:1403–8. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Berl MM, Moore EN, Ritzl EK, Rosenberger LR, Weinstein SL, et al. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69:1761–71. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore W. Functional anatomy of cognitve development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–5. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Pugliese M, Grandin CB, Braniecki SH, Kondapaneni P, Hunter K, et al. Cortical localization of reading in normal children: an fMRI language study. Neurology. 2001;57:47–54. doi: 10.1212/wnl.57.1.47. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, McClelland S, 3rd, McKhann GM, 2nd, Williams AC, Goodman RR. Distribution of auditory and visual naming sites in nonlesional temporal lobe epilepsy patients and patients with space-occupying temporal lobe lesions. Epilepsia. 2007a;48:531–8. doi: 10.1111/j.1528-1167.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, Goodman RR, Williams A, Perrine K, Devinsky O, et al. Evidence for cortical reorganization of language in patients with hippocampal sclerosis. Brain. 2007b;130:2942–50. doi: 10.1093/brain/awm187. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, McKhann GM, 2nd, Perrine K, Goodman RR. Brain stimulation reveals critical auditory naming cortex. Brain. 2005;128:2742–9. doi: 10.1093/brain/awh621. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol. 1999;45:430–8. doi: 10.1002/1531-8249(199904)45:4<430::aid-ana3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Fritz NE, Gonzalez Perez PA, Elger CE, Weber B. Shift-back of right into left hemisphere language dominance after control of epileptic seizures: evidence for epilepsy driven functional cerebral organization. Epilepsy Res. 2006;70:257–62. doi: 10.1016/j.eplepsyres.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Henry TR, Mazziotta JC, Engel JJ. The functional anatomy of frontal lobe epilepsy studied with PET. Adv Neurol. 1992;57:449–63. [PubMed] [Google Scholar]
- Hertz-Pannier L, Gaillard WD, Mott SH, Cuenod CA, Bookheimer SY, Weinstein S, et al. Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study. Neurology. 1997;48:1003–12. doi: 10.1212/wnl.48.4.1003. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–43. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Time course of semantic processes during sentence comprehension: an fMRI study. Neuroimage. 2007;36:924–32. doi: 10.1016/j.neuroimage.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janszky J, Mertens M, Janszky I, Ebner A, Woermann FG. Left-sided interictal epileptic activity induces shift of language lateralization in temporal lobe epilepsy: an fMRI study. Epilepsia. 2006;47:921–7. doi: 10.1111/j.1528-1167.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activity modulated by sentence comprehension. Science. 1996;274:114–16. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Just MA, Newman SD, Keller TA, McEleney A, Carpenter PA. Imagery in sentence comprehension: an fMRI study. Neuroimage. 2004;21:112–24. doi: 10.1016/j.neuroimage.2003.08.042. [DOI] [PubMed] [Google Scholar]
- Kim YK, Lee DS, Lee SK, Chung CK, Chung JK, Lee MC. (18)F-FDG PET in localization of frontal lobe epilepsy: comparison of visual and SPM analysis. J Nucl Med. 2002;43:1167–74. [PubMed] [Google Scholar]
- Kim YK, Lee DS, Lee SK, Kim SK, Chung CK, Chang KH, et al. Differential features of metabolic abnormalities between medial and lateral temporal lobe epilepsy: quantitative analysis of (18)F-FDG PET using SPM. J Nucl Med. 2003;44:1006–12. [PubMed] [Google Scholar]
- Lehericy S, Cohen L, Bazin B, Samson S, Giacomini E, Rougetet R, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54:1625–33. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- Liu RS, Lemieux L, Bell GS, Bartlett PA, Sander JW, Sisodiya SM, et al. A longitudinal quantitative MRI study of community-based patients with chronic epilepsy and newly diagnosed seizures: methodology and preliminary findings. Neuroimage. 2001;14:231–43. doi: 10.1006/nimg.2001.0773. [DOI] [PubMed] [Google Scholar]
- Malow BA, Blaxton TA, Sato S, Brookheimer SY, kufta CV, Figlozzi CM, et al. Cortical Stimulation elicits regionals distinctions in auditory and visual naming. Epilepsia. 1996;37:245–52. doi: 10.1111/j.1528-1157.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Breitenstein C, Wienbruch C, Elbert T, Rockstroh B. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. Neuroimage. 2008;39:2038–46. doi: 10.1016/j.neuroimage.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Muller RA, Kleinhans N, Kemmotsu N, Pierce K, Courchesne E. Abnormal variability and distribution of functional maps in autism: an FMRI study of visuomotor learning. Am J Psychiatry. 2003;160:1847–62. doi: 10.1176/appi.ajp.160.10.1847. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316–26. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Plante E, Schmithorst VJ, Holland SK, Byars AW. Sex differences in the activation of language cortex during childhood. Neuropsychologia. 2006;44:1210–21. doi: 10.1016/j.neuropsychologia.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Pollard D. The annals of statistics. Vol. 9. Beachwood, OH: Institute of Mathematical Statistics; 1981. [Google Scholar]
- Pouratian N, Bookheimer SY, Rex DE, Martin NA, Toga AW. Utility of preoperative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations. J Neurosurg. 2002;97:21–32. doi: 10.3171/jns.2002.97.1.0021. [DOI] [PubMed] [Google Scholar]
- Price CJ, Crinion J, Friston KJ. Design and analysis of fMRI studies with neurologically impaired patients. J Magn Reson Imaging. 2006;23:816–26. doi: 10.1002/jmri.20580. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–43. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Raboyeau G, De Boissezon X, Marie N, Balduyck S, Puel M, Bezy C, et al. Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology. 2008;70:290–8. doi: 10.1212/01.wnl.0000287115.85956.87. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, Sommer I, Rutten GJ, Kahn R. Combined analysis of language tasks in fMRI improves assessment of hemispheric dominance for language functions in individual subjects. Neuroimage. 2001;13:719–33. doi: 10.1006/nimg.2000.0722. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann NY Acad Sci. 1977;299:355–69. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Ray S, Turi RH. Determination of number of clusters in k-means clustering and application in colour image segmentation. In:. Proceedings of the 4th International Conference on Advances in Pattern Recognition and Digital Techniques (ICAPRDT'99); Calcutta, India. 1999. pp. 137–43. [Google Scholar]
- Rugg-Gunn FJ, Eriksson SH, Boulby PA, Symms MR, Barker GJ, Duncan JS. Magnetization transfer imaging in focal epilepsy. Neurology. 2003;60:1638–45. doi: 10.1212/01.wnl.0000065891.93179.cc. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn FJ, Eriksson SH, Symms MR, Barker GJ, Duncan JS. Diffusion tensor imaging of cryptogenic and acquired partial epilepsies. Brain. 2001;124:627–36. doi: 10.1093/brain/124.3.627. [DOI] [PubMed] [Google Scholar]
- Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–84. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Schlosser MJ, Luby M, Spencer DD, Awad IA, McCarthy G. Comparative localization of auditory comprehension by using functional magnetic resonance imaging and cortical stimulation. J Neurosurg. 1999;91:626–35. doi: 10.3171/jns.1999.91.4.0626. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, Pegna AJ, Annoni JM, Khateb A. Group analysis and the subject factor in functional magnetic resonance imaging: analysis of fifty right-handed healthy subjects in a semantic language task. Hum Brain Mapp. 2008;29:461–77. doi: 10.1002/hbm.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, et al. Sex differences in functional organization of the brain for language. Nature. 1995;373:607–9. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122(Pt 11):2033–46. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Niemann G, Wildgruber D, Erb M, Krageloh-Mann I. Early left periventricular brain lesions induce right hemispheric organization of speech. Neurology. 2001;57:122–5. doi: 10.1212/wnl.57.1.122. [DOI] [PubMed] [Google Scholar]
- Staudt M, Lidzba K, Grodd W, Wildgruber D, Erb M, Krageloh-Mann I. Right-hemispheric organization of language following early left-sided brain lesions: functional MRI topography. Neuroimage. 2002;16:954–67. doi: 10.1006/nimg.2002.1108. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–44. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Theodore WH. Antiepileptic drugs and cerebral glucose metabolism. Epilepsia. 1988;29(Suppl 2):S48–55. doi: 10.1111/j.1528-1157.1988.tb05797.x. [DOI] [PubMed] [Google Scholar]
- Thiel A, Schumacher B, Wienhard K, Gairing S, Kracht LW, Wagner R, et al. Direct demonstration of transcallosal disinhibition in language networks. J Cereb Blood Flow Metab. 2006;26:1122–7. doi: 10.1038/sj.jcbfm.9600350. [DOI] [PubMed] [Google Scholar]
- Thivard L, Hombrouck J, du Montcel ST, Delmaire C, Cohen L, Samson S, et al. Productive and perceptive language reorganization in temporal lobe epilepsy. Neuroimage. 2005;24:841–51. doi: 10.1016/j.neuroimage.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Flowers DL, Verbalis A, Miranda M, Gareau L, Eden GF. The neural basis of hyperlexic reading. An FMRI case study. Neuron. 2004;41:11–25. doi: 10.1016/s0896-6273(03)00803-1. [DOI] [PubMed] [Google Scholar]
- Voets NL, Adcock JE, Flitney DE, Behrens TE, Hart Y, Stacey R, et al. Distinct right frontal lobe activation in language processing following left hemisphere injury. Brain. 2006;129:754–66. doi: 10.1093/brain/awh679. [DOI] [PubMed] [Google Scholar]
- Warburton L. Management of stroke: a practical guide for the prevention, evaluation and treatment of acute stroke. J Neurol Neurosurg Psychiatry. 1999;66:696A. doi: 10.1136/jnnp.66.5.696a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B, Wellmer J, Reuber M, Mormann F, Weis S, Urbach H, et al. Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain. 2006;129:346–51. doi: 10.1093/brain/awh694. [DOI] [PubMed] [Google Scholar]
- Wilke M, Lidzba K. LI-tool: a new toolbox to assess lateralization in functional MR-data. J Neurosci Methods. 2007;163:128–36. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Scott SK, Blank C, Mummery CJ, Murphy K, Warburton EA. Separate neural subsystems within ‘Wernicke's area’. Brain. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, et al. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61:699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]
- Wood AG, Saling MM, Abbott DF, Jackson GD. A neurocognitive account of frontal lobe involvement in orthographic lexical retrieval: an fMRI study. Neuroimage. 2001;14:162–9. doi: 10.1006/nimg.2001.0778. [DOI] [PubMed] [Google Scholar]
- Xiong J, Rao S, Jerabek P, Zamarripa F, Woldorff M, Lancaster J, et al. Intersubject variability in cortical activations during a complex language task. Neuroimage. 2000;12:326–39. doi: 10.1006/nimg.2000.0621. [DOI] [PubMed] [Google Scholar]
- Yetkin FZ, Swanson S, Fischer M, Akansel G, Morris G, Mueller W, et al. Functional MR of frontal lobe activation: comparison with Wada language results. AJNR Am J Neuroradiol. 1998;19:1095–8. [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Thomas KM, Davidson MC, Casey BJ, Heier LA, Ulug AM. MR quantitation of volume and diffusion changes in the developing brain. AJNR Am J Neuroradiol. 2005;26:45–9. [PMC free article] [PubMed] [Google Scholar]