Abstract
Clinical outcomes of nerve grafting are often inferior to those of end-to-end nerve repair. This may be due, in part, to the routine use of cutaneous nerve to support motor axon regeneration. In previous work, we have demonstrated that Schwann cells express distinct sensory and motor phenotypes, and that these promote regeneration in a modality-specific fashion. Intra-operative modification of graft Schwann cell phenotype might therefore improve clinical outcomes. This paper demonstrates the feasibility of electroporating genes into intact nerve to modify Schwann cell gene expression. Initial trials established 70 V, 5 ms as optimum electroporation parameters. Intact, denervated, and reinnervated rat tibial nerves were electroporated with the YFP gene and evaluated serially by counting S-100 positive cells that expressed YFP. In intact nerve, a mean of 28% of Schwann cells expressed the gene at 3 days, falling to 20% at 7 days with little expression at later times. There were no significant differences among the three groups at each time period. Electronmicroscopic evaluation of treated, intact nerve revealed only occasional demyelination and axon degeneration. Intraoperative electroporation of nerve graft is thus a practical means of altering Schwann cell gene expression without the risks inherent in viral transfection.
INTRODUCTION
Peripheral nerve injuries compromise the function of over 40,000 Americans each year (Kreiger et al., 1981). While sharp lacerations with narrow zones of injury can be treated with end-to-end nerve repair, more extensive injuries often require the use of nerve grafts to replace damaged neural tissue. Nerve grafts perform two primary functions: they guide regenerating axons along longitudinally-oriented Schwann cell tubes, and they produce trophic factors needed to support the regeneration process. Grafts are usually fashioned from cutaneous nerves, as they are located superficially and their loss is well-tolerated. In spite of recent advances in microsurgical technique, however, current grafting techniques lag behind end-to-end repair in their ability to restore function (Brushart, 2008). Strategies to improve the guidance or trophic function of nerve grafts could thus benefit thousands each year.
A variety of growth factors are produced by Schwann cells, the glia of the peripheral nervous system. These factors play a crucial role in promoting regeneration through both intact and grafted nerve (reviewed in Boyd and Gordon, 2003). Until recently, it was assumed that mature Schwann cells expressed one of only two possible phenotypes: myelinating, or non-myelinating (Jessen and Mirsky, 2005). This view has been challenged by recent experiments in which the Schwann cells of cutaneous nerve and ventral root were found to differ substantially in their growth factor expression (Hoke et al., 2006). Furthermore, the ability of nerve graft to promote the regeneration of sensory or motor axons was found to be modality-specific; sensory axon regeneration was best supported by grafts of cutaneous nerve, and motor axon regeneration by grafts of ventral root. These findings may explain the compromise of function when cutaneous nerve grafts are used clinically to reconstruct damaged motor nerve. Manipulation of graft Schwann cell phenotype to provide optimal trophic support for a target axon population could thus improve clinical outcomes when grafting is necessary.
Schwann cell gene expression has been modified experimentally both in vitro and in vivo. Though both electroporation and viral transfection have been used to modify Schwann cells in vitro, the process for grafting with cultured, autologous Schwann cells requires multiple steps and at least two operative procedures (Haastert et al., 2007b). These experiments demonstrate, however, that Schwann cells can express growth factors after electroporation of their respective genes. In vivo manipulation of Schwann cell gene expression has so far been limited to viral transfection (reviewed in Federici and Boulis, 2007), a technique that confers the risk of malignant transformation and death (Hacein-Bey-Abina et al., 2003, Raper et al., 2003). Electroporation of intact graft thus offers a safe, rapid alternative for the intra-operative manipulation of Schwann cell phenotype.
MATERIALS AND METHODS
DNA preparation
Plasmid YFP DNA (pCS2-Venus) was prepared with the QIAGEN Plasmid Maxi Kit (QIAGEN, Valencia, CA) according to standard procedures (Nagai et al., 2002, Willard et al., 2007). The DNA was solubilized in PBS and Fast Green (Sigma, FW 808.86) at a final concentration of 0.05% Fast Green and 2μg/μl plasmid DNA.
Electroporation in vivo
Experiments were performed on female Sprague Dawley rats (9–12 weeks, 200–250 gms, Charles River, Wilmington, MA). Rats were anesthetized by intraperitoneal injection of ketamine (87 mg/kg) and xylazine (13 mg/kg). All procedures were performed under aseptic conditions and were approved by the Johns Hopkins Animal Care and Use Committee. Animals were housed in an ALAC-approved facility and given a rodent diet and water ad libitum.
To determine the optimal conditions for in vivo electroporation we applied 12 different combinations of voltage and pulse length to 46 tibial nerve grafts in 23 animals. The tibial nerve was exposed in the mid-thigh by splitting the overlying muscles. The perineurium was punctured with an 8-0 surgical needle and 1.5μl of plasmid DNA was injected into the intact tibial nerve with a glass micropipette (World Precision Instruments, Inc., Sarasota, FL) using a picospritzer (Picospritzer II, Parker Instrumentation, Cleveland, OH). The pipette was first advanced proximally and then distally to distribute the DNA evenly within a 1.2 cm length of nerve (Figure 1). Following injection, the puncture site was marked with a 10-0 nylon suture to define the center of the electroporated zone. The nerve was then cut 8 mm distal to the injection site so that it could be placed between the moistened electrode plates (20% sucrose in 0.1 M Sorensen’s Buffer solution) (Figure 1). No leakage of Fast Green was noted, confirming the localization of the plasmid to the treated segment. An electric pulse generator (BTX Electro Square Porator ECM 830) was used to deliver eight unipolar electrical pulses, each separated by a 100 msec interval, through forceps electrodes with disc-shaped plates (BTX Tweezertrodes 522, Harvard Apparatus, Holliston, MA). Voltages of 40, 50, 60, 70, 80, or 100 were delivered for 2 or 5ms. The nerve was then irrigated with 0.9% saline bi-biotic solution (Neomycin 40mg, Polymyxin B 2HTU in 1000ml 0.9% saline), transected 8 mm proximal to the injection site, and immediately repaired with 10-0 nylon sutures (Figure 1- graft). The distal end of the graft was sewn to the adductor brevis muscle to prevent graft contraction. Overlying muscles and skin were reapproximated, and the animals were allowed to recover. Control nerves received DNA without electroporation (n=2), or were electroporated (70V/5ms) after injection of 0.9% saline + 0.05% Fast Green only (n=2).
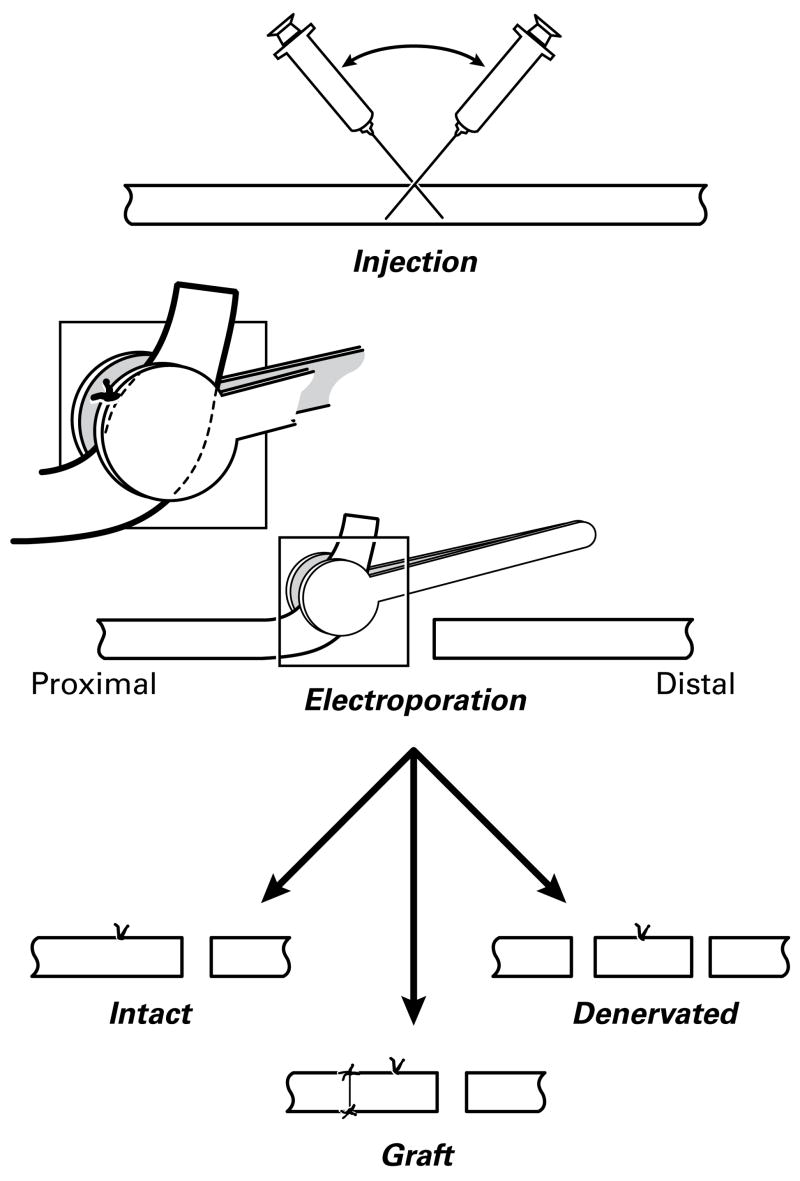
Figure 1.
Electroporation of rat tibial nerve. Proximal is to the left. After plasmid injection, the site of penetration is marked with a suture that is used as a landmark to center the electroporation paddles. The nerve is transected 8 mm distal to the suture and reflected to facilitate this process. It is then replaced in the wound without further manipulation (Intact group), or transected again 8mm proximal to the suture. This detached segment of nerve is then sutured back to the proximal stump so that it can be reinnervated (Graft group) or left detached (Denervated group). The Graft preparation was used both for the preliminary calibration studies as part of the long-term expression experiments.
Long-term gene expression was evaluated in intact nerve (IN), denervated nerve (DN), and grafted nerve (GN) (Figure 1). Eight rats (16 nerves) were prepared in each group. In the intact nerve group, only the distal cut was made to allow placement of the nerve between the electrodes; the proximal nerve was left intact. In the denervated nerve group, the proximal nerve stump was ligated to prevent regeneration and both proximal and distal ends of the graft were sewn to muscle to prevent graft contraction. Graft preparations were prepared as described above. Exposure of the nerve, plasmid injection, and electroporation were performed in long-term groups as described for the optimization experiments above, using 70V/5 msec as the electroporation parameters. Additional intact and graft preparations were harvested 3, 7, 14, or 21 days after electroporation for electronmicroscopic evaluation of graft ultrastructure.
Histological preparation
Tibial nerves were harvested 3 days after electroporation in the optimization experiments, and 3, 7, 14, or 21 days after electroporation in the long-term expression experiments. They were placed in 4% paraformaldehyde at 4 degrees Celsius for 24 hrs, then cryoprotected in 20% sucrose in 0.1 M Sorensen’s Buffer (1 part 0.1 M sodium phosphate monobasic, 4 parts 0.1 M sodium phosphate dibasic) overnight. A 1cm piece of the nerve, centered on the puncture site, was embedded in OCT (Electron Microscopy Sciences, Hatfield, PA) and cut longitudinally at a thickness of 10μm. Section were mounted on glass slides and immunostained to demonstrate basal lamina (laminin) or Schwann cells (S-100). Sections were washed with phosphate-buffered saline (PBS) and permeabilized with PBS containing 50% methanol, 1% Triton X-100 for 1h at ambient temperature. Nonspecific antibody binding was blocked with 3% bovine serum albumin in PBS containing 0.25% Triton X-100 and 10 mM glycine for 3h. Following blocking, the sections were incubated with rabbit polyclonal antibodies against laminin (Sigma) or S-100 (Dako cytometer) diluted 250 fold in PBS containing 1% bovine serum albumin, 0.08% Triton X-100 and 10 mM glycine for 18h at 4oC. The binding was visualized with Alexa 568 conjugated goat anti-rabbit IgG (Invitrogen). The slides were overlain with coverslips using Vectastain (Vector Labs).
Electron Microscopy
Nerves for electron microscopy were fixed in 4% glutaraldehyde in 0.1 M Sorenson’s phosphate buffer, rinsed, impregnated with osmium, and embedded in Epon-Araldite. One-micron thick sections were stained with 1% toluidine blue. Thin sections (60—70 nm) were stained with uranyl acetate (2.5% in 50% ethanol) and lead citrate (3%), and photographed at 3000–10,000 magnifications with an Hitachi H-600 electron microscope. Negatives were scanned at 600 dpi and contrast and brightness levels were adjusted in photoshop.
Evaluation of tissue sections to determine transfection rate
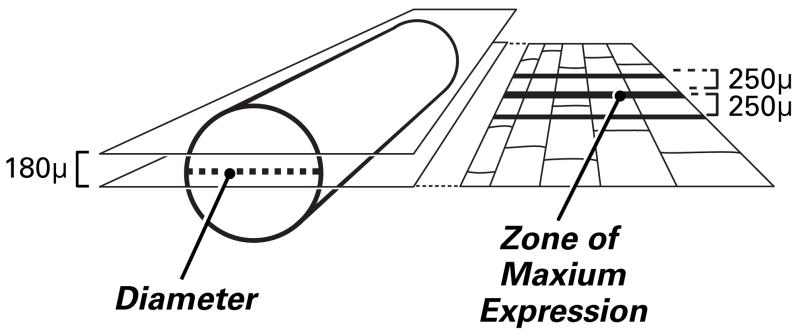
Two full-width longitudinal sections of each nerve were evaluated (Figure 2). These lay 90 μm on either side of a plane that defined the diameter of the nerve. Each 10 μm nerve section was viewed with fluorescent light (yellow GFP: excitation: 490nm, emission: 520 and G-2E/C excitation: 525, emission: 600) by an observer unaware of specimen identity. The percentage of transfected cells was calculated as follows: the area of maximal YFP expression was identified and bisected by a line drawn perpendicular to a long axis of the nerve. Counts were made of labeled and unlabeled SC tubes that intersected this line (Figure 3), and that intersected parallel lines drawn 250 μm proximally and distally. The following formula was used to quantify transfection: (Σ all YFP labeled tubes)/(Σ all Laminin labeled tubes) ×100). Statistical analysis was performed using two tailed students t-test. All data are presented as means and standard error of the mean.
Figure 2.
Determining transfection efficiency. In each nerve specimen, transfected Schwann cells were counted in two parallel, longitudinal sections that lay 90u on either side of the nerve diameter. Counts were made of both transfected and non-transfected Schwann cells that were intersected by a line through the zone of maximum expression, the area with the largest number of labeled Schwann cells (usually at the sight of injection), and by two lines placed 250u proximal and distal to this line.
Figure 3.

The green channel (left), red channel (center) and merged image (right) of a fluorescent photograph of intact nerve 3 days after electroporation. Ten micrometer section, original magnification 40x. YFP expression overlaps the distribution of S-100 positive (red) Schwann cells. The center image is marked to indicate the technique used to count Schwann cell tubes.
RESULTS
Optimization experiments
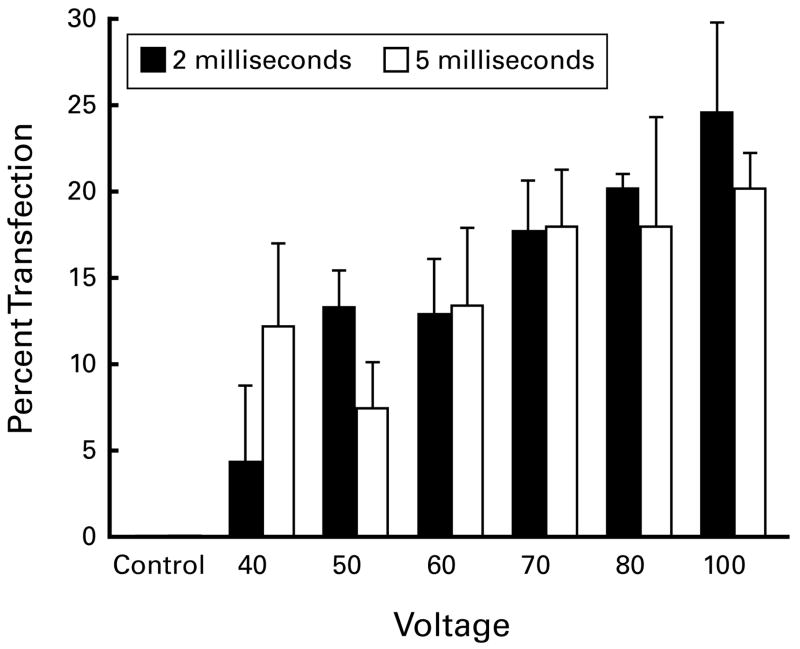
Twelve groups of 2–5 nerves were prepared at various combinations of stimulus voltage and duration to optimize conditions for the later experiments. There was no expression when DNA was injected but not electroporated (Figure 4). Three days after electroporation, mean transfection rates ranged from 4.4% (40V/2ms) to 24.7% (100V/2ms). Within each group, rates of transfection varied considerably, for instance from 5.6% to 21.6% (mean 12.3, SEM 4.8) using 40V/5ms, or from 5.6% to 26.4% (mean 13.5, SEM 4.5) using 60V/5ms. Comparisons among the groups showed significant differences between 50V/2ms and 70V/2ms (p=0.04), 50V/2ms and 70V/5ms (p=0.04), 50V/2ms and 80V/2ms (p=0.04), and 50V/2ms and 100V/5ms (p=0.03). Small numbers of animals were used in this experiment, as the goal was a broad appreciation of electroporation parameters. Further differences among groups would doubtless be found if more animals were used. Several of the specimens subjected to 80V and 100V were visibly damaged by the procedure. We thus chose 70V/5 msec as the most suitable combination for experiments on long-term gene expression.
Figure 4.
Optimization Experiments. Voltage and pulse length were varied to determine the parameters for long-term expression experiments. Twelve groups of 2–5 nerves were prepared. Although short-term expression increased with increasing voltage, some nerves were visibly damaged at 80 and 100 volts. Seventy volts, 5 ms. were thus chosen as optimal parameters.
Long-term gene expression experiments
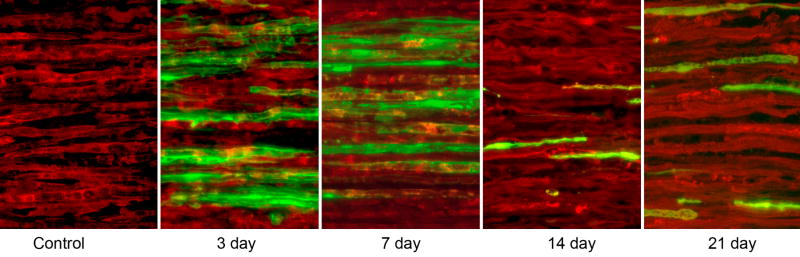
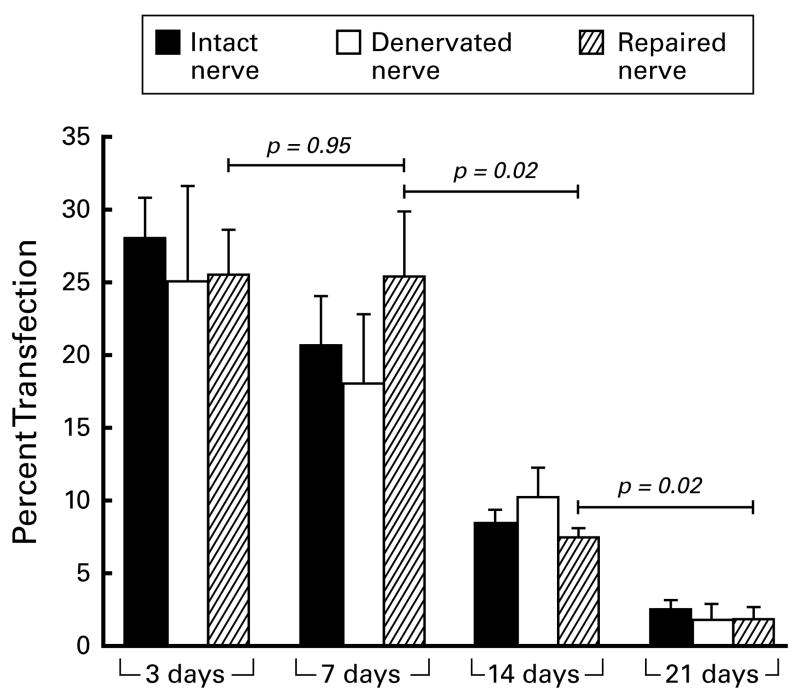
Long-term gene expression experiments (Figures 5,6) revealed no significant differences among the three groups (intact nerve, IN; denervated nerve, DN; or grafted nerve, GN) at any time period. Transfection rates where highest 3 days after electroporation (28%, IN; 25%, DN; and 26%, GN), and were similar at 7 days. By 14 days, transfection rates in intact nerve (p=0.021) and grafted nerve (p=0.020) had diminished significantly from their values at 7 days. YFP expression was further reduced at 21 days, and was significantly less than that at 14 days for all groups (IN: p=0.03; DN: p=0.01; GN: p=0.02).
Figure 5.
The time course of gene expression. Ten micrometer sections viewed at 20×; Schwann cells are labeled red with antibodies to S-100, and cells expressing the electroporated gene for YFG are labeled green. The co-localization of these markers indicates that Schwann cells are responsible for the observed YFP expression. No YFP expression was noted within S-100 –negative cells within the endoneurium.
Figure 6.
The percentage of Schwann cells transfected as a function of time. Four nerves were prepared per group. There was little difference among intact, denervated, and grafted nerve at each time period. Expression was similar at 3 and 7 days, then decreased significantly at later times.
Immunofluorescence staining
Immunoflourescence staining revealed that YFP co-localized with S-100 positive cells that were longitudinally-oriented within basal lamina tubes and had the characteristic morphology of Schwann cells (Figure 3,5).
Nerve ultrastructure
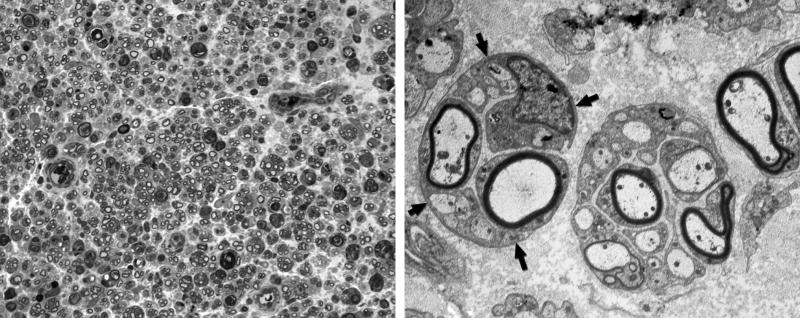
Nerve ultrastructure was evaluated by light and electronmicroscopy to assess potential damage from the trauma of electroporation. Three days after electroporation of intact nerve, there was moderate endoneurial edema, but no significant ultrastructural changes were seen (Figure 7). By 7 days, there was increased edema and there appeared to be dropout of some fibers (Figure 8). Many Schwann cells and their myelinated axons were ultrastructurally normal, but there was evidence of segmental demyelination and sprout formation by occasional axons that were still myelinated. In two specimens of intact nerve 14 days after electroporation there was axon loss around the margin of the nerve, with preservation of normal structure centrally (Figure 9), while other nerves appeared structurally normal. The appearance of Wallerian degeneration in denervated and grafted nerve was indistinguishable from that in control nerve at early time periods. By 21days, robust regeneration with formation of multiple regenerating units was seen in grafted nerve (Figure 10), indicative of a healthy regeneration response.
Figure 7.
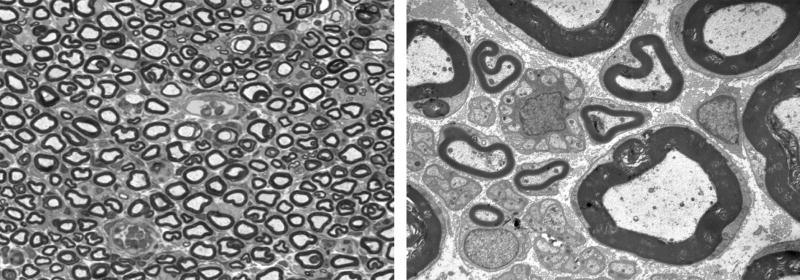
Intact nerve, 3 days after electroporation. Left: One micrometer plastic section, 100×. No significant fiber loss is noted, and myelinated axons are closely packed. Right: Electronmicrograph, 4,000×. Myelinated and unmyelinated fibers are intact and there is sparse endoneurial space between axons.
Figure 8.
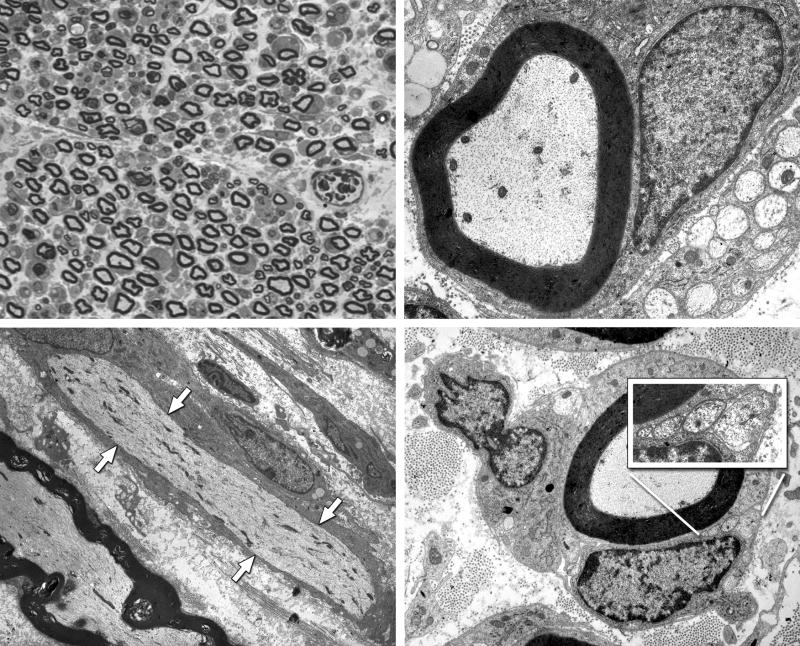
Intact nerve, 7 days after electroporation. Upper Left: One micrometer plastic section, 100×. Endoneurial edema results in increased space between axons, and some myelinated profiles are lost. Upper Right: Electronmicrograph, 10,000×. Many myelinated axons and their accompanying Schwann cells are morphologically normal. Lower Left: Electronmicrograph of longitudinal section, 4,000×. The axon that runs from upper left to lower right, indicated by white arrows, is intact, but has undergone segmental demyelination. The axon at the lower left has maintained its myelin sheath. Lower Right: Many axons that remain myelinated are accompanied by unmyelinated sprouts within the same Schwann cell basal lamina (inset).
Figure 9.
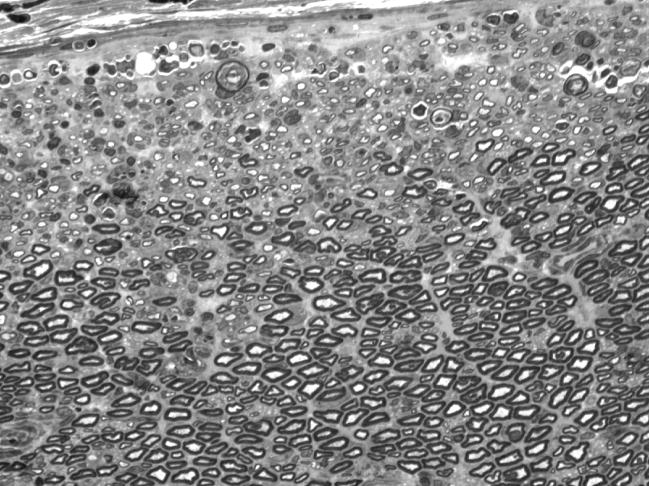
Intact nerve, 14 days after electroporation. One micrometer plastic section, 100×, taken from the nerve that was damaged most severely by electroporation. Axonal loss and/or demyelination is prominent near the perineurium (upper edge), but more central portions of the nerve appear normal. Endoneurial edema has resolved as evidenced by the tight packing of myelinated axons.
Figure 10.
Grafted nerve, 21 days after electroporation. Left: One micrometer plastic section, 100×. The nerve is populated with regenerating units, groups of regenerating axons contained within the basal lamina of Schwann cell tubes that have been cleared by Wallerian degeneration. Right: Electronmicrograph, 4,000×. Two regenerating units with fibers in various stages of myelination. The arrows outline the Schwann cell basal lamina that contains a single regenerating unit.
DISCUSSION
The term gene therapy refers to a variety of techniques used to manipulate cellular protein synthesis. Although these techniques have the potential to increase the treatment options for inherited and acquired disease, they remain largely experimental. The goals of gene therapy targeting the peripheral nervous system include support of neuronal survival, promotion of axonal regeneration, enhancement of regeneration specificity, and prevention end-organ atrophy (Haastert and Grothe, 2007a).
A variety of viral and non-viral gene transfer techniques have been developed to achieve these goals. In peripheral nerve, adenovirus (Araki et al., 2006, Boulis et al., 1999, Boulis et al., 2002, Boulis et al., 2003a, Boulis et al., 2003b, Dijkhuizen et al., 1998, Glatzel et al., 2000, Hermens et al., 1997, Joung et al., 2000, Rubin et al., 2003, Shy et al., 1995, Sorensen et al., 1998, Watanabe et al., 2006), adeno-associated virus (Boulis et al., 2003a, Boulis et al., 2003b), herpes simples virus (Palmer et al., 2000), liposome-mediated gene transfer (Kato et al., 2005), and receptor-mediated gene transfer techniques (Barati et al., 2006) have been successful in vivo. Although virus-mediated gene transfer is highly effective, its clinical applicability is limited because of safety concerns; death and iatrogenic leukemia have been reported as a complication of viral-mediated gene transfer in clinical trials (Jaichandran et al., 2006, Marshall, 1999, Raper et al., 2003). In comparison, plasmid and oligonucleotide-based gene transfer methods have the advantage of reduced immunogenicity, minimal integration into the genome, and reduced potential for environmental spread, which makes these techniques attractive for clinical use. Transfection efficiency and effectiveness, however, are limited, challenging the clinical applicability of this approach (Isaka and Imai, 2007).
Electroporation, or electropermeabilization, increases the effectiveness of plasmid-based gene transfer (reviewed in Cemazar et al., 2006, Favard, Dean and Rols, 2007, Gehl, 2003). Electrical pulses delivered directly to a target tissue reversibly increase cell membrane permeability by creating a network of temporary pores. Molecules that cannot normally cross the membrane can then enter through these pores during the few seconds that they are present. Electroporation is highly target specific; gene expression is limited to cells that are exposed to both plasmid DNA and electricity. So far, application of electroporation to the treatment of peripheal nerve injury has been limited to the fabrication of prosthetic nerve graft. Schwann cells have been electroporated in vitro, then re-implantated into rat sciatic nerve defects within a tubular prosthesis (Haastert et al., 2006a, Haastert et al., 2006b, Timmer et al., 2003). This technique, referred to as ex vivo gene therapy, facilitates meticulous control of transfection conditions and culture environment. Schwann cells can be assessed for survival and appropriate gene expression before re-implantation is performed, enhancing control of growth factor production within the tube. Nevertheless, ex vivo cell modification requires multiple procedures: Schwann cells must be harvested, the cell population must be purified, expanded, and transfected, and only then can the modified cells be transplanted to bridge a neural gap. These cells orient themselves along the longitudinal axis of the tube to myelinate axons; functional recovery, however, has not yet reached the level provided by autologous nerve graft (Rodriguez et al., 2000).
The present work extends the application of electroporation to include the transfection of Schwann cells in vivo, within the normal three dimensional structure of peripheral nerve. The co-localization of all YFP expression with S-100 and the morphology and orientation of expressing cells indicate that myelinating Schwann cells were the primary targets of transfection. The intimate relationship between Schwann cells and their axonal partners will maximize the availability of novel gene products to the axon. Lentivirus, in contrast, has been shown to transfect fibroblasts when it is introduced into human peripheral nerve in vitro (Tannemaat et al., 2007). The distribution of fibroblast products within the peripheral nerve, and thus the relative effectiveness of this technique, have yet to be evaluated.
Preliminary experiments in the rat tibial nerve indicated that 70 v, 5 msec resulted in the greatest YFP expression without injuring the nerve, and these parameters were used to evaluate long-term expression. The observed transfection rates were 25–28% three days after electroporation. These compare favorably to transfection rates of 20–40% obtained by electroporating Schwann cells in vitro (Haastert et al., 2007b). Inconsistent transgene expression is frequently observed in gene therapy of peripheral nerve, regardless of the technique used (Tannemaat et al., 2007). This has been attributed to the barrier imposed on uniform DNA injection by peripheral nerve structure. During preliminary trials we encountered difficulties with backflow of the DNA solution at the injection site and uneven distribution of the fluid throughout the nerve. We added Fast Green to the solution so that we could determine its distribution (Blits et al., 2000), and found that advancing the micropipette proximally and distally from one central puncture site resulted in more even distribution.
In these experiments, YFP expression was robust for the first week, but declined rapidly thereafter. Lentivirus, in contrast, modifies Schwann cell gene expression for several weeks (reviewed in Hermens and Verhaagen, 1998; Eggers et al., 2008). Expression of several growth factors by peripheral nerve in vivo is downregulated soon after Schwann cells are contacted by regenerating axons (Hoke et al., 2006), suggesting that prolonged growth factor expression near a site of nerve repair could hinder, rather than promote, axonal elongation beyond this area. This possibility is borne out by recent experiments in which lentiviral- mediated expression of GDNF hampered axon regeneration (Eggers et al., 2008). The more limited period of expression that results from electroporation may thus prove to be advantageous in some circumstances.
Transfected nerve was examined electronmicroscopically to assess the morphologic consequences of the electroporation process. Intact nerve developed intraneural edema, and modest axon loss was noted in some specimens. Occasional axons underwent segmental demyelination, and others generated collateral sprouts in the presence of intact myelin. Overall, however, intact peripheral nerve survived the trauma of electroporation quite well. No electroporation effects could be detected in nerve that was denervated, or denervated and undergoing reinnervation. By 21 days reinnervated nerve was populated with regenerating units consistent with normal support of regeneration.
We determined transfection rates in intact nerve, denervated nerve, and grafted nerve to evaluate the effectiveness of electroporation in potential clinical settings. Upregulation of specific genes in the intact proximal nerve stump, where protein products can be transported to parent neurons, could be used to prevent axotomy-related neuron death or to stimulate the neuronal regeneration response. Electroporation of denervated nerve mimics the situation after proximal injury, in which long portions of peripheral nerve are denervated for months before regenerating axons arrive. Modification of grafted nerves will be clinically useful to prolong their ability to support regeneration, and to modify the growth factor environment of sensory grafts to better support motor axon regeneration. The relatively short duration of gene expression resulting from electroporation suggests that the technique will be most useful when applied to the proximal stump or short nerve graft, and less applicable to treatment of long-term denervated nerve graft.
Electroporation of genes into intact peripheral nerve is a single-stage procedure with high clinical potential. Nerve graft can be harvested at the beginning of a reconstructive procedure, electroporated in the operating room while the recipient nerve is prepared, and then returned to the patient after it has been modified to upregulate specific genes. The present experiments demonstrate expression of a fluorescent marker rather than a growth factor. Work in vitro, however, has shown that Schwann cell growth factor production, and thus phenotype, can be manipulated successfully with electroporation (Hastert et al., 2006a; Haastert et al., 2007b). This technique avoids the possible complications of viral transfection, and maintains normal peripheral nerve architecture. Its’ ready applicability suggests new approaches to nerve repair and reconstruction, such as proximal stump transfection to minimize neuron loss or augment the regeneration response, and modification of cutaneous nerve to better support motor axon regeneration. Work is ongoing in our laboratory to increase transfection rates, prolong expression, and minimize tissue damage. We anticipate that electroporation of intact peripheral nerve will be a robust technique with multiple clinical applications.
Acknowledgments
The authors thank Mr. Norman Barker for photography and Ms. Catherine Weaver for preparation of illustrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki K, Shiotani A, Watabe K, Saito K, Moro K, Ogawa K. Adenoviral GDNF gene transfer enhances neurofunctional recovery after recurrent laryngeal nerve injury. Gene Ther. 2006;13:296–303. doi: 10.1038/sj.gt.3302665. [DOI] [PubMed] [Google Scholar]
- Barati S, Hurtado PR, Zhang SH, Tinsley R, Ferguson IA, Rush RA. GDNF gene delivery via the p75(NTR) receptor rescues injured motor neurons. Exp Neurol. 2006;202:179–188. doi: 10.1016/j.expneurol.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Blits B, Dijkhuizen PA, Boer GJ, Verhaagen J. Intercostal nerve implants transduced with an adenoviral vector encoding neurotrophin-3 promote regrowth of injured rat corticospinal tract fibers and improve hindlimb function. Exp Neurol. 2000;164:25–37. doi: 10.1006/exnr.2000.7413. [DOI] [PubMed] [Google Scholar]
- Boulis NM, Turner DE, Imperiale MJ, Feldman EL. Neuronal survival following remote adenovirus gene delivery. J Neurosurg. 2002;96:212–219. doi: 10.3171/spi.2002.96.2.0212. [DOI] [PubMed] [Google Scholar]
- Boulis NM, Willmarth NE, Song DK, Feldman EL, Imperiale MJ. Intraneural colchicine inhibition of adenoviral and adeno-associated viral vector remote spinal cord gene delivery. Neurosurgery. 2003a;52:381–387. doi: 10.1227/01.neu.0000044459.24519.3e. [DOI] [PubMed] [Google Scholar]
- Boulis NM, Turner DE, Dice JA, Bhatia V, Feldman EL. Characterization of adenoviral gene expression in spinal cord after remote vector delivery. Neurosurgery. 1999;45:131–137. doi: 10.1097/00006123-199907000-00029. [DOI] [PubMed] [Google Scholar]
- Boulis NM, Noordmans AJ, Song DK, Imperiale MJ, Rubin A, Leone P, During M, Feldman EL. Adeno-associated viral vector gene expression in the adult rat spinal cord following remote vector delivery. Neurobiol Dis. 2003b;14:535–541. doi: 10.1016/j.nbd.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- Brushart TM. Nerve regeneration. New York: Oxford University Press; 2009. in press. [Google Scholar]
- Cemazar M, Golzio M, Sersa G, Rols MP, Teissie J. Electrically-assisted nucleic acids delivery to tissues in vivo: Where do we stand? Curr Pharm Des. 2006;12:3817–3825. doi: 10.2174/138161206778559740. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen PA, Pasterkamp RJ, Hermens WT, de Winter F, Giger RJ, Verhaagen J. Adenoviral vector-mediated gene delivery to injured rat peripheral nerve. J Neurotrauma. 1998;15:387–397. doi: 10.1089/neu.1998.15.387. [DOI] [PubMed] [Google Scholar]
- Eggers R, Hendriks W, Tannemaat M, van Heerikhulze J, Pool C, Carlstedt T, Zaldumbide A, Hoeben R, Boer G, Verhaagem J. Neuroregenerative effects of lentiviral vector-mediated GDNF expression in reimplanted ventral roots. Mol Cell Neurosci. 2008 doi: 10.1016/j.mcn.2008.05.018. Epub 1–19. [DOI] [PubMed] [Google Scholar]
- Favard C, Dean DS, Rols MP. Electrotransfer as a non viral method of gene delivery. Curr Gene Ther. 2007;7:67–77. doi: 10.2174/156652307779940207. [DOI] [PubMed] [Google Scholar]
- Federici T, Boulis N. Gene therapy for peripheral nervous system diseases. Curr Gene Ther. 2007;7:239–248. doi: 10.2174/156652307781369083. [DOI] [PubMed] [Google Scholar]
- Gehl J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177:437–447. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- Glatzel M, Flechsig E, Navarro B, Klein MA, Paterna JC, Bueler H, Aguzzi A. Adenoviral and adeno-associated viral transfer of genes to the peripheral nervous system. Proc Natl Acad Sci U S A. 2000;97:442–447. doi: 10.1073/pnas.97.1.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haastert K, Grothe C. Gene therapy in peripheral nerve reconstruction approaches. Curr Gene Ther. 2007a;7:221–228. doi: 10.2174/156652307780859035. [DOI] [PubMed] [Google Scholar]
- Haastert K, Mauritz C, Chaturvedi S, Grothe C. Human and rat adult schwann cell cultures: Fast and efficient enrichment and highly effective non-viral transfection protocol. Nat Protoc. 2007b;2:99–104. doi: 10.1038/nprot.2006.486. [DOI] [PubMed] [Google Scholar]
- Haastert K, Mauritz C, Matthies C, Grothe C. Autologous adult human schwann cells genetically modified to provide alternative cellular transplants in peripheral nerve regeneration. J Neurosurg. 2006a;104:778–786. doi: 10.3171/jns.2006.104.5.778. [DOI] [PubMed] [Google Scholar]
- Haastert K, Lipokatic E, Fischer M, Timmer M, Grothe C. Differentially promoted peripheral nerve regeneration by grafted schwann cells over-expressing different FGF-2 isoforms. Neurobiol Dis. 2006b;21:138–153. doi: 10.1016/j.nbd.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Hermens WT, Verhaagen J. Viral vectors, tools for gene transfer in the nervous system. Prog Neurobiol. 1998;55:399–432. doi: 10.1016/s0301-0082(98)00007-0. [DOI] [PubMed] [Google Scholar]
- Hermens WT, Giger RJ, Holtmaat AJ, Dijkhuizen PA, Houweling DA, Verhaagen J. Transient gene transfer to neurons and glia: Analysis of adenoviral vector performance in the CNS and PNS. J Neurosci Methods. 1997;71:85–98. doi: 10.1016/s0165-0270(96)00129-x. [DOI] [PubMed] [Google Scholar]
- Hoke A, Redett RJ, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaka Y, Imai E. Electroporation-mediated gene therapy. Expert Opin Drug Deliv. 2007;4:561–571. doi: 10.1517/17425247.4.5.561. [DOI] [PubMed] [Google Scholar]
- Jaichandran S, Yap ST, Khoo AB, Ho LP, Tien SL, Kon OL. In vivo liver electroporation: Optimization and demonstration of therapeutic efficacy. Hum Gene Ther. 2006;17:362–375. doi: 10.1089/hum.2006.17.362. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Joung I, Kim HS, Hong JS, Kwon H, Kwon YK. Effective gene transfer into regenerating sciatic nerves by adenoviral vectors: Potentials for gene therapy of peripheral nerve injury. Mol Cells. 2000;10:540–545. doi: 10.1007/s10059-000-0540-4. [DOI] [PubMed] [Google Scholar]
- Kato N, Nemoto K, Nakanish K, Morishita R, Kaneda Y, Uenoyama M, Ikeda T, Fujikawa K. Nonviral HVJ (hemagglutinating virus of japan) liposome-mediated retrograde gene transfer of human hepatocyte growth factor into rat nervous system promotes functional and histological recovery of the crushed nerve. Neurosci Res. 2005;52:299–310. doi: 10.1016/j.neures.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kreiger N, Kelsey JL, Harris C, Pastides H. Injuries to the upper extremity: Patterns of occurrence. Clin Plast Surg. 1981;8:13–19. [PubMed] [Google Scholar]
- Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- Naga T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Palmer JA, Branston RH, Lilley CE, Robinson MJ, Groutsi F, Smith J, Latchman DS, Coffin RS. Development and optimization of herpes simplex virus vectors for multiple long-term gene delivery to the peripheral nervous system. J Virol. 2000;74:5604–5618. doi: 10.1128/jvi.74.12.5604-5618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Rodriguez FJ, Verdu E, Ceballos D, Navarro X. Nerve guides seeded with autologous schwann cells improve nerve regeneration. Exp Neurol. 2000;161:571–584. doi: 10.1006/exnr.1999.7315. [DOI] [PubMed] [Google Scholar]
- Rubin A, Moble B, Hogikyan N, Bell K, Sullivan K, Boulis N, Feldman E. Delivery of an adenoviral vector to the crushed recurrent laryngeal nerve. Laryngoscope. 2003;113:985–989. doi: 10.1097/00005537-200306000-00013. [DOI] [PubMed] [Google Scholar]
- Shy ME, Tani M, Shi YJ, Whyatt SA, Chbihi T, Scherer SS, Kamholz J. An adenoviral vector can transfer lacZ expression into schwann cells in culture and in sciatic nerve. Ann Neurol. 1995;38:429–436. doi: 10.1002/ana.410380313. [DOI] [PubMed] [Google Scholar]
- Sorensen J, Haase G, Krarup C, Gilgenkrantz H, Kahn A, Schmalbruch H. Gene transfer to schwann cells after peripheral nerve injury: A delivery system for therapeutic agents. Ann Neurol. 1998;43:205–211. doi: 10.1002/ana.410430210. [DOI] [PubMed] [Google Scholar]
- Tannemaat MR, Boer GJ, Verhaagen J, Malessy MJ. Genetic modification of human sural nerve segments by a lentiviral vector encoding nerve growth factor. Neurosurgery. 2007;61:1286–94. doi: 10.1227/01.neu.0000306108.78044.a2. [DOI] [PubMed] [Google Scholar]
- Timmer M, Robben S, Muller-Ostermeyer F, Nikkhah G, Grothe C. Axonal regeneration across long gaps in silicone chambers filled with schwann cells overexpressing high molecular weight FGF-2. Cell Transplant. 2003;12:265–277. doi: 10.3727/000000003108746821. [DOI] [PubMed] [Google Scholar]
- Watanabe TS, Ohtori S, Koda M, Aoki Y, Doya H, Shirasawa H, Yamazaki M, Moriya H, Takahashi K, Yamashita T. Adenoviral gene transfer in the peripheral nervous system. J Orthop Sci. 2006;11:64–69. doi: 10.1007/s00776-005-0971-z. [DOI] [PubMed] [Google Scholar]
- Willard MD, Willard FS, Li X, Cappell SD, Snider WD, Siderovski DP. Selective role for RGS12 as a Ras/Raf/MEK scaffold in nerve growth factor-mediated differentiation. EMBO J. 2007;26:2029–2040. doi: 10.1038/sj.emboj.7601659. [DOI] [PMC free article] [PubMed] [Google Scholar]