Abstract
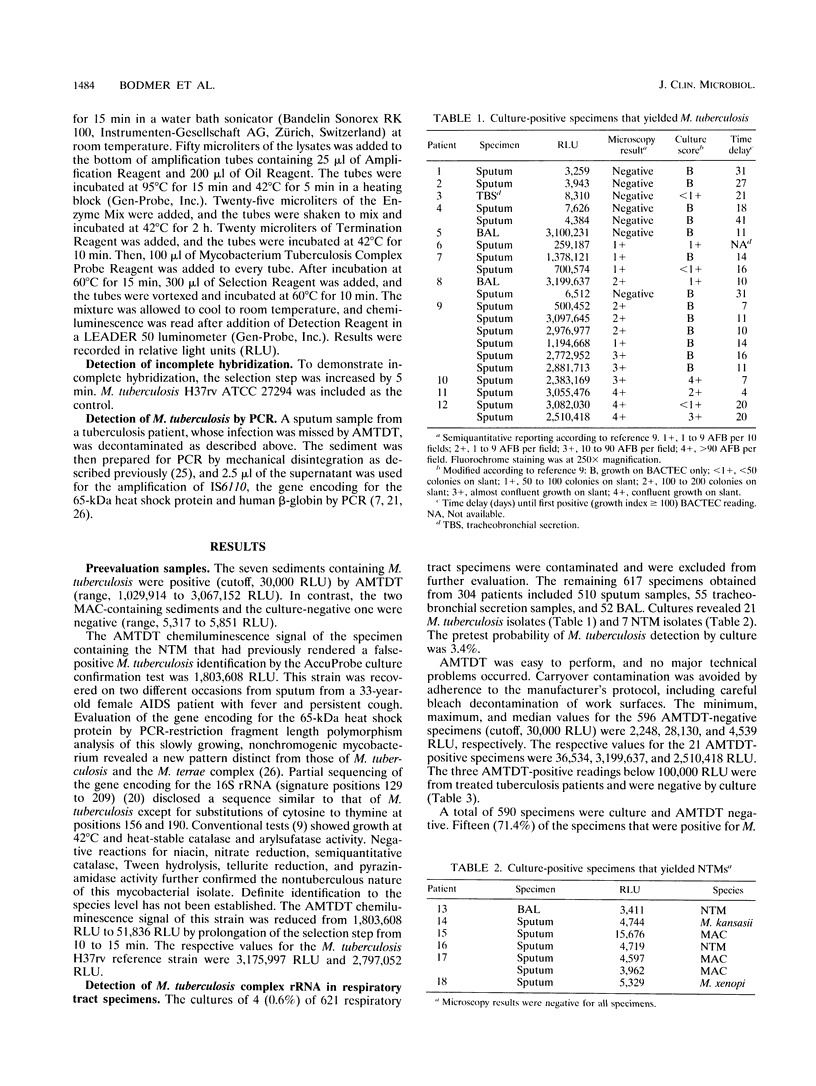
A prospective 2-month trial involving 617 respiratory tract specimens was conducted to compare sensitivity, specificity, and predictive values of the newly developed Gen-Probe Amplified Mycobacterium Tuberculosis Direct Test kit (AMTDT; Gen-Probe, Inc., San Diego, Calif.) for the rapid detection of Mycobacterium tuberculosis and of fluorescent acid-fast staining versus combined BACTEC 12B and solid-medium cultures as the "gold standard." A total of 590 specimens were culture and AMTDT negative. Twenty-one (3.4%) cultures yielded M. tuberculosis. Of these, 15 (71.4%) were detected by AMTDT, whereas 6 (28.6%) were missed. M. tuberculosis did not grow in six (28.6%) of AMTDT-positive specimens derived from three patients under treatment for tuberculosis. AMTDT exhibited a sensitivity, a specificity, a negative predictive value, and a positive predictive value of 71.4, 99, 99, and 71.4%, respectively. In comparison, the same values for fluorescent microscopy were 66.7, 98.3, 98.8, and 58.3%, respectively. AMTDT was easy to perform and highly specific. However, a screening test would require an improved sensitivity and, when feasible, the implementation of an internal amplification control.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anargyros P., Astill D. S., Lim I. S. Comparison of improved BACTEC and Lowenstein-Jensen media for culture of mycobacteria from clinical specimens. J Clin Microbiol. 1990 Jun;28(6):1288–1291. doi: 10.1128/jcm.28.6.1288-1291.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarridge J. E., 3rd, Shawar R. M., Shinnick T. M., Plikaytis B. B. Large-scale use of polymerase chain reaction for detection of Mycobacterium tuberculosis in a routine mycobacteriology laboratory. J Clin Microbiol. 1993 Aug;31(8):2049–2056. doi: 10.1128/jcm.31.8.2049-2056.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker E. Molecular epidemiology meets the fourth world. Lancet. 1993 Oct 2;342(8875):817–818. doi: 10.1016/0140-6736(93)92689-q. [DOI] [PubMed] [Google Scholar]
- Genewein A., Telenti A., Bernasconi C., Mordasini C., Weiss S., Maurer A. M., Rieder H. L., Schopfer K., Bodmer T. Molecular approach to identifying route of transmission of tuberculosis in the community. Lancet. 1993 Oct 2;342(8875):841–844. doi: 10.1016/0140-6736(93)92698-s. [DOI] [PubMed] [Google Scholar]
- Goto M., Oka S., Okuzumi K., Kimura S., Shimada K. Evaluation of acridinium-ester-labeled DNA probes for identification of Mycobacterium tuberculosis and Mycobacterium avium-Mycobacterium intracellulare complex in culture. J Clin Microbiol. 1991 Nov;29(11):2473–2476. doi: 10.1128/jcm.29.11.2473-2476.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans P. W., van Soolingen D., Dale J. W., Schuitema A. R., McAdam R. A., Catty D., van Embden J. D. Insertion element IS986 from Mycobacterium tuberculosis: a useful tool for diagnosis and epidemiology of tuberculosis. J Clin Microbiol. 1990 Sep;28(9):2051–2058. doi: 10.1128/jcm.28.9.2051-2058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas V., Alden M. J., Curry J. I., Kamisango K., Knott C. A., Lankford R., Wolfe J. M., Moore D. F. Detection and identification of Mycobacterium tuberculosis directly from sputum sediments by amplification of rRNA. J Clin Microbiol. 1993 Sep;31(9):2410–2416. doi: 10.1128/jcm.31.9.2410-2416.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner P., Springer B., Vogel U., Meier A., Wrede A., Kiekenbeck M., Bange F. C., Böttger E. C. Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J Clin Microbiol. 1993 Nov;31(11):2882–2889. doi: 10.1128/jcm.31.11.2882-2889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun L., Espinasse F., Poveda J. D., Vincent-Levy-Frebault V. Evaluation of nonradioactive DNA probes for identification of mycobacteria. J Clin Microbiol. 1992 Sep;30(9):2476–2478. doi: 10.1128/jcm.30.9.2476-2478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. D., Todd J., Lopez J., Ford E., Janda J. M. Genotypic identification of pathogenic Mycobacterium species by using a nonradioactive oligonucleotide probe. J Clin Microbiol. 1991 Jun;29(6):1276–1278. doi: 10.1128/jcm.29.6.1276-1278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Lévy-Frébault V. V., Cattier B., Legras A., Goudeau A. False positive result of Mycobacterium tuberculosis complex DNA probe hybridization with a Mycobacterium terrae isolate. Eur J Clin Microbiol Infect Dis. 1993 Apr;12(4):309–310. doi: 10.1007/BF01967270. [DOI] [PubMed] [Google Scholar]
- Middlebrook G., Reggiardo Z., Tigertt W. D. Automatable radiometric detection of growth of Mycobacterium tuberculosis in selective media. Am Rev Respir Dis. 1977 Jun;115(6):1066–1069. doi: 10.1164/arrd.1977.115.6.1066. [DOI] [PubMed] [Google Scholar]
- Noordhoek G. T., van Embden J. D., Kolk A. H. Questionable reliability of the polymerase chain reaction in the detection of Mycobacterium tuberculosis. N Engl J Med. 1993 Dec 30;329(27):2036–2036. doi: 10.1056/NEJM199312303292713. [DOI] [PubMed] [Google Scholar]
- Rogall T., Wolters J., Flohr T., Böttger E. C. Towards a phylogeny and definition of species at the molecular level within the genus Mycobacterium. Int J Syst Bacteriol. 1990 Oct;40(4):323–330. doi: 10.1099/00207713-40-4-323. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Spence D. P., Hotchkiss J., Williams C. S., Davies P. D. Tuberculosis and poverty. BMJ. 1993 Sep 25;307(6907):759–761. doi: 10.1136/bmj.307.6907.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenti A., Imboden P., Marchesi F., Lowrie D., Cole S., Colston M. J., Matter L., Schopfer K., Bodmer T. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993 Mar 13;341(8846):647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- Telenti A., Imboden P., Marchesi F., Schmidheini T., Bodmer T. Direct, automated detection of rifampin-resistant Mycobacterium tuberculosis by polymerase chain reaction and single-strand conformation polymorphism analysis. Antimicrob Agents Chemother. 1993 Oct;37(10):2054–2058. doi: 10.1128/aac.37.10.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenti A., Marchesi F., Balz M., Bally F., Böttger E. C., Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993 Feb;31(2):175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Crawford J. T., Huebner R. E., Geiter L. J., Horsburgh C. R., Jr, Good R. C. The resurgence of tuberculosis: is your laboratory ready? J Clin Microbiol. 1993 Apr;31(4):767–770. doi: 10.1128/jcm.31.4.767-770.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannier A. M., Tarrand J. J., Murray P. R. Mycobacterial cross contamination during radiometric culturing. J Clin Microbiol. 1988 Sep;26(9):1867–1868. doi: 10.1128/jcm.26.9.1867-1868.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


