Abstract
Microglia are cells of myeloid origin that populate the CNS during early development and form the brain's innate immune cell type. They perform homoeostatic activity in the normal CNS, a function associated with high motility of their ramified processes and their constant phagocytic clearance of cell debris. This debris clearance role is amplified in CNS injury, where there is frank loss of tissue and recruitment of microglia to the injured area. Recent evidence suggests that this phagocytic clearance following injury is more than simply tidying up, but instead plays a fundamental role in facilitating the reorganization of neuronal circuits and triggering repair. Insufficient clearance by microglia, prevalent in several neurodegenerative diseases and declining with ageing, is associated with an inadequate regenerative response. Thus, understanding the mechanism and functional significance of microglial-mediated clearance of tissue debris following injury may open up exciting new therapeutic avenues.
Keywords: neuroinflammation; microglia; neurodegeneration; regeneration; phagocytosis; multiple sclerosis, Alzheimer disease
Introduction
Over several decades the question of whether microglia and brain macrophages play harmful or beneficial roles in CNS injury and disease has been widely debated and reviewed (Streit, 2005, 2006; Block et al., 2007; Hanisch and Kettenmann, 2007). In our view it is clear that they can fulfil both roles and we do not intend to argue for one position against the other. Instead, we start with the premise that microglia perform many beneficial roles in diseases and review how recent advances in our understanding of microglial biology relate to these, highlighting how phagocytic removal of tissue debris has an important function in creating a pro-regenerative environment within the CNS. Microglia engaged in phagocytosis generally assume a macrophage phenotype, which is indistinguishable from the macrophage phenotype assumed by monocyte-derived cells. Recent evidence demonstrates that transition of monocyte-derived cells into microglia is a very rare event that only occurs under very defined host conditions (Mildner et al., 2007). In general microgliosis arises as a result of a proliferative response of resident microglia, present within the CNS due to invasion by myeloid precursors during development (Ajami et al., 2007; Ransohoff, 2007). Therefore, in this article the term macrophage will be used to infer a cell of predominantly CNS origin, if not explicitly defined as blood- or monocyte-derived macrophage.
Microglial motility
Under pathological conditions such as infectious diseases, stroke or neurodegenerative processes, microglia become activated, migrate to and within the lesion site, release a wide range of soluble factors that include cytotoxins, neurotrophins and immunomodulary factors and clear cellular debris by phagocytosis. Until recently it was thought that, in contrast to their frenzied activity in pathology, microglial cells under normal conditions are quiescent and non-motile cells. However, in vivo imaging on living mice has revealed that their highly ramified processes are remarkably motile, continuously and randomly undergoing cycles of filopodia-like protrusion formation, extension and withdrawal of bulbous tips (Davalos et al., 2005; Nimmerjahn et al., 2005). This high motility of the processes enables microglia to effectively monitor the status of the local surroundings and possibly to endocytose small cellular debris or budded vesicular structures, including that from apoptotic cells, from the microenvironment. Thus microglia might behave like monocyte-derived macrophages, whose filopodia can act as phagocytic or endocytic tentacles, efficiently pulling engulfed vesicular material towards the cell body (Kress et al., 2007). In addition to the high motility of their processes under normal conditions, they polarize and converge their processes at sites of brain injury attracted by extracellular ATP and mediated via microglial purinoreceptors (Davalos et al., 2005; Haynes et al., 2006). Recently, it was shown by in vivo two-photon microscopy that not only the processes, but also the cell bodies of microglial cells are activated and recruited to newly formed amyloid-β (Aβ) plaques within 1–2 days in an animal model of Alzheimer's disease (Meyer-Luehmann et al., 2008).
An important function of microglial cells responding and migrating towards the chemokine ligand of CX3CR1 appears to be the support of endangered neurons since deficiency in the chemokine receptor CX3CR1 resulted in increased neuronal death in animal models of amyotrophic lateral sclerosis and Parkinson's disease (Cardona et al., 2006). The precise mechanisms by which CX3CR1-positive microglia might assist compromised neurons have yet to be determined, although it seems likely that it will relate in part to the release of neuroprotective and trophic factors.
Microglial production of trophic factors and protective cytokines
Microglial cells are able to produce and release a plethora of soluble mediators ranging from cytotoxic mediators to trophic factors, which can exert deleterious as well as beneficial effects on the surrounding tissue. Important insights into this dual nature are derived from in vitro experiments using organotypic hippocampal slice cultures, where it has been shown that microglia become neurotoxic following treatment with lipopolysaccharides (LPS) but become neuroprotective when pre-activated with interleukin-4 (IL-4) (Butovsky et al., 2006). The protective effect of IL-4 conditioned microglia is associated with a downregulation of tumour necrosis factor-α (TNF-α) and an upregulation of insulin-like growth factor-1 (IGF-1) gene transcripts. IGF-1 has neuroprotective effects but also exerts survival and pro-regenerative activities on oligodendrocyte-lineage cells, preventing acute glutamate-mediated toxicity and promoting oligodendrocyte differentiation from precursor cells in vitro (Ness and Wood, 2002; Hsieh et al., 2004; Butovsky et al., 2006). Brain derived neurotrophic factor (BDNF), having both protective and growth promoting effects on neurons, was suggested to be produced by microglial cells and to stimulate axonal sprouting towards a wound edge (Batchelor et al., 2002). Recent data also indicate that microglial-derived BDNF is implicated in neuropathic pain by causing a shift in the neuronal anion gradient of spinal lamina I neurons, thus contributing to tactile allodynia (Coull et al., 2005). However, it is unclear whether microglia in vivo produce and release sufficient amount of BDNF for these effects on neurons.
Under certain conditions microglial cells are able to produce anti-inflammatory cytokines such as IL-10 and transforming growth factor-β (TGF-β), which have neuroprotective effects in experimental animal models of traumatic injury and stroke (Streit, 2005; Hanisch and Kettenmann, 2007). Often, a clear distinction between cytokines that are either harmful or beneficial cannot be made since the primarily cytotoxic pro-inflammatory cytokines IL-1β and TNF-α released from activated microglia can directly or indirectly evoke a neuroprotective or pro-myelin regenerative response. For example, TNF-α has been shown to protect neurons against Aβ mediated toxicity (Barger, 1995) under pathological conditions and the absence of glial derived TNF-α revealed a role in homoeostatic synaptic scaling under physiological conditions (Stellwagen and Malenka, 2006).
The release of cytokines, chemokines and other soluble mediators is the first step for successful repair and contributes to the creation of an environment conducive for regeneration. The factors secreted attract phagocytic and repair-promoting effector and precursor cells, which are able to replace damaged tissue. This process is especially evident during remyelination, the regenerative event in which new myelin sheaths are restored to demyelinated axons and that can occur with impressive efficiency in experimental models and clinical disease (Ludwin, 1978, 1980; Woodruff and Franklin, 1999; Sim et al., 2002; Patrikios et al., 2006; Patani et al., 2007). Studies of remyelination in animals lacking pro-inflammatory cytokines such as TNF-α (Arnett et al., 2001, 2003) and IL-1β (Mason et al., 2001) have suggested that inflammatory cytokines and, as will be discussed later, the inflammatory response to demyelination are required to trigger efficient remyelination. The remyelination-enhancing effects of IL-1β and TNF-α could be due to direct effects or indirectly mediated via the induction of IGF-1 (Arnett et al., 2001, 2003), although IGF-I is likely to be a redundant component of environmental factors governing remyelination (O’Leary et al., 2002). In addition to trophic and pro-regenerative effects of secretory factors directly or indirectly derived from microglia, the phagocytic clearance of debris is also instrumental for repair as discussed in the following sections.
Microglial phagocytic receptors
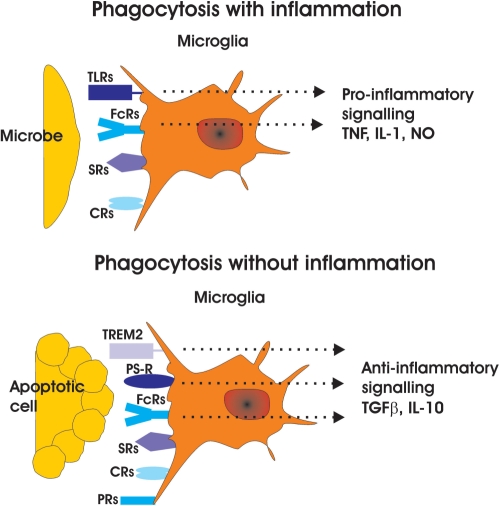
There are two distinct functional types of phagocytic receptors. First, receptors recognizing microbes such as toll like receptors (TLRs) which support removal of pathogens and simultaneously stimulates a pro-inflammatory response in the phagocytes (Ravichandran, 2003), and second, receptors recognizing apoptotic cellular material such as receptors that recognize phosphatidylserine (PS) and which are important for ingesting apoptotic cell corpses and stimulate an anti-inflammatory response in phagocytes (Ravichandran, 2003). This silent phagocytosis that takes place without inducing inflammation is one of the major beneficial functions of phagocytes (Fig. 1).
Fig. 1.
Microglial phagocytic receptors. Phagocytosis is associated with inflammation during uptake of microbes, while phagocytosis of apoptotic cells is executed without inflammation. Recognition of microbes induces a microglial phagocytic response, which is associated with release of pro-inflammatory mediators such as TNF and NO. Particularly, TLRs recognize microbial patterns leading to pro-inflammatory activity release in microglial cells. Furthermore, Fc-receptor (FcR) engagement antibodies binding induces pro-inflammatory activity dependent of the Fc-receptor subtyp and antibody isotype. Recognition and phagocytosis of apoptotic cells induces an anti-inflammatory cytokine profile in microglia. Phosphatidylserine receptors (PRs) recognizing phosphatidylserine residues in apoptotic membranes stimulated microglial production and release of TGF-β and IL-10. Triggering receptor expressed on myeloid cells-2 (TREM2) induces anti-inflammatory activity of microglia. Purine receptors (PRs) such as P2Y6 are recognizing UDP. Phagocytic receptors: PRs = purine receptors; PSRs = phosphatidylserine receptors; CRs = complement receptors; TLRs = toll like receptors; FcR = Fc-receptors; SRs = scavenger receptors; TREM2 = triggering receptor expressed on myeloid cells-2. Soluble mediators: TNF = tumour necrosis factor-α; IL-1 =interleukin-1β; NO = nitric oxide; TGF-β = transforming growth factor-β; IL-10 = interleukin-10.
The specificity of these phagocytic receptors and their respective ligands are often unknown but are gradually beginning to emerge. For example, T-cell immunoglobulin- and mucin-domain-containing molecule-4 (Tim4) has recently been shown to recognize phosphatidylserine residues (Miyanishi et al., 2007). In addition, a number of microglia-specific phagocytic receptors have been observed recently, including microglial metabotropic P2Y6 receptor that recognizes the nucleotide UDP released from injured neurons and stimulates microglial phagocytosis (Koizumi et al., 2007). Furthermore, triggering receptor expressed on myeloid cells-2 (TREM2)-mediated signalling in microglia has been shown in vitro to facilitate debris clearance in the absence of inflammation (Takahashi et al., 2005). The paramount importance of these receptors has recently become clear as patients with a loss of function mutation of either TREM2 or DAP12 develop an inflammatory neurodegenerative disease leading to death at the fourth or fifth decade of life. Thus, even though the ligand of TREM2 is unknown, microglial TREM2/DAP12-mediated phagocytosis appears to be an essential function for CNS tissue homoeostasis (Neumann and Takahashi, 2007).
Microglial phagocytosis during restructuring of neuronal connections
Selective synapse elimination and axon pruning are vital late-stage refinements in the formation of functional neural circuits. In the brain of the adult fruitfly Drosophila a program involving glia acts to achieve pruning of the axonal connection of the mushroom body γ neuron (Broadie, 2004). Phagocytic glial cells actively invade the mushroom body lobes and engulf axonal varicosities prior to the axonal degeneration and accumulate acidic degradative organelles at the time of axonal pruning (Awasaki and Ito, 2004; Awasaki et al., 2006). Insect glial cells appear to be active phagocytes, which engulf the axons and synapses by an extrinsic mechanism (Awasaki and Ito, 2004; Awasaki et al., 2006). While microglial cells in mammals are the principal phagocytes in the CNS, Schwann cells of the peripheral nervous system (PNS) can assist blood-derived macrophages in removing myelin debris and have been recognized as playing a key role in synapse removal during development of the PNS (Bishop et al., 2004). During development of the murine CNS, apoptotic neurons and their connections, which have been established in excess, are actively and rapidly removed by microglia (Frade and Barde, 1998; Marin-Teva et al., 2004). At the initial but still reversible stages of programmed cell death of developing Purkinje neurons, caspase-3-mediated activation of Ca-independent phospholipase A2 results in the production of pysophosphatidylcholine and exposure of phosphatidylserine on the cell membrane, leading to active cell death and removal by microglial cells (Marin-Teva et al., 2004).
Recent data indicate that microglia via its complement receptor C3 might be involved in synapse removal of unwanted synapses that have been tagged by complement for elimination during development (Stevens et al., 2007). Complement C1q and C3, both components of the classical complement cascade, are expressed by distinct synapses throughout the postnatal CNS. Mice deficient in C1q or C3 exhibited large sustained defects in CNS synapse elimination, as shown by the failure of anatomical refinement of retinogeniculate connections and the retention of excess retinal innervation by lateral geniculate neurons. Our knowledge of the removal mechanism of synapses and axons during reorganization of the normal and injured adult mammalian CNS is still incomplete, nevertheless it is becoming increasingly clear that microglia play a central role.
Microglial phagocytosis in acute CNS injury
In acute injury, microglia has been shown to react within a few hours with a migratory response towards the lesion. For example, in an in vitro model of entorhinal cortex injury microglia migrated towards the zone of axonal degeneration where loss of the denervated dendrites of interneurons occurred (Rappert et al., 2004). This migration is functionally significant since in chemokine receptor CXCR3 deficient mice, where microglia do not migrate, no loss of dendrites was observed (Rappert et al., 2004). Thus, denervated neuronal dendrites are not retracted autonomously, but require a trigger signal or active removal by microglia. Similarly, in response to an experimental axonal lesion to facial nerve motoneurons in rats, a glial cell mediated removal or ‘stripping’ of synapses from the perikaryon and dendrites of affected cells has been reported (Streit, 2005). It was recently suggested that microglia and major histocompatibility complex (MHC) class I related receptors take up a main role in the process of synapse removal after motoneuron injury (Cullheim and Thams, 2007).
In most cases of acute injury deposition of tissue debris is observed due to cell death. In general tissue debris does not linger for long periods after tissue damage due to efficient removal by macrophages. However, in the CNS the myelin debris associated with Wallerian degeneration can persist for very long time periods (Miklossy and Van der Loos, 1991; Vargas and Barres, 2007). In the CNS microglia are the first cell type engaged in phagocytosis. However, their phagocytic capacity as compared to blood-borne macrophages might be limited (Mosley and Cuzner, 1996; Popovich et al., 1999). In the second instance, blood-borne macrophages assist and could significantly contribute to the removal of debris (Amat et al., 1996; Stoll and Jander, 1999). The capacity of macrophages to phagocytose myelin can be altered by environmental mediators. After treatment with TNF-α a massive reduction of the amount of myelin ingested by macrophages via their complement receptor type 3 (CR3) occurred in vitro (Bruck et al., 1992). Immunofluorescence analysis indicated that TNF-α caused a reduction of CR3. Similarly, in vivo experiments have demonstrated that pre-activation of macrophages transplanted into transected optic nerve has profound effects on the rate of myelin clearance (Lazarov-Spiegler et al., 1998). Myelin contains several growth inhibitory molecules such as Nogo A, which exhibit inhibitory effects on axonal re-growth (Schwab, 2004). Thus, the rapid removal of myelin-associated inhibitors is important for establishing an environment beneficial for axon regeneration. A number of observations suggest that insufficient myelin clearance in the CNS after acute injury may contribute to the failure of axonal regeneration, while efficient myelin clearance in the PNS during Wallerian degeneration by Schwann cells and invading and resident macrophages facilitates axonal regeneration (David and Lacroix, 2003; Vargas and Barres, 2007). In support of this notion, it was observed that the transected optic nerve of amphibians exhibits a rapid phagocytic response, which leads to an effective clearance of myelin debris and finally, successful axonal regeneration (Battisti et al., 1995; Perry et al., 1995).
Recent evidence indicates that the presence of myelin molecules not only inhibits axonal outgrowth but also affects the differentiation of oligodendrocyte precursor cells into mature oligodendrocytes during remyelination (Kotter et al., 2005). Thus, the myelin debris generated during demyelination needs to be rapidly removed by phagocytic cells, for remyelination to proceed efficiently. This is reflected in the strong correlation between the efficiency of remyelination and the effectiveness of myelin debris removal, both occurring in young animals more effectively than in older adult animals or young animals in which additional myelin was experimentally added (Shields et al., 1999; Kotter et al., 2005, 2006; Dubois-Dalcq et al., 2005).
Microglial phagocytosis in multiple sclerosis
Phagocytically active macrophages, identified by staining against myelin degradation products or lysosomal lipids, have been extensively described in multiple sclerosis lesions (Li et al., 1993; Bruck et al., 1995). Most of these myelin-laden macrophages are localized in the perivascular areas in active inflammatory lesions. Phagocytic cells have also been analysed in experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis. During the first attack of acute and chronic relapsing EAE, immunostaining with an antibody against lysosomal membranes of phagocytes demonstrated at the ultrastructural level that these phagocytes were seen to contain degraded myelin products in lysosomes (Bauer et al., 1994). However, the functional role of phagocytosis in EAE is unclear. Recently, a beneficial role of the microglial phagocytic TREM2 receptors has also been demonstrated in EAE. Antibody-mediated blockade of TREM2 during the effector phase of EAE results in disease exacerbation with more diffuse CNS inflammatory infiltrates and demyelination in the brain parenchyma (Piccio et al., 2007). In another study, intravenous transplantation of myeloid precursor cells genetically engineered to over-express TREM2 at the clinical peak of EAE improved myelin removal in the lesioned spinal cord, created an anti-inflammatory cytokine profile in the lesions and facilitated recovery (Takahashi et al., 2007). Thus, debris clearance by resident endogenous microglia appears to be insufficient and it is possible to promote recovery in EAE by the transplantation of phagocytic TREM2 positive cells that contribute to the clearance of debris.
Microglial phagocytosis in Alzheimer disease
In Alzheimer disease microglia can be beneficial by phagocytosing Aβ or harmful by secretion of neurotoxins. Recently it was shown in an animal model of Alzheimer disease plaque formation that microglia accumulation is associated with rapid appearance and local toxicity of Aβ plaques (Meyer-Luehmann et al., 2008) (Fig. 2). Using in vivo multiphoton microscopy plaques were revealed to form over 24 h followed within 1–2 days by microglial activation and recruitment to the plaque, and finally the appearance of dysmorphic neurites over the next days to weeks. In an animal model of tauopathy, exhibiting certain aspects of neurofibrillary tangle formation of Alzheimer disease, early microglia activation was associated with loss of synapses preceding tangle formation (Yoshiyama et al., 2007). In APP/PS1 and APP23 transgenic mice, bone marrow-derived myeloid cells were recruited to senile plaques and differentiated into microglial-like cells after irradiation and bone marrow transplantation (Malm et al., 2005; Stalder et al., 2005). Invading bone marrow-derived cells gradually obtained morphologies and lineage markers very similar to resident microglia. However, CNS invasion of bone marrow derived cells might be induced by irradiation performed for the transplantation of bone marrow cells, and not a consequence of the disease process (Mildner et al., 2007). Furthermore, it is unclear whether bone marrow-derived cells develop a real microglia phenotype, since resident microglia underwent a distinct developmental program coming from the yolk sac and invaded the CNS during early stages of development. Recently, it was shown that the chemokine receptor CCR2 expressed on microglia, invading blood-derived macrophages and circulating monocytes is required for accumulation of these CD11b+ cells in the CNS in a transgenic mouse model of Alzheimer disease (El Khoury et al., 2007). Diseased mice deficient in CCR2 demonstrated increased perivascular Aβ deposits and died prematurely possibly due to amyloid angiopathy, indicating that CCR2 function on circulating monocytes or microglia is required for prevention or clearance of perivascular Aβ deposits (El Khoury et al., 2007). Thus, there is certain evidence that either local microglia or invading blood-derived macrophages restrict Aβ deposits in an animal model of Alzheimer disease.
Fig. 2.
Detrimental effects of myelin debris and extracellular aggregates (A) Inhibitory activity of myelin debris in multiple sclerosis. Immune mediated demyelination and oligodendrocyte injury liberates myelin. Myelin debris inhibits axonal re-growth and regeneration. Furthermore, myelin debris inhibits oligodendrocyte precursor cell differentiation. (B) Neurotoxic activity of Aβ plaques in Alzheimer disease. Extracellular Aβ directly damages synapses and stimulates microglial to release neurotoxic mediators such as TNF-α and nitric oxide (NO). Microglial cytotoxic mediators induces synaptic and axonal injury.
Microglial phagocytosis in ageing
Ageing is associated with senescence of microglia and impaired microglial clearance functions. In particular, data indicate that microglia in aged rodent and human brains show a replicative senescence with a reduced self-renewal capacity (Streit, 2006). Microglia in aged animals were characterized by the presence of lipofuscin granules, decreased processes complexity, altered granularity and increased mRNA expression of pro-inflammatory cytokines such as TNF-α and IL-1β (Sierra et al., 2007). Furthermore, older rats compared to young rats showed delayed recruitment of phagocytic cells and less clearance of myelin after a toxin-induced demyelination lesion (Zhao et al., 2006), which correlates with the slower remyelination in older animals (Sim et al., 2002). Thus, microglia dysfunction occurring as a result of ageing might contribute to the exacerbation of chronic neurodegenerative diseases and Aβ plaque load in Alzheimer disease and a reduced repair capacity in aged individuals (Fig. 3).
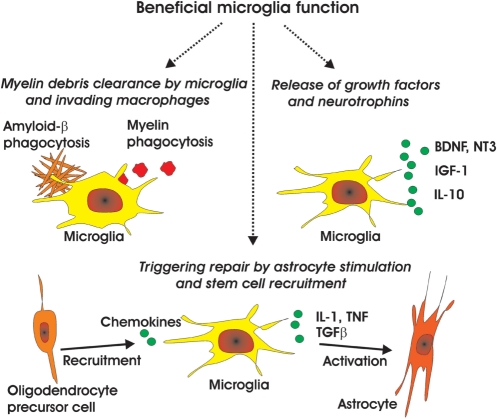
Fig. 3.
Beneficial microglia function. Microglial beneficial function is mediated via several effector mechanisms. Firstly, microglia and invading macrophages clear myelin debris or extracellular aggregates. Secondly, microglia initiate the repair process by stimulating neighbouring astrocytes to produce trophic support factors and by recruiting stem and precursor cells. Thirdly, microglial cells produce a variety of neurotrophins, growth factors and anti-inflammatory cytokines stimulating sprouting of axons and myelin repair. Soluble mediators: BDNF = brain derived neurotrophic factor; NT3 = neurotrophin-3; IGF-1 = insulin-like growth factor-1; IL-10 = interleukin-10; IL-1 = interleukin-1; TNF = tumour necrosis factor-α; TGF-β = transforming growth factor-β.
Conclusion
The removal of non-functional or degenerated tissue is an essential role of microglia. This response is most strikingly seen following injury in adulthood and can be viewed as an exaggerated version of a normal physiological task performed by microglia to remove superfluous cells undergoing apoptosis in development and adulthood. If phagocytosis is compromised as it is evident in loss-of-function mutations of either TREM2 or DAP12, this results in a chronic degenerative CNS disease. In the context of a homoeostatic role for microglial phagocytosis, the clearance function fits comfortably with a pro-regenerative contribution to the complex events occurring in the damaged CNS. At present this is most clearly evident in the inefficient remyelination associated with inhibition of precursor differentiation and in impaired axon regeneration in the presence by uncleared myelin debris. Similarly, limited clearance of affected tissue or dysfunction of microglia are features of several neurodegenerative diseases and are exacerbated with ageing. These relatively diverse lines of evidence point to the generic importance of the microglia-mediated phagocytic removal of debris in creating environments most conducive to intrinsic regenerative processes. Allowing these to occur will require a deeper understanding of the mechanisms and functional significance of microglia and macrophage-mediated clearance of tissue debris following injury from which new CNS regenerative medicines may emerge.
Acknowledgements
The group of H.N. is supported by the Hertie Foundation, the Rose Foundation, the Deutsche Forschungsgemeinschaft, the BMBF and the European Union (LSHM-CT-2005-018637). The group of R.J.M.F is mainly supported by The UK MS Society, The National MS Society, Research into Ageing and The Wellcome Trust. M.R.K's group receives funding from Wings for Life and the Medical University Vienna.
Glossary
Abbreviations
- Aβ
amyloid-β
- BDNF
brain derived neurotrophic factor
- CR3
complement receptor type 3
- EAE
experimental autoimmune encephalomyelitis
- IGF-1
insulin-like growth factor-1
- IL-4
interleukin-4
- TLRs
toll like receptors
- TNF-α
tumour necrosis factor-α
- TREM-2
triggering receptor expressed on myeloid cells-2
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–43. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Amat JA, Ishiguro H, Nakamura K, Norton WT. Phenotypic diversity and kinetics of proliferating microglia and astrocytes following cortical stab wounds. Glia. 1996;16:368–82. doi: 10.1002/(SICI)1098-1136(199604)16:4<368::AID-GLIA9>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–22. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- Arnett HA, Wang Y, Matsushima GK, Suzuki K, Ting JP. Functional genomic analysis of remyelination reveals importance of inflammation in oligodendrocyte regeneration. J Neurosci. 2003;23:9824–32. doi: 10.1523/JNEUROSCI.23-30-09824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T, Ito K. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol. 2004;14:668–77. doi: 10.1016/j.cub.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, et al. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–67. doi: 10.1016/j.neuron.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Barger SE. Establishing a nursing center: learning from the literature and the experiences of others. J Prof Nurs. 1995;11:203–12. doi: 10.1016/s8755-7223(95)80021-2. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Porritt MJ, Martinello P, Parish CL, Liberatore GT, Donnan GA, et al. Macrophages and microglia produce local trophic gradients that stimulate axonal sprouting toward but not beyond the wound edge. Mol Cell Neurosci. 2002;21:436–53. doi: 10.1006/mcne.2002.1185. [DOI] [PubMed] [Google Scholar]
- Battisti WP, Wang J, Bozek K, Murray M. Macrophages, microglia, and astrocytes are rapidly activated after crush injury of the goldfish optic nerve: a light and electron microscopic analysis. J Comp Neurol. 1995;354:306–20. doi: 10.1002/cne.903540211. [DOI] [PubMed] [Google Scholar]
- Bauer J, Sminia T, Wouterlood FG, Dijkstra CD. Phagocytic activity of macrophages and microglial cells during the course of acute and chronic relapsing experimental autoimmune encephalomyelitis. J Neurosci Res. 1994;38:365–75. doi: 10.1002/jnr.490380402. [DOI] [PubMed] [Google Scholar]
- Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–61. doi: 10.1016/j.neuron.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Broadie K. Axon pruning: an active role for glial cells. Curr Biol. 2004;14:R302–4. doi: 10.1016/j.cub.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Bruck W, Bruck Y, Friede RL. TNF-alpha suppresses CR3-mediated myelin removal by macrophages. J Neuroimmunol. 1992;38:9–17. doi: 10.1016/0165-5728(92)90085-y. [DOI] [PubMed] [Google Scholar]
- Bruck W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA, et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38:788–96. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–60. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–24. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–21. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Cullheim S, Thams S. The microglial networks of the brain and their role in neuronal network plasticity after lesion. Brain Res Rev. 2007;55:89–96. doi: 10.1016/j.brainresrev.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- David S, Lacroix S. Molecular approaches to spinal cord repair. Annu Rev Neurosci. 2003;26:411–40. doi: 10.1146/annurev.neuro.26.043002.094946. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M, Ffrench-Constant C, Franklin RJM. Enhancing central nervous system remyelination in multiple sclerosis. Neuron. 2005;48:9–12. doi: 10.1016/j.neuron.2005.09.004. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–8. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35–41. doi: 10.1016/s0896-6273(00)80432-8. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–9. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol. 2004;164:111–22. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–5. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter MR, Li WW, Zhao C, Franklin RJM. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26:328–32. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter MR, Zhao C, van Rooijen N, Franklin RJM. Macrophage-depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol Dis. 2005;18:166–75. doi: 10.1016/j.nbd.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Kress H, Stelzer EH, Holzer D, Buss F, Griffiths G, Rohrbach A. Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity. Proc Natl Acad Sci USA. 2007;104:11633–8. doi: 10.1073/pnas.0702449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov-Spiegler O, Solomon AS, Schwartz M. Peripheral nerve-stimulated macrophages simulate a peripheral nerve-like regenerative response in rat transected optic nerve. Glia. 1998;24:329–37. [PubMed] [Google Scholar]
- Li H, Newcombe J, Groome NP, Cuzner ML. Characterization and distribution of phagocytic macrophages in multiple sclerosis plaques. Neuropathol Appl Neurobiol. 1993;19:214–23. doi: 10.1111/j.1365-2990.1993.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Ludwin SK. Central nervous system demyelination and remyelination in the mouse: an ultrastructural study of cuprizone toxicity. Lab Invest. 1978;39:597–612. [PubMed] [Google Scholar]
- Ludwin SK. Chronic demyelination inhibits remyelination in the central nervous system. An analysis of contributing factors. Lab Invest. 1980;43:382–7. [PubMed] [Google Scholar]
- Malm TM, Koistinaho M, Parepalo M, Vatanen T, Ooka A, Karlsson S, et al. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis. 2005;18:134–42. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–47. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1beta promotes repair of the CNS. J Neurosci. 2001;21:7046–52. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, et al. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–4. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy J, Van der Loos H. The long-distance effects of brain lesions: visualization of myelinated pathways in the human brain using polarizing and fluorescence microscopy. J Neuropathol Exp Neurol. 1991;50:1–15. doi: 10.1097/00005072-199101000-00001. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–53. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–9. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- Mosley K, Cuzner ML. Receptor-mediated phagocytosis of myelin by macrophages and microglia: effect of opsonization and receptor blocking agents. Neurochem Res. 1996;21:481–7. doi: 10.1007/BF02527713. [DOI] [PubMed] [Google Scholar]
- Ness JK, Wood TL. Insulin-like growth factor I, but not neurotrophin-3, sustains Akt activation and provides long-term protection of immature oligodendrocytes from glutamate-mediated apoptosis. Mol Cell Neurosci. 2002;20:476–88. doi: 10.1006/mcne.2002.1149. [DOI] [PubMed] [Google Scholar]
- Neumann H, Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol. 2007;184:92–9. doi: 10.1016/j.jneuroim.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- O’Leary MT, Hinks GL, Charlton HM, Franklin RJM. Increasing local levels of IGF-I mRNA expression using adenoviral vectors does not alter oligodendrocyte remyelination in the CNS of aged rats. Mol Cell Neurosci. 2002;19:32–42. doi: 10.1006/mcne.2001.1062. [DOI] [PubMed] [Google Scholar]
- Patani R, Balaratnam M, Vora A, Reynolds R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol Appl Neurobiol. 2007;33:277–87. doi: 10.1111/j.1365-2990.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- Patrikios P, Stadelmann C, Kutzelnigg A, Rauschka H, Schmidbauer M, Laursen H, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–72. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- Perry VH, Bell MD, Brown HC, Matyszak MK. Inflammation in the nervous system. Curr Opin Neurobiol. 1995;5:636–41. doi: 10.1016/0959-4388(95)80069-7. [DOI] [PubMed] [Google Scholar]
- Piccio L, Buonsanti C, Mariani M, Cella M, Gilfillan S, Cross AH, et al. Blockade of TREM-2 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol. 2007;37:1290–301. doi: 10.1002/eji.200636837. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–65. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. Microgliosis: the questions shape the answers. Nat Neurosci. 2007;10:1507–9. doi: 10.1038/nn1207-1507. [DOI] [PubMed] [Google Scholar]
- Rappert A, Bechmann I, Pivneva T, Mahlo J, Biber K, Nolte C, et al. CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. J Neurosci. 2004;24:8500–9. doi: 10.1523/JNEUROSCI.2451-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran KS. “Recruitment signals” from apoptotic cells: invitation to a quiet meal. Cell. 2003;113:817–20. doi: 10.1016/s0092-8674(03)00471-9. [DOI] [PubMed] [Google Scholar]
- Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14:118–24. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Shields SA, Gilson JM, Blakemore WF, Franklin RJM. Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination. Glia. 1999;28:77–83. doi: 10.1002/(sici)1098-1136(199910)28:1<77::aid-glia9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–24. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJM. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–9. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder AK, Ermini F, Bondolfi L, Krenger W, Burbach GJ, Deller T, et al. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J Neurosci. 2005;25:11125–32. doi: 10.1523/JNEUROSCI.2545-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–9. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–47. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia and neuroprotection: implications for Alzheimer's disease. Brain Res Brain Res Rev. 2005;48:234–9. doi: 10.1016/j.brainresrev.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglial senescence: does the brain's immune system have an expiration date? Trends Neurosci. 2006;29:506–10. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4:e124. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–57. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–79. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Woodruff RH, Franklin RJM. Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide, and complement/anti-galactocerebroside: a comparative study. Glia. 1999;25:216–28. doi: 10.1002/(sici)1098-1136(19990201)25:3<216::aid-glia2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–51. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Zhao C, Li WW, Franklin RJM. Differences in the early inflammatory responses to toxin-induced demyelination are associated with the age-related decline in CNS remyelination. Neurobiol Aging. 2006;27:1298–307. doi: 10.1016/j.neurobiolaging.2005.06.008. [DOI] [PubMed] [Google Scholar]