Abstract
Postembryonic de novo organogenesis represents an important competence evolved in plants that allows their physiological and developmental adaptation to changing environmental conditions. The phytohormones auxin and cytokinin (CK) are important regulators of the developmental fate of pluripotent plant cells. However, the molecular nature of their interaction(s) in control of plant organogenesis is largely unknown. Here, we show that CK modulates auxin-induced organogenesis (AIO) via regulation of the efflux-dependent intercellular auxin distribution. We used the hypocotyl explants-based in vitro system to study the mechanism underlying de novo organogenesis. We show that auxin, but not CK, is capable of triggering organogenesis in hypocotyl explants. The AIO is accompanied by endogenous CK production and tissue-specific activation of CK signaling. CK affects differential auxin distribution, and the CK-mediated modulation of organogenesis is simulated by inhibition of polar auxin transport. CK reduces auxin efflux from cultured tobacco cells and regulates expression of auxin efflux carriers from the PIN family in hypocotyl explants. Moreover, endogenous CK levels influence PIN transcription and are necessary to maintain intercellular auxin distribution in planta. Based on these findings, we propose a model in which auxin acts as a trigger of the organogenic processes, whose output is modulated by the endogenously produced CKs. We propose that an important mechanism of this CK action is its effect on auxin distribution via regulation of expression of auxin efflux carriers.
Keywords: PIN expression, two-component signalling, root meristem, auxin maxima
Postembryonic de novo organogenesis represents an important developmental adaptation evolved in plants. Regeneration of entire bodies in hydras (1) or organs in amphibians (2) has been described. However, in the animal kingdom, these examples are rather exceptional. In contrast, plants evolved postembryonic formation of new organs from differentiated tissues as a strategy that allows physiological and developmental adaptation to changing environmental conditions. However, this strategy requires action by factors that are specifically able to induce developmental programs, leading to the formation of entire organs from virtually differentiated cells.
The interaction of auxin and cytokinin (CK) during plant organogenesis is a phenomenon known for a long time. In their pioneering work, Skoog and Miller (3) identified auxin-to-CK concentration ratios as an important factor regulating the developmental fate of plant tissue explants. Since that time, the role of both growth factors in plant development has been extensively studied. For auxin action, a model involving a spatial and temporal pattern of intercellular auxin distribution and concentration maxima is well established, and the molecular and cellular factors mediating auxin distribution have been identified (4, 5). Differential auxin distribution has been shown to mediate multiple aspects of plant development, such as apical/basal axis formation (6), root patterning (7, 8), tropisms (9–11), and organogenesis (12–15). CK is an important regulator of shoot (16) and root architecture (17–22), and it also regulates seed development (23), abiotic stress (24), and plant senescence (25). CK signaling is mediated by two-component phosphorelay in Arabidopsis (for an in-depth recent review, see ref. 26). However, the molecular factors acting downstream of the CK signaling pathway remain mostly unknown.
Here, we use de novo auxin-induced organogenesis (AIO) as a model for characterization of the interactions between CKs and auxin in regulation of plant development. We show that auxin triggers organogenesis and that CK modulates its output through its effect on auxin distribution, which is realized by CK-dependent regulation of expression of auxin transport components.
Results
CK Modulates Auxin-Induced de Novo Organogenesis via Two-Component Signalling.
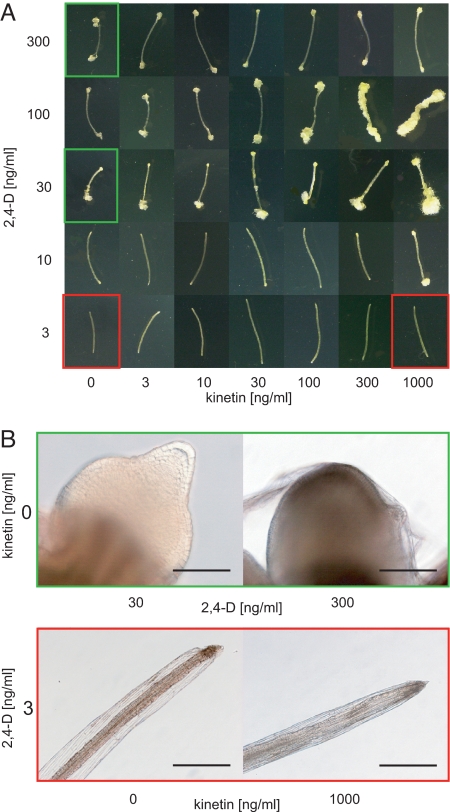
We have used the well-known phenomenon of distinct effects of different CK-to-auxin ratios on the development of plant explants in vitro (3, 27) and adapted this system to study the mechanism underlying de novo organogenesis. Placement of Arabidopsis hypocotyls on the media with threshold auxin concentration has resulted in the formation of newly induced root-like organs, even in the absence of exogenous CK. The threshold auxin concentration was identified as the lowest auxin concentration leading to the formation of well-distinguishable organs at different CK concentrations and was identified to be 30 ng/mL (135 nM) for 2,4-dichlorophenoxyacetic acid [2,4-D] and 100 ng/mL (537 nM) for naphthalene-1-acetic acid [NAA] (Fig. 1A). In the root-like structures induced by NAA, all important morphological traits of genuine roots could be recognized (i.e., columella, lateral root cap, quiescent center, epidermis, cortex, endodermis, stele). In 2,4-D–induced organs, only the columella-like cells could be distinguished. However, in both 2,4-D– and NAA-induced organs, the columella-like cells revealed DR5 activity, which is consistent with the situation in genuine roots. With an increasing concentration of CK in the media, we observed a decreasing ability of hypocotyl explants to form root-like structures and their gradual disorganization (Fig. 1A and Fig. S1, for more details see later in the text). At the CK (kinetin) concentration of 300 ng/mL (1.4 μM, further referred to as the CK threshold), only disorganized callus was produced (Fig. 1A), with very rare remnants of distinguishable root-like organs (Fig. S1). After a prolonged period of cultivation at these CK and auxin concentrations, the calli turned green, and new shoots have occasionally been formed from the disorganized tissue (data not shown). However, at auxin concentrations below the organ-inducing threshold, CK alone was unable to induce any organogenic response (Fig. 1 A and B). This suggests that auxin triggers organogenesis, whereas CK modulates it.
Fig. 1.
CK modulates AIO. (A) Formation of root-like organs and calli in hypocotyls grown on different combinations of 2,4-D and kinetin. (B) Details of the root-like organs (green frames in A) formed in the absence of kinetin and at different 2,4-D concentrations. In contrast, no organogenic response was observed at the low auxin concentration and any of the tested kinetin concentrations (red frames in A). (Scale bars: 100 μm in green frame and 400 μm in red frame.)
To address the involvement of CK signaling in the observed phenomenon, we analyzed expression of the primary CK response genes (i.e., the A-type ARR genes) (28) in hypocotyl explants using quantitative real-time (qRT) PCR in the presence of auxin threshold [NAA (100 ng/mL)] and increasing CK concentration. With the exception of ARR3, which peaked at 30 ng/mL (139 nM) kinetin, we have found a gradual increase in the expression levels of all inspected A-type ARRs with increasing CK concentration; a particularly steep increase of expression was observed at the CK threshold concentration (Fig. 2A). Next, we addressed the involvement of CK perception and its specificity in the observed morphogenic effect. We analyzed the organogenic response in hypocotyl explants isolated from mutants in CK receptors AHK2, AHK3, and AHK4 (29, 30). All single, and particularly double, mutants showed increased resistance to CK in terms of modulation of organogenesis in comparison to corresponding WT (Fig. 2B). Differences in the strength of the phenotype in particular single and double ahk mutants suggest a certain specificity of individual signaling pathways in the CK-dependent modulation of organogenesis, with a dominant effect of AHK4, followed by AHK3 and AHK2 (AHK4 ≥ AHK3 > AHK2; Fig. 2B). These findings are in accordance with previous observations (29, 30), thus confirming the suitability of our experimental setup. Collectively, these results show that CKs modulate the auxin-induced organogenic response in Arabidopsis via two-component signaling.
Fig. 2.
CK modulates organogenesis via a two-component system. (A) Relative expression of CK primary response genes, A-type ARRs with the increasing CK concentration in the presence of NAA (537 nM). The statistical significance of identified differences in comparison to the absence of exogenous CKs (t test) at alpha 0.05 and 0.01 is designated (* and **, respectively); error bars show SDs. (B) Phenotypes of root-like organs induced by NAA (537 nM) at the increasing CK concentrations in WT (Col-0 and Ws ecotypes) and different single and double CK receptor mutants. All mutants are of Col-0 ecotype except for ahk4 and ahk3 ahk4, which carry ahk4–1 allele from Ws (see Materials and Methods). (Scale bar: 50 μm.)
AIO Is Accompanied by the Production of Endogenous CKs and Tissue-Specific Activation of CK Signaling.
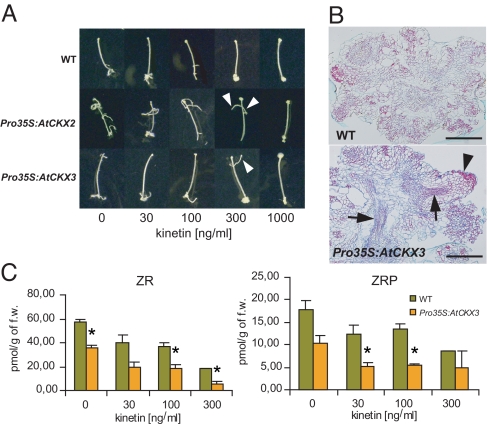
The expression of A-type ARRs even in the absence of exogenous CKs (Fig. 2A and data not shown) suggests that the AIO might be accompanied by endogenous CK production and subsequent activation of CK signaling. To identify the potential importance of endogenous CKs in AIO, we inspected organogenesis in hypocotyl explants with endogenous CKs depleted via ectopic overexpression of CYTOKININ OXIDASE/DEHYDROGENASE genes (19). In Pro35S:AtCKX2 and Pro35S:AtCKX3 explants, we observed partial resistance to CK, as manifested by increased competence of hypocotyl explants to form root-like organs (Fig. S1) and formation of root-like structures even at the CK threshold concentration (Fig. 3 A and B). Because kinetin has been found to be only a poor substrate of CKX (31), this effect seems to be attributable to a decrease of endogenous CKs rather than to inactivation of exogenously applied CKs. To confirm that, we have measured levels of endogenous CKs in hypocotyl explants cultivated in the absence and presence of exogenous CKs. In the WT hypocotyl explants, endogenous CKs [from active CKs, predominantly trans-zeatin-9-riboside (ZR) and ZR phosphate] were found in the hypocotyls grown at the organogenesis-inducing (threshold) auxin concentration in the absence of exogenous CK. The amounts of most of the endogenous CKs were substantially reduced in the Pro35S:AtCKX3 hypocotyl explants (Fig. 3C and Fig. S2). Surprisingly, the addition of exogenous CKs led to the further reduction of endogenously produced CKs in both WT and AtCKX3 overexpressing hypocotyl explants (Fig. 3C). This is presumably attributable to up-regulation of endogenous AtCKX expression by exogenous CKs (32). These data show that AIO is accompanied by the production of endogenous CKs that affect its developmental output.
Fig. 3.
Auxin induces production of endogenous CKs that contribute to AIO. (A) Formation of root-like organs induced by NAA (537 nM). Note that in Pro35S:AtCKX2 and Pro35S:AtCKX3 lines, there are still root-like organs distinguishable even at the CK threshold concentration (arrowheads), which is not the case in WT. (B) Structure of calli induced by auxin (537 nM NAA) at the CK threshold (1.4 μM kinetin). In the Pro35S:AtCKX3 line, there are still patterned organs distinguishable (arrowhead) in comparison to WT, where only almost completely disorganized tissue could be detected. Arrows point to the patterned vascular tissue in Pro35S:AtCKX3 calli. (Scale bar: 200 μm.) (C) Levels of endogenous CKs after induction of organogenesis by NAA (537 nM) at different exogenous CK concentrations. The statistical significance of identified differences in comparison to WT (t test) at alpha 0.05 is designated (*); error bars show SDs. For the data on all analyzed CK metabolites, see Fig. S2 and Table S1.
To gain insight into the potential tissue specificity of CK production and action during AIO, we have inspected expression of ARR5, one of the earliest expressed CK primary response genes (28). In agreement with our qRT-PCR data, we have observed the activity of ARR5 promoter in ProARR5:GUS hypocotyl explants even in the absence of exogenous CKs (Fig. S3A). GUS activity in hypocotyl explants was delimited to the induced root-like organs, suggesting tissue specificity of CK signaling leading to up-regulation of ARR5 expression. Expression of ARR5 was reduced in both Pro35S:AtCKX2 and Pro35S:AtCKX3 lines (Fig. S3A and data not shown, respectively). Altogether, these findings indicate that AIO is accompanied by tissue-specific activation of the CK signaling pathway and endogenous CK production that contributes to the CK-dependent modulation of AIO. Thus, the CK effect on AIO in our system is a sum of both endogenous and exogenous CKs.
CK Affects Auxin Distribution During de Novo Organogenesis.
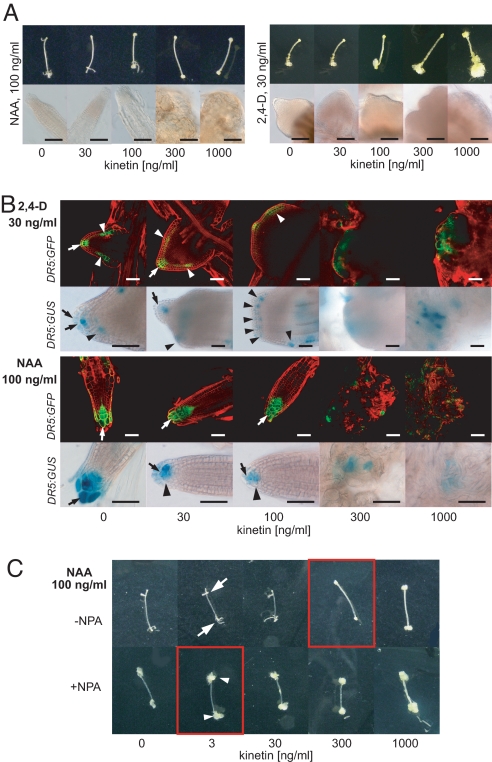
To identify a mechanism of CK action during AIO, we inspected its effect on the morphology of auxin-induced organs in more detail. Interestingly, we found important differences in CK effect on AIO induced by either 2,4-D or NAA. In the absence of exogenous CKs, NAA induces formation of root-like structures with a cellular pattern that resembles Arabidopsis roots. The increasing CK concentration led to a decrease in the number of NAA-induced organs (Fig. S1); however, the morphology of formed organs was only slightly affected by CK concentration below the CK threshold (Fig. 4A). In contrast, 2,4-D induced formation of only poorly specified root-like organs that only partially resembled Arabidopsis roots, and the increasing CK concentration led to a gradual loss of organ structure and patterning (Fig. 4A). However, in both cases, the CK threshold led to the loss of organ formation and only unorganized callus was formed (Fig. 4A). The 2 types of auxin used, 2,4-D and NAA, differ in the mechanism of their transport in plant cells. While 2,4-D must be taken up into cells actively by AUX/LAX importers (11), NAA enters cells almost entirely via passive diffusion (33). On the other hand, NAA, but not 2,4-D, gets out from cells easily via auxin efflux carriers (33, 34), and can thus be more efficiently transported between cells. Thus, these different effects of CK on NAA- and 2,4-D–induced organogenesis indicated the involvement of auxin transport in this process.
Fig. 4.
CK has effects on auxin distribution during de novo organogenesis. (A) Phenotype of root-like organs after induction of organogenesis in hypocotyl explants either by NAA (Left) or 2,4-D (Right). Note the higher organization and patterning resembling Arabidopsis roots in the case of root-like organs induced by NAA. (Scale bar: 100 μm.) (B) Spatial pattern of auxin maxima as visualized by activity of DR5 on 2,4-D– (Upper) and NAA-induced organs (Lower). Note the formation of ectopic auxin maxima (arrowheads), their spreading and disorganization, and the more pronounced loss of the organ patterning and structure in the case of organs induced by 2,4-D. The auxin maxima are gradually less pronounced in case of NAA-induced root-like organs (arrowhead). Note the almost complete disappearance of DR5 maxima and loss of organ patterning at the CK threshold concentration [300 ng/mL (1.4 μM) kinetin]. Arrows depict the columella-like cells. (Scale bar: 50 μm.) (C) NPA (10 μM) partially mimics the effect of the CK threshold. Note the large amount of calli (arrowheads) formed even at the lowest kinetin concentration [3 ng/mL (14 nM)] in comparison to well-recognizable root-like organs at the same kinetin concentration in the absence of NPA (arrows); this resembles the effect of the CK threshold in the absence of NPA (compare figures in red frames). For details, see Fig. S3.
Transport-dependent control of the spatial and temporal pattern of auxin distribution in plant tissues plays an important role in multiple aspects of organogenesis in planta (13). Thus, we examined the potential CK effect on the formation of local auxin maxima as visualized by the activity of the auxin response reporter DR5 (35) in organs induced by 2,4-D or NAA. The NAA-induced organs displayed single auxin maxima at the “root tip,” which resembles the situation in Arabidopsis root primordia (13, 36). With an increasing CK concentration, the auxin maxima in NAA-induced organs were only slightly affected; they became diffuse and weaker, as visible particularly in DR5rev:GUS (Fig. 4B). On the other hand, 2,4-D–induced organs formed with multiple ectopically located auxin maxima in additional “root tips”. The increasing CK concentration resulted in the formation of less focused auxin maxima and their spreading and disorganization. That correlated well with changes in the shape of 2,4-D–induced root-like organs, (i.e., gradual loss of the organ structure and patterning) (Fig. 4B). At the CK-threshold concentration, almost complete loss of auxin maxima formation was observed in both NAA- and 2,4-D–induced calli (Fig. 4B). Thus, the apparently higher sensitivity of 2,4-D–induced organs to CK-mediated morphogenic effect very probably reflects lower efficiency of efflux carriers to relocate 2,4-D in comparison to NAA. However, at concentrations reaching or higher than the CK threshold, the auxin efflux capacity decreases below the level necessary for formation of defined auxin maxima and, consequently, results in loss of organ patterning in both 2,4-D– and NAA-induced organogenesis.
Moreover, treatment with 1-naphthylphthalamic acid (NPA), a potent inhibitor of polar auxin transport at the level of auxin efflux (37, 38), partially mimics the effect of exogenous CKs (Fig. 4C). In the presence of NPA (10 μM) and absence of exogenous CKs, NAA induces the formation of root-like organs similar to those induced by 2,4-D. However, these organs were more sensitive to both endogenous and exogenous CKs. That was manifested by the formation of a large amount of callus and a higher degree of organ structure disintegration even at the lowest CK concentration applied, thus resembling the CK threshold (Fig. 4C and Fig. S3). Accordingly, in the absence of exogenous CKs, NPA led to the formation of organs whose structure was better preserved and more resembled roots in Pro35S:AtCKX2 hypocotyl explants in comparison to WT (Fig. S3B). This suggests partially synergistic but distinct effects of NPA and CKs on the AIO. Taken together, our results indicate a correlation between auxin distribution and its organogenic effect during de novo AIO and suggest an interference of CKs with the formation of the cellular efflux-dependent local auxin maxima. Nevertheless, it is obvious, and must be considered, that apart from the role of CKs in the regulation of auxin distribution, CKs also affect organogenesis via other mechanisms (e.g., regulation of cell proliferation) (3).
CK Affects the Expression of PIN Auxin Efflux Carriers and the Cellular Efflux of Auxin.
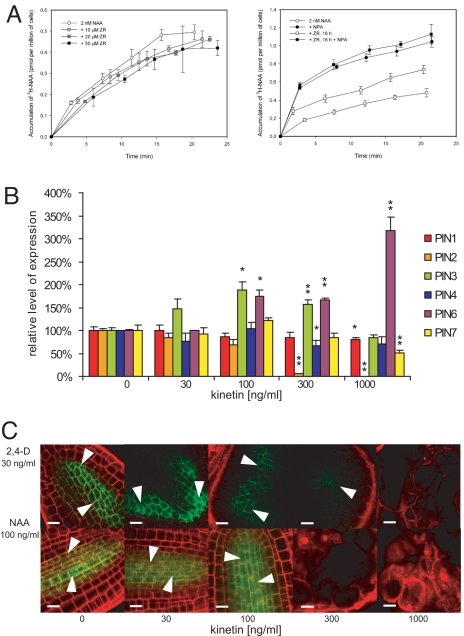
Our results suggest that, as shown for in planta organogenesis (12, 13, 15), auxin efflux is also required for control of auxin distribution during de novo-induced organogenesis in our in vitro system. Therefore, we tested the scenario that CK influences AIO through an effect on auxin efflux. Accumulation of radioactively labeled NAA ([3H]NAA) in cultured tobacco BY-2 cells is a reliable system for the measuring and quantification of auxin efflux (34). When CKs were added to the cell suspension just at the beginning of the assay (i.e., together with [3H]NAA), no CK effect on the [3H]NAA accumulation was observed. However, pretreatment of BY-2 cells with CKs (5 μM ZR for 16 h) led to the increase of the [3H]NAA accumulation (i.e., to a decrease of its efflux) (Fig. 5A). Similar results were obtained with various other CKs (Fig. S4). Thus, CKs similar to established auxin transport inhibitors, such as NPA, inhibit auxin efflux. However, the long lag time of CK effect suggests that CKs regulate auxin efflux in BY-2 cells via regulation of expression of genes for efflux carriers or regulatory proteins rather than via direct interference with efflux activity. CK enhances ethylene biosynthesis (39), and the involvement of ethylene in the regulation of auxin transport has been reported (40, 41). Therefore, we analyzed CK effects on the auxin efflux in the presence of aminoethoxy vinyl glycine (AVG), an inhibitor of ethylene production (42). No difference in auxin accumulation in BY-2 cells treated with CK was observed between the absence and presence of AVG (Fig. S4), showing that CKs act on auxin efflux independent of regulation of ethylene biosynthesis.
Fig. 5.
CKs modulate auxin efflux and PIN expression. (A) Accumulation of [3H]NAA in BY-2 cells. Although there is no apparent effect on the auxin accumulation in BY-2 cells when CK of different concentrations (10, 20, and 50 μM ZR) was added together with [3H]NAA (Left), the 16-hour pretreatment with 5 μM ZR led to an increase in the accumulation of [3H]NAA (Right); 2 nM [3H]NAA was used in both cases. This effect could be mimicked by addition of 10 μM NPA (Right). Error bars show SDs. (B) Relative transcription of PIN auxin transporters in hypocotyl explants at different CK concentrations and at the auxin threshold (NAA; 537 nM) as measured by qRT-PCR. The statistical significance of identified differences in comparison to the absence of exogenous CKs (t test) at alpha 0.05 and 0.01 is designated (* and **, respectively), and error bars show SDs. (C) PIN1-GFP signal (green, arrowheads) in the root-like organs of ProPIN1:PIN1-GFP hypocotyl explants at the different CK concentrations. Note that the signal is getting weaker and diffuse and disappears at the CK threshold in the case of both 2,4-D and NAA. (Scale bar: 10 μm.)
Next, we addressed the possible mechanisms by which CKs modulate auxin efflux. Auxin carriers from the PIN family were identified to be the rate-limiting regulators of the cellular auxin efflux (34), and their key role in generating differential local auxin distribution has been demonstrated (5). Because cellular output of CK signaling occurs at the level of regulation of gene expression, we tested possible regulation of PIN expression by CKs. Using qRT-PCR, we have observed differential transcription of individual PIN genes in hypocotyl explants cultivated in the presence of auxin threshold (537 nM NAA) and different CK concentrations. Although the expression of PIN3 peaked at 100 ng/mL CK (464 nM kinetin) and decreased with further increasing CK concentrations, the expression of PIN6 was up-regulated at the same CK concentration (100 ng/mL) and further increased at 1,000 ng/mL CK (4.6 μM kinetin) (Fig. 5B). The transcription of root-specific PIN2 (10) dramatically decreased at the CK threshold, presumably reflecting the loss of root identity and formation of only undifferentiated calli (Fig. 5B). Interestingly, PIN1 transcription was only slightly down-regulated even at the highest CK concentration (4.6 μM kinetin) (Fig. 5B). However, in both 2,4-D– and NAA-induced root-like organs, the signal of PIN1-GFP was getting weaker and more diffuse with the increasing CK concentrations (Fig. 5C). The PIN1-GFP signal was lost in calli at the CK threshold concentration, and only residual PIN1-GFP, apparently not associated with plasma membrane, was occasionally detectable (data not shown); for quantification of the CK effect on PIN1-GFP expression, see Fig. S5. Thus, CKs seem to affect the expression of PIN genes, possibly at both transcriptional and posttranscriptional levels. Taken together, these results show that CKs regulate expression of PIN auxin efflux carriers during de novo AIO, which provides a plausible mechanism for CK-dependent regulation of auxin efflux.
Endogenous CKs Are Required for Differential Auxin Distribution in Arabidopsis Roots.
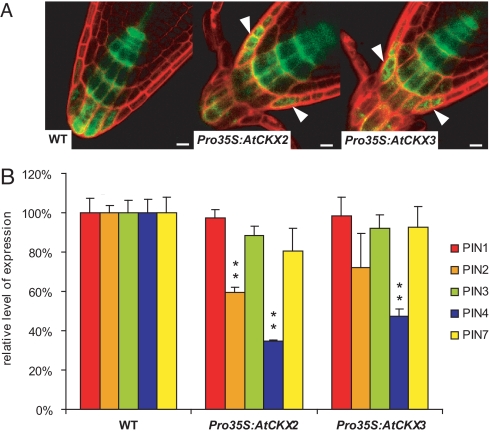
Our results imply that CKs can affect auxin distribution during de novo organogenesis via regulation of auxin efflux from cells. In root development, differential auxin distribution has been shown to regulate activity and patterning of the root meristem (7, 36). Thus, we addressed whether endogenous CKs are required for auxin distribution and root meristem patterning in planta. We examined the formation of local auxin maxima (visualized by DR5 activity) in CK-deficient Pro35S:AtCKX2 and Pro35S:AtCKX3 plants. In the root tips of these plants, DR5rev:GFP expression in columella expanded more laterally in comparison to that of control (Fig. 6A). We analyzed 2 lines of each transformant, Pro35S::AtCKX2 and Pro35S::AtCKX3. For Pro35S::AtCKX2, 30 and 33 aberrant roots were scored out of 38 and 40 inspected roots, respectively (30 of 38 roots and 33 of 40 roots). For Pro35S::AtCKX3, the result was similar (32 of 41 roots and 19 of 23 roots). In WT background, only 5 of 39 inspected roots revealed aberrations in the DR5rev:GFP expression pattern. Accordingly, the first 5 columella cells were significantly enlarged in the longitudinal direction in several independent Pro35S:AtCKX2 and Pro35S:AtCKX3 lines (Fig. S6). This presumably reflects the dose-dependent role of auxin in the regulation of cell elongation (43) and provides additional evidence for a disturbed auxin gradient in the root tip of Pro35S:AtCKX2(3) lines. We also tested whether endogenous CK levels influence PIN transcription or polar PIN localization. In the roots of 6-day-old seedlings of both Pro35S:AtCKX2 and Pro35S:AtCKX3 lines, polar localization of PIN2 and PIN4 proteins did not differ from that of controls (Fig. S7), but we have found a strong decrease in the PIN2 and PIN4 mRNA levels (Fig. 6B). Based on these data, we conclude that distinct levels of endogenous CKs are necessary to maintain expression of PIN auxin efflux carriers in the root tip, thus regulating formation of local auxin maxima and root meristem development. These data show that the mechanism of CK-dependent regulation of PIN transcription and control of differential auxin distribution that we identified during de novo organogenesis also applies for processes in planta.
Fig. 6.
Endogenous CK levels are required for local auxin maxima formation and mediate PIN gene expression in Arabidopsis roots. (A) Depletion of endogenous CKs in Pro35S:AtCKX2 and Pro35S:AtCKX3 lines leads to the defects in auxin response gradients as visualized by DR5rev:GFP. Note the lateral expansion of the maxima in transgenic lines (arrowheads) in comparison to WT. (Scale bar: 10 μm.) (B) Relative PIN transcription measured by qRT-PCR in Pro35S:AtCKX2 and Pro35S:AtCKX3 roots in comparison to WT. The statistical significance of differences (t test) at alpha 0.01 is marked by **; error bars show SDs.
Discussion
Our work addresses the mechanism underlying the role of the phytohormones auxin and CK in plant organogenesis. We show that in contrast to CK, auxin is able to induce a de novo organogenic response in hypocotyl explants. This is in accordance with the recent recognition of auxin and/or its gradients as a general trigger for the change in the developmental program in plants (4, 15). We have found that the auxin-induced organogenic response is accompanied by production of endogenous CKs and the tissue-specific activation of the CK signaling pathway. The activation of ARR5 expression in the absence of exogenous CKs was also observed in root explants (44). This further confirms our conclusions and implies that auxin might induce similar developmental programs in root and hypocotyl explants, thus strengthening the role of auxin as a universal trigger of organogenesis.
Formation of lateral roots represents one of the examples for postembryonal de novo organogenesis in plants. Recent reports (20, 22) suggest potential involvement of CKs in the regulation of auxin efflux during lateral root formation. Exogenous CKs are supposed to down-regulate expression of all inspected PIN genes at early stages of lateral root primordia development (20). However, this does not seem to be the case in the roots of Pro35S:AtCKX2(3) lines, in which at least PIN2 and PIN4 are down-regulated after endogenous CK depletion. Our results reflect predominantly the context of primary root meristem, because we have analyzed PIN expression in the roots of 6-day-old seedlings, in which only a few lateral roots and lateral root primordia have yet been formed. This implies that CKs affect the expression of individual PIN carriers differentially in particular plant tissues and that complex interactions between CKs and individual members of the auxin-efflux machinery should be further characterized in a spatiotemporal context.
Our results suggest that in addition to recently identified interaction between CK and auxin on the level of signaling (45), CKs modulate auxin distribution via regulation of auxin efflux. This type of regulation represents a thus far unidentified mechanism for well-known CK-auxin interactions during plant development. We propose that changes in endogenous CK levels form an intrinsic part of the auxin-induced organogenic response and that CK-mediated modulation of auxin distribution via regulation of auxin efflux is one of the mechanisms underlying the auxin-CK interaction during organogenesis in plants.
Materials and Methods
Plant Materials.
Unless otherwise stated, all plant material used was Arabidopsis thaliana, ecotype Col-0. For the hypocotyl explant assay, ahk2–1, ahk3–1, and ahk4–1 (30) single-mutant lines and ahk2–1 ahk3–1, ahk3–1 ahk4–1 (30), and ahk2–2TK cre1–12 (29) double-mutant lines were used. For details of preparation of transgenic lines used, see SI Text.
Hypocotyl Explants Assay.
Plants were cultivated 1 day in the light and 5 days in the dark in Petri dishes with Murashige and Skoog medium, including Gamborg B5 vitamins in growth chambers (Percival) at 21 °C. Hypocotyls were isolated by removing cotyledons and roots and were placed on Petri dishes with cultivation medium as described (27) and enriched with respective hormone concentrations. Kinetin, 2,4-D, and NAA were purchased from Sigma-Aldrich. Hypocotyl explants were cultivated for 21 days under long-day conditions (16 h light at 21 °C and 8 h dark at 19 °C), a light intensity of 100 μM·m−2·s−1, and 80% relative humidity.
[3H]NAA Accumulation in BY-2 Cells.
The [3H]NAA accumulation assay was performed as described (38).
Supplementary Material
Acknowledgments.
We thank Yka Helariutta and Chiharu Ueguchi for providing us with seeds of ahk mutants, Eva Benkova for DR5rev:GUS and DR5rev:GFP lines, Kristin Bilyeu for AtCKX3 cDNA, Joseph Kieber for ProARR5:GUS construct, and Guido Jach for pGJ-Bar plasmid. We are grateful to Ivo Lukeš and Tomáš Jendrulek (Olympus C&S, spol. s r. o.) for excellent technical support. This work was supported by the Ministry of Education, Youth, and Sports of the Czech Republic, Project Nos. LC06034 (to M.P., P.K., J. Horák, P.S., K.H., E.Z., and J. Hejátko) and MSM0021622415 (to P.R., J.D., J.F. and J. Hejátko) and by the Grant Agency of the Czech Republic, Project No. 204/08/H054 (to J. Horák and J. Hejátko).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811539106/DCSupplemental.
References
- 1.Gierer A, et al. Regeneration of hydra from reaggregated cells. Nat New Biol. 1972;239:98–101. doi: 10.1038/newbio239098a0. [DOI] [PubMed] [Google Scholar]
- 2.Endo T, Bryant SV, Gardiner DM. A stepwise model system for limb regeneration. Dev Biol. 2004;270:135–145. doi: 10.1016/j.ydbio.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Skoog F, Miller CO. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;54:118–130. [PubMed] [Google Scholar]
- 4.Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka H, Dhonukshe P, Brewer PB, Friml J. Spatiotemporal asymmetric auxin distribution: A means to coordinate plant development. Cell Mol Life Sci. 2006;63:2738–2754. doi: 10.1007/s00018-006-6116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friml J, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 7.Friml J, et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- 8.Blilou I, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 9.Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 10.Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchant A, et al. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 1999;18:2066–2073. doi: 10.1093/emboj/18.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heisler MG, et al. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol. 2005;15:1899–1911. doi: 10.1016/j.cub.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 13.Benkova E, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 14.Reinhardt D, et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- 15.Dubrovsky JG, et al. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci USA. 2008;105:8790–8794. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurakawa T, et al. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature. 2007;445:652–655. doi: 10.1038/nature05504. [DOI] [PubMed] [Google Scholar]
- 17.Scheres B, et al. Mutations affecting the radial organization of the Arabidopsis root display specific defects throughout the embryonic axis. Development. 1995;121(1):53–62. [Google Scholar]
- 18.Mahonen AP, et al. Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science. 2006;311:94–98. doi: 10.1126/science.1118875. [DOI] [PubMed] [Google Scholar]
- 19.Werner T, et al. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laplaze L, et al. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell. 2007;19:3889–3900. doi: 10.1105/tpc.107.055863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dello Ioio R, et al. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr Biol. 2007;17:678–682. doi: 10.1016/j.cub.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 22.Kuderova A, et al. Effects of conditional IPT-dependent cytokinin overproduction on root architecture of Arabidopsis seedlings. Plant Cell Physiol. 2008;49:570–582. doi: 10.1093/pcp/pcn029. [DOI] [PubMed] [Google Scholar]
- 23.Riefler M, Novak O, Strnad M, Schmulling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell. 2006;18:40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran LS, et al. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc Natl Acad Sci USA. 2007;104:20623–20628. doi: 10.1073/pnas.0706547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HJ, et al. Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:814–819. doi: 10.1073/pnas.0505150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.To JP, Kieber JJ. Cytokinin signaling: Two-components and more. Trends Plant Sci. 2008;13:85–92. doi: 10.1016/j.tplants.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Kubo M, Kakimoto T. The cytokinin-hypersensitive genes of Arabidopsis negatively regulate the cytokinin-signaling pathway for cell division and chloroplast development. Plant J. 2000;23:385–394. doi: 10.1046/j.1365-313x.2000.00796.x. [DOI] [PubMed] [Google Scholar]
- 28.D'Agostino IB, Deruere J, Kieber JJ. Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol. 2000;124:1706–1717. doi: 10.1104/pp.124.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higuchi M, et al. In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci USA. 2004;101:8821–8826. doi: 10.1073/pnas.0402887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimura C, et al. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell. 2004;16:1365–1377. doi: 10.1105/tpc.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popelkova H, et al. Kinetic and chemical analyses of the cytokinin dehydrogenase-catalysed reaction: Correlations with the crystal structure. Biochem J. 2006;398:113–124. doi: 10.1042/BJ20060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werner T, Kollmer I, Bartrina I, Holst K, Schmulling T. New insights into the biology of cytokinin degradation. Plant Biology. 2006;8:371–381. doi: 10.1055/s-2006-923928. [DOI] [PubMed] [Google Scholar]
- 33.Delbarre A, Muller P, Imhoff V, Guern J. Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta. 1996;198:532–541. doi: 10.1007/BF00262639. [DOI] [PubMed] [Google Scholar]
- 34.Petrasek J, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 35.Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabatini S, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- 37.Katekar GF, Geissler AE. Auxin transport inhibitors: IV. Evidence of a common mode of action for a proposed class of auxin transport inhibitors: The phytotropins. Plant Physiol. 1980;66:1190–1195. doi: 10.1104/pp.66.6.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrasek J, et al. Do phytotropins inhibit auxin efflux by impairing vesicle traffic? Plant Physiol. 2003;131:254–263. doi: 10.1104/pp.012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chae HS, Faure F, Kieber JJ. The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell. 2003;15:545–559. doi: 10.1105/tpc.006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruzicka K, et al. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell. 2007;19:2197–2212. doi: 10.1105/tpc.107.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swarup R, et al. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell. 2007;19:2186–2196. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher-plants. Annu Rev Plant Physiol Plant Mol Biol. 1984;35:155–189. [Google Scholar]
- 43.Hardtke CS. Transcriptional auxin-brassinosteroid crosstalk: Who's talking? BioEssays. 2007;29:1115–1123. doi: 10.1002/bies.20653. [DOI] [PubMed] [Google Scholar]
- 44.Gordon SP, et al. Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development. 2007;134:3539–3548. doi: 10.1242/dev.010298. [DOI] [PubMed] [Google Scholar]
- 45.Muller B, Sheen J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature. 2008;453:1094–1097. doi: 10.1038/nature06943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.