Abstract
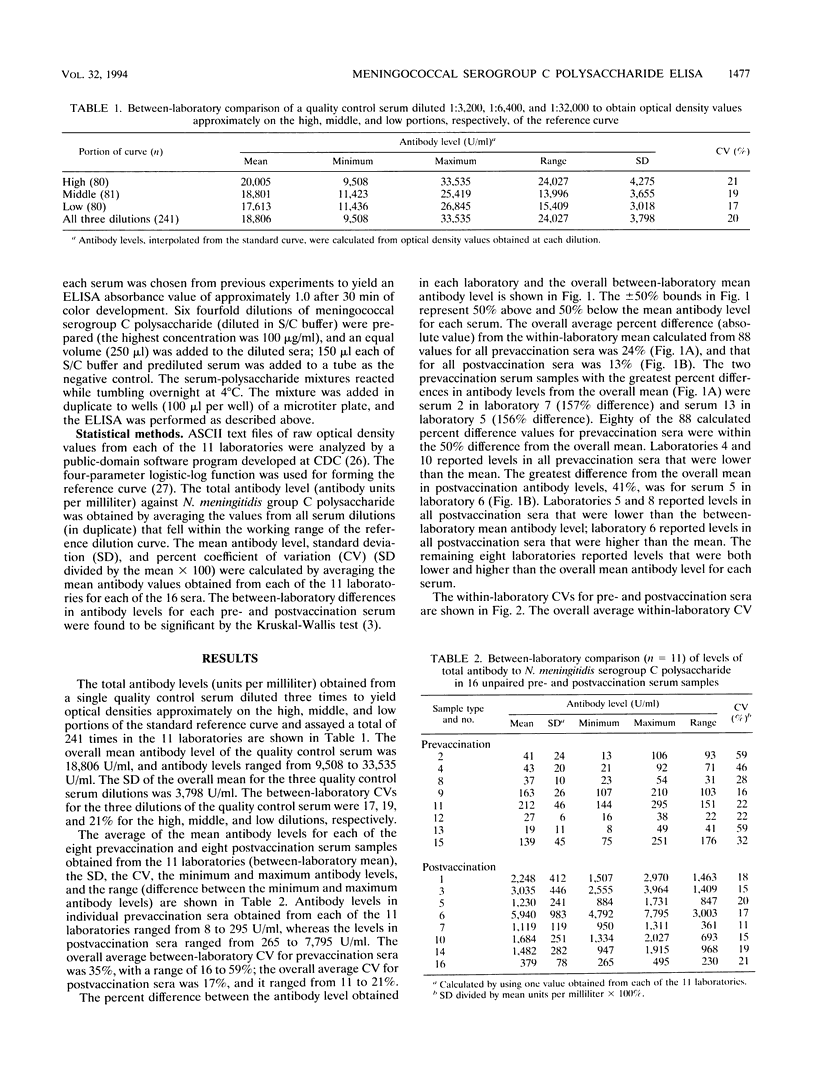
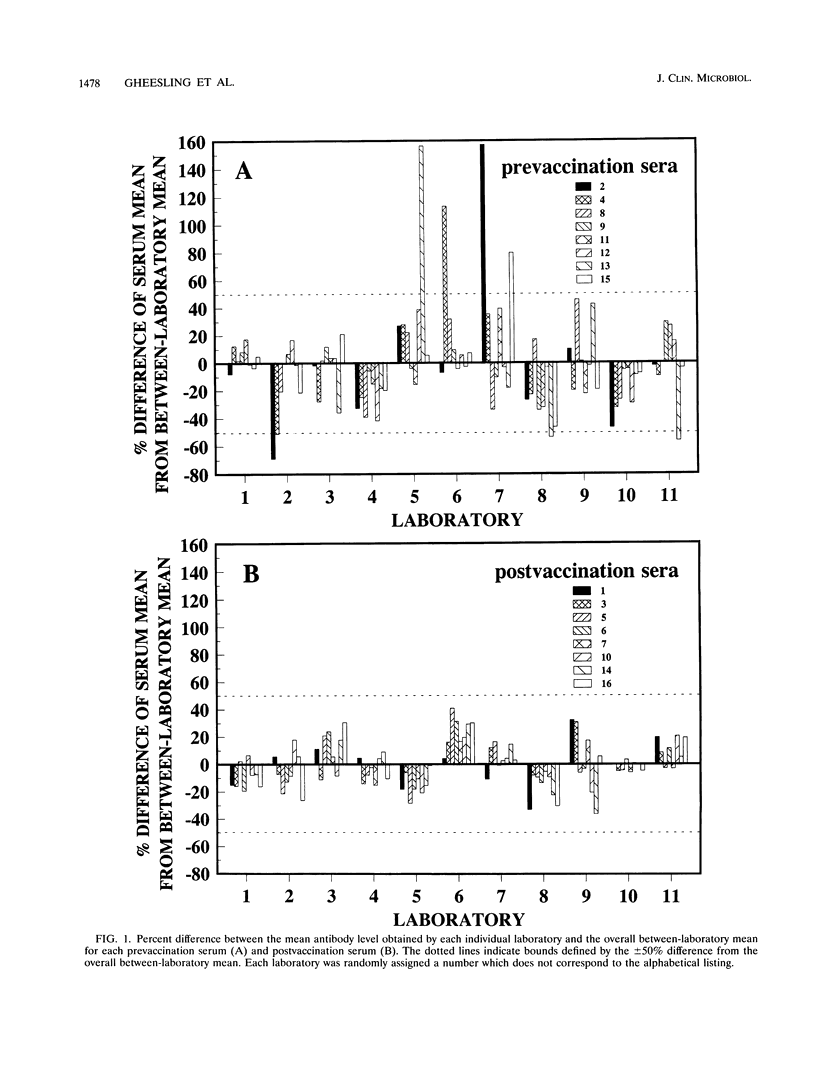
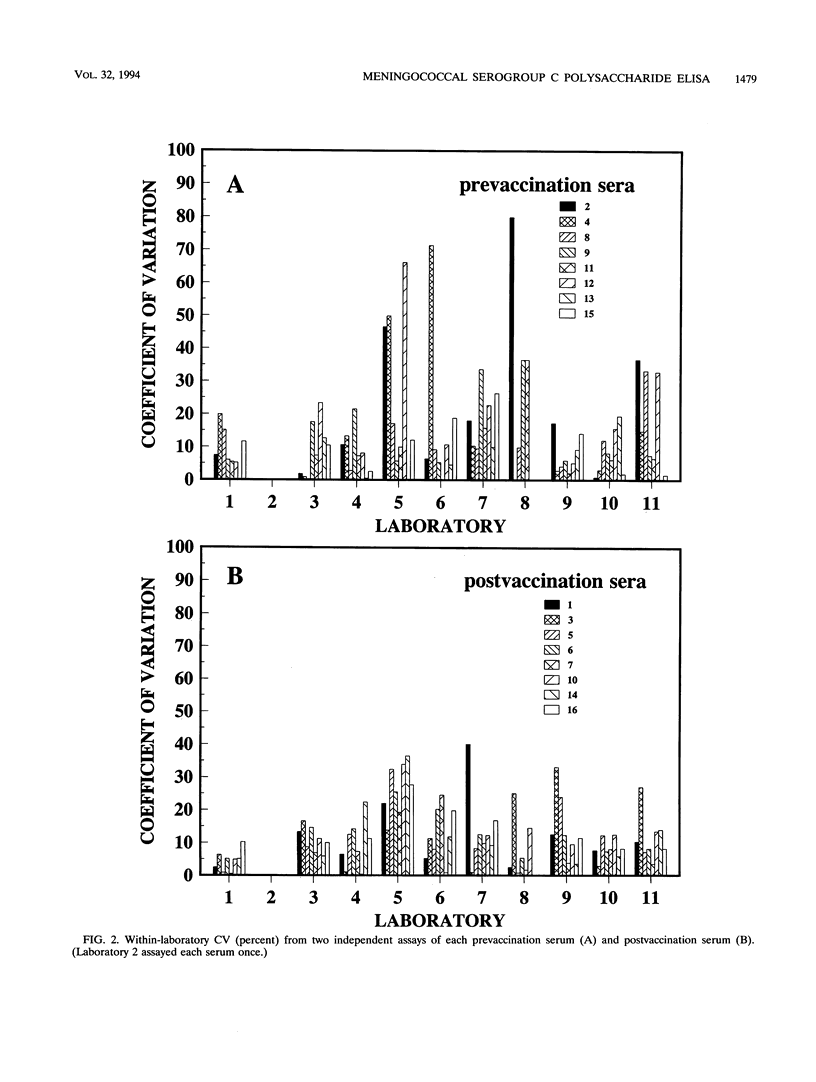
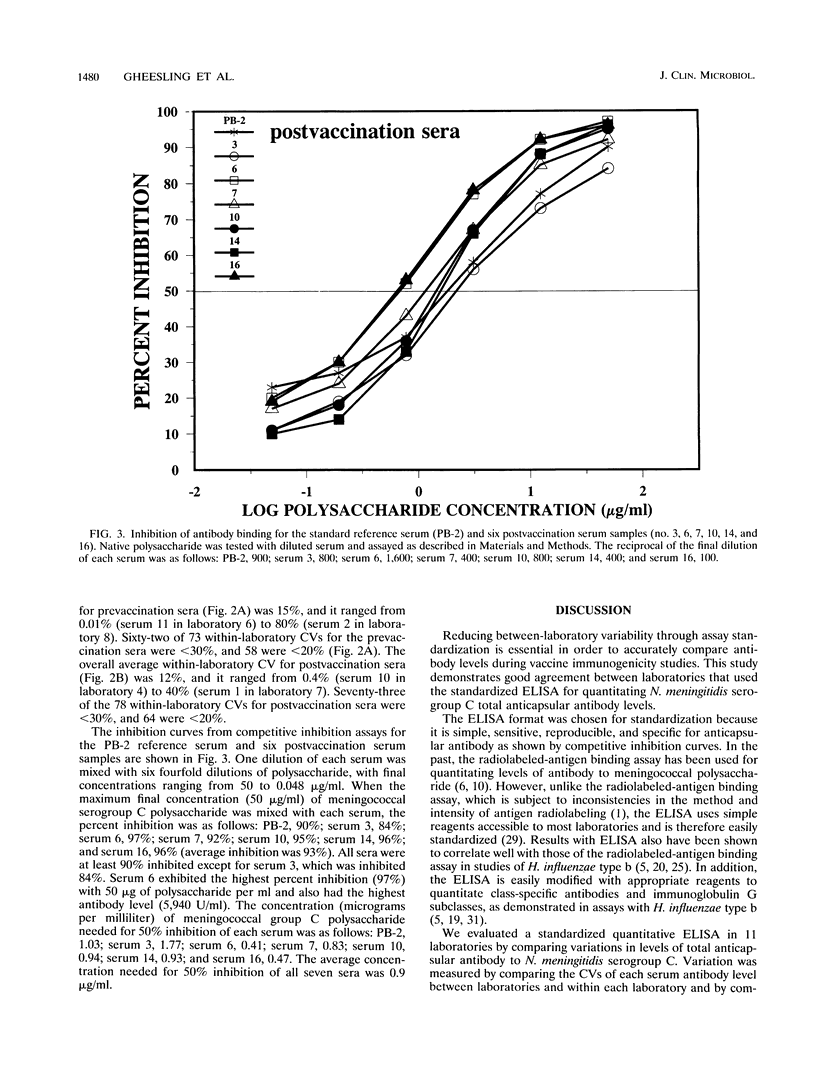
A standardized enzyme-linked immunosorbent assay (ELISA) was used by 11 laboratories to measure levels of total serum antibody to Neisseria meningitidis serogroup C capsular polysaccharide in 16 unpaired pre- and postvaccination serum samples. Twelve serum samples were from adults, and four were from children aged 2, 3, 5, and 9. The between-laboratory coefficient of variation for pre- and postvaccination sera ranged from 16 to 59% and 11 to 21%, respectively. The average percent difference (absolute value) from the between-laboratory means for all prevaccination sera measured by each laboratory was 24%, whereas the average percent difference was 13% for all postvaccination sera. A postvaccination quality control serum was diluted three times to give optical densities on the high, middle, and low portions of the standard reference curve. The three dilutions were assayed by the 11 laboratories a total of 241 times and yielded an overall coefficient of variation of 20%. Antibody-binding inhibition curves showed that the standardized ELISA was specific for N. meningitidis serogroup C capsular polysaccharide antibody. Fifty percent inhibition of seven serum samples was obtained after reaction with an average concentration of 0.9 micrograms of meningococcal serogroup C polysaccharide per ml; an average of 93% inhibition was obtained with 50 micrograms of polysaccharide per ml. The acceptance and use of this standardized ELISA will reduce between-laboratory assay variability and ensure a more accurate and reproducible assessment of immunogenicity for vaccines under development.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Insel R. A., Porcelli S., Ward J. I. Immunochemical variables affecting radioantigen-binding assays of antibody to Haemophilus influenzae type b capsular polysaccharide in childrens' sera. J Infect Dis. 1987 Oct;156(4):582–590. doi: 10.1093/infdis/156.4.583. [DOI] [PubMed] [Google Scholar]
- Arakere G., Frasch C. E. Specificity of antibodies to O-acetyl-positive and O-acetyl-negative group C meningococcal polysaccharides in sera from vaccinees and carriers. Infect Immun. 1991 Dec;59(12):4349–4356. doi: 10.1128/iai.59.12.4349-4356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artenstein M. S., Gold R., Zimmerly J. G., Wyle F. A., Schneider H., Harkins C. Prevention of meningococcal disease by group C polysaccharide vaccine. N Engl J Med. 1970 Feb 19;282(8):417–420. doi: 10.1056/NEJM197002192820803. [DOI] [PubMed] [Google Scholar]
- Barra A., Schulz D., Aucouturier P., Preud'homme J. L. Measurement of anti-Haemophilus influenzae type b capsular polysaccharide antibodies by ELISA. J Immunol Methods. 1988 Nov 25;115(1):111–117. doi: 10.1016/0022-1759(88)90317-1. [DOI] [PubMed] [Google Scholar]
- Brandt B. L., Wyle F. A., Artenstein M. S. A radioactive antigen-binding assay for Neisseria meningitidis polysaccharide antibody. J Immunol. 1972 Apr;108(4):913–920. [PubMed] [Google Scholar]
- Carlone G. M., Frasch C. E., Siber G. R., Quataert S., Gheesling L. L., Turner S. H., Plikaytis B. D., Helsel L. O., DeWitt W. E., Bibb W. F. Multicenter comparison of levels of antibody to the Neisseria meningitidis group A capsular polysaccharide measured by using an enzyme-linked immunosorbent assay. J Clin Microbiol. 1992 Jan;30(1):154–159. doi: 10.1128/jcm.30.1.154-159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino P., Viti S., Podda A., Velmonte M. A., Nencioni L., Rappuoli R. Development and phase 1 clinical testing of a conjugate vaccine against meningococcus A and C. Vaccine. 1992;10(10):691–698. doi: 10.1016/0264-410x(92)90091-w. [DOI] [PubMed] [Google Scholar]
- Evans J. R., Artenstein M. S., Hunter D. H. Prevalence of meningococcal serogroups and description of three new groups. Am J Epidemiol. 1968 May;87(3):643–646. doi: 10.1093/oxfordjournals.aje.a120854. [DOI] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Frasch C. E., Zahradnik J. M., Wang L. Y., Mocca L. F., Tsai C. M. Antibody response of adults to an aluminum hydroxide-adsorbed Neisseria meningitidis serotype 2b protein-group B polysaccharide vaccine. J Infect Dis. 1988 Oct;158(4):710–718. doi: 10.1093/infdis/158.4.710. [DOI] [PubMed] [Google Scholar]
- Gold R., Lepow M. L., Goldschneider I., Draper T. F., Gotshlich E. C. Kinetics of antibody production to group A and group C meningococcal polysaccharide vaccines administered during the first six years of life: prospects for routine immunization of infants and children. J Infect Dis. 1979 Nov;140(5):690–697. doi: 10.1093/infdis/140.5.690. [DOI] [PubMed] [Google Scholar]
- Gold R., Lepow M. L., Goldschneider I., Draper T. L., Gotschlich E. C. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J Clin Invest. 1975 Dec;56(6):1536–1547. doi: 10.1172/JCI108235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969 Jun 1;129(6):1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg D. P., Ward J. I., Burkart K., Christenson P. D., Guravitz L., Marcy S. M. Factors influencing immunogenicity and safety of two Haemophilus influenzae type b polysaccharide vaccines in children 18 and 24 months of age. Pediatr Infect Dis J. 1987 Jul;6(7):660–665. doi: 10.1097/00006454-198707000-00008. [DOI] [PubMed] [Google Scholar]
- Herrmann D. J., Hamilton R. G., Barington T., Frasch C. E., Arakere G., Mäkelä O., Mitchell L. A., Nagel J., Rijkers G. T., Zegers B. Quantitation of human IgG subclass antibodies to Haemophilus influenzae type b capsular polysaccharide. Results of an international collaborative study using enzyme immunoassay methodology. J Immunol Methods. 1992 Apr 8;148(1-2):101–114. doi: 10.1016/0022-1759(92)90163-n. [DOI] [PubMed] [Google Scholar]
- Jeffcoate S. L., Das R. E. Interlaboratory comparison of radioimmunoassay results. Variation produced by different methods of calculation. Ann Clin Biochem. 1977 Sep;14(5):258–260. doi: 10.1177/000456327701400170. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J Immunol. 1981 Sep;127(3):1011–1018. [PubMed] [Google Scholar]
- Käyhty H., Mäkelä O., Eskola J., Saarinen L., Seppälä I. Isotype distribution and bactericidal activity of antibodies after immunization with Haemophilus influenzae type b vaccines at 18-24 months of age. J Infect Dis. 1988 Nov;158(5):973–982. doi: 10.1093/infdis/158.5.973. [DOI] [PubMed] [Google Scholar]
- Lagergård T., Trollfors B., Claesson B. A., Schneerson R., Robbins J. B. Comparison between radioimmunoassay and direct and indirect enzyme-linked immunosorbent assays for determination of antibodies against Haemophilus influenzae type b capsular polysaccharide. J Clin Microbiol. 1988 Dec;26(12):2554–2557. doi: 10.1128/jcm.26.12.2554-2557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepow M. L., Goldschneider I., Gold R., Randolph M., Gotschlich E. C. Persistence of antibody following immunization of children with groups A and C meningococcal polysaccharide vaccines. Pediatrics. 1977 Nov;60(5):673–680. [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- Peltola H. Meningococcal disease: still with us. Rev Infect Dis. 1983 Jan-Feb;5(1):71–91. doi: 10.1093/clinids/5.1.71. [DOI] [PubMed] [Google Scholar]
- Peterman J. H., Butler J. E. Application of theoretical considerations to the analysis of ELISA data. Biotechniques. 1989 Jun;7(6):608–615. [PubMed] [Google Scholar]
- Phipps D. C., West J., Eby R., Koster M., Madore D. V., Quataert S. A. An ELISA employing a Haemophilus influenzae type b oligosaccharide-human serum albumin conjugate correlates with the radioantigen binding assay. J Immunol Methods. 1990 Dec 31;135(1-2):121–128. doi: 10.1016/0022-1759(90)90264-v. [DOI] [PubMed] [Google Scholar]
- Plikaytis B. D., Turner S. H., Gheesling L. L., Carlone G. M. Comparisons of standard curve-fitting methods to quantitate Neisseria meningitidis group A polysaccharide antibody levels by enzyme-linked immunosorbent assay. J Clin Microbiol. 1991 Jul;29(7):1439–1446. doi: 10.1128/jcm.29.7.1439-1446.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruslin F. H., To S. E., Winston R., Rodman T. C. Caveats and suggestions for the ELISA. J Immunol Methods. 1991 Mar 1;137(1):27–35. doi: 10.1016/0022-1759(91)90390-2. [DOI] [PubMed] [Google Scholar]
- Siber G. R., Priehs C., Madore D. V. Standardization of antibody assays for measuring the response to pneumococcal infection and immunization. Pediatr Infect Dis J. 1989 Jan;8(1 Suppl):S84–S91. [PubMed] [Google Scholar]
- Steward M. W., Lew A. M. The importance of antibody affinity in the performance of immunoassays for antibody. J Immunol Methods. 1985 Apr 22;78(2):173–190. doi: 10.1016/0022-1759(85)90074-2. [DOI] [PubMed] [Google Scholar]
- Trollfors B., Lagergård T., Claesson B. A., Thornberg E., Martinell J., Schneerson R. Characterization of the serum antibody response to the capsular polysaccharide of Haemophilus influenzae type b in children with invasive infections. J Infect Dis. 1992 Dec;166(6):1335–1339. doi: 10.1093/infdis/166.6.1335. [DOI] [PubMed] [Google Scholar]
- Ward J. I., Greenberg D. P., Anderson P. W., Burkart K. S., Christenson P. D., Gordon L. K., Kayhty H., Kuo J. S., Vella P. Variable quantitation of Haemophilus influenzae type b anticapsular antibody by radioantigen binding assay. J Clin Microbiol. 1988 Jan;26(1):72–78. doi: 10.1128/jcm.26.1.72-78.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



