Abstract
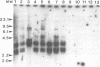
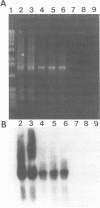
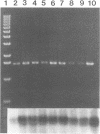
A PCR assay for the detection of Serpulina hyodysenteriae in diagnostic specimens was developed on the basis of sequence analysis of a recombinant clone designated pRED3C6. Clone pRED3C6, which contained a 2.3-kb DNA fragment unique to S. hyodysenteriae, was identified by screening a plasmid library of S. hyodysenteriae isolate B204 genomic DNA in Escherichia coli by colony immunoblot with the mouse monoclonal antibody 10G6/G10, which was produced against cell-free supernatant antigens from the same isolate. Southern blot analysis of HindIII-digested genomic DNA of S. hyodysenteriae serotypes 1 through 7 and of four weakly beta-hemolytic intestinal spirochetes, including Serpulina innocens, with the 2.3-kb DNA fragment of pRED3C6 indicated that the cloned sequence was present exclusively in the seven serotypes of S. hyodysenteriae. An oligonucleotide primer pair for PCR amplification of a 1.55-kb fragment and an internal oligonucleotide probe were designed and synthesized on the basis of sequence analysis of the 2.3-kb DNA fragment of pRED3C6. Purified genomic DNAs from reference isolates of S. hyodysenteriae serotypes 1 through 9, S. innocens, weakly beta-hemolytic intestinal spirochetes belonging to genotypic groups distinct from those of reference Serpulina spp., other cultivable reference isolates of the order Spirochaetales, and enteric bacteria including Escherichia coli, Salmonella spp., Campylobacter spp., and Bacteroides vulgatus were amplified with the oligonucleotide primer pair in a hot-start PCR. The 1.55-kb products were obtained only in the presence of genomic DNA from each of the nine serotypes of S. hyodysenteriae. The specificity of the 1.55-kb products for S. hyodysenteriae was confirmed on the basis of production of a restriction endonuclease pattern of the PCR products identical to the predicted restriction map analysis of pRED3C6 and positive hybridization signal with the S. hyodysenteriae-specific internal oligonucleotide probe. By using total DNA obtained from normal swine feces inoculated with decreasing concentrations of S. hyodysenteriae cells, the sensitivity of the PCR assay was calculated to be between 1 and 10 organisms per 0.1 g of feces. The PCR assay was 1,000 times more sensitive than conventional culture of dysenteric feces on selective medium. There was complete agreement between the results of PCR assays and anaerobic culture on selective agar medium with diagnostic specimen (n = 9) obtained from six farms on which there were cases with clinical signs suggestive of swine dysentery. Detection of S. hyodysenteriae by PCR amplification of DNA has great potential for rapid identification of S. hyodysenteriae in diagnostic specimens.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achacha M., Messier S. Identification of Treponema hyodysenteriae and Treponema innocens using two four-hour identification systems. J Vet Diagn Invest. 1991 Jul;3(3):211–214. doi: 10.1177/104063879100300304. [DOI] [PubMed] [Google Scholar]
- Bélanger M., Jacques M. Evaluation of the An-Ident system and an indole spot test for the rapid differentiation of porcine treponemes. J Clin Microbiol. 1991 Aug;29(8):1727–1729. doi: 10.1128/jcm.29.8.1727-1729.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs B. G., Hampson D. J. Use of a whole chromosomal probe for identification of Treponema hyodysenteriae. Res Vet Sci. 1991 May;50(3):286–289. doi: 10.1016/0034-5288(91)90125-8. [DOI] [PubMed] [Google Scholar]
- Cwyk W. M., Canale-Parola E. Treponema succinifaciens sp. nov., an anaerobic spirochete from the swine intestine. Arch Microbiol. 1979 Sep;122(3):231–239. doi: 10.1007/BF00411285. [DOI] [PubMed] [Google Scholar]
- Duhamel G. E., Bernard R. J., Mathiesen M. R., Eskridge K. M. Comparison of six commercially available transport media for maintenance of Serpulina (Treponema) hyodysenteriae. J Vet Diagn Invest. 1992 Jul;4(3):285–292. doi: 10.1177/104063879200400310. [DOI] [PubMed] [Google Scholar]
- Hampson D. J., Cutler R., Lee B. J. Virulent Serpulina hyodysenteriae from a pig in a herd free of clinical swine dysentery. Vet Rec. 1992 Oct 3;131(14):318–319. doi: 10.1136/vr.131.14.318. [DOI] [PubMed] [Google Scholar]
- Hugo F., Arvand M., Reichwein J., Mackman N., Holland I. B., Bhakdi S. Identification with monoclonal antibodies of hemolysin produced by clinical isolates of Escherichia coli. J Clin Microbiol. 1987 Jan;25(1):26–30. doi: 10.1128/jcm.25.1.26-30.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D., Wood T. An evaluation of the API ZYM system as a means of classifying spirochaetes associated with swine dysentery. Vet Rec. 1979 Apr 28;104(17):383–384. doi: 10.1136/vr.104.17.383. [DOI] [PubMed] [Google Scholar]
- Jacques M., Girard C., Higgins R., Goyette G. Extensive colonization of the porcine colonic epithelium by a spirochete similar to Treponema innocens. J Clin Microbiol. 1989 May;27(5):1139–1141. doi: 10.1128/jcm.27.5.1139-1141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen N. S., Casey T. A., Stanton T. B. Detection and identification of Treponema hyodysenteriae by using oligodeoxynucleotide probes complementary to 16S rRNA. J Clin Microbiol. 1990 Dec;28(12):2717–2721. doi: 10.1128/jcm.28.12.2717-2721.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. J., Miller J. N., George W. L. Microbiological and biochemical characterization of spirochetes isolated from the feces of homosexual males. J Clin Microbiol. 1986 Dec;24(6):1071–1074. doi: 10.1128/jcm.24.6.1071-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyon J. M., Harris D. L., Glock R. D. Enteropathogenicity of various isolates of Treponema hyodysenteriae. Infect Immun. 1977 Feb;15(2):638–646. doi: 10.1128/iai.15.2.638-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkle R. A., Harris D. L., Kinyon J. M. Autoclaved liquid medium for propagation of Treponema hyodysenteriae. J Clin Microbiol. 1986 Oct;24(4):669–671. doi: 10.1128/jcm.24.4.669-671.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkle R. A., Kinyon J. M. Improved selective medium for the isolation of Treponema hyodysenteriae. J Clin Microbiol. 1988 Nov;26(11):2357–2360. doi: 10.1128/jcm.26.11.2357-2360.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. I., Hampson D. J., Combs B. G., Lymbery A. J. Genetic relationships between isolates of Serpulina (Treponema) hyodysenteriae, and comparison of methods for their subspecific differentiation. Vet Microbiol. 1993 Jan;34(1):35–46. doi: 10.1016/0378-1135(93)90005-r. [DOI] [PubMed] [Google Scholar]
- Lemcke R. M., Burrows M. R. A disc growth-inhibition test for differentiating Treponema hyodysenteriae from other intestinal spirochaetes. Vet Rec. 1979 Jun 16;104(24):548–551. doi: 10.1136/vr.104.24.548. [DOI] [PubMed] [Google Scholar]
- Li Z. S., Bélanger M., Jacques M. Serotyping of Canadian isolates of Treponema hyodysenteriae and description of two new serotypes. J Clin Microbiol. 1991 Dec;29(12):2794–2797. doi: 10.1128/jcm.29.12.2794-2797.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymbery A. J., Hampson D. J., Hopkins R. M., Combs B., Mhoma J. R. Multilocus enzyme electrophoresis for identification and typing of Treponema hyodysenteriae and related spirochaetes. Vet Microbiol. 1990 Mar;22(1):89–99. doi: 10.1016/0378-1135(90)90127-h. [DOI] [PubMed] [Google Scholar]
- Lysons R. J. Microscopic agglutination: a rapid test for identification of Treponema hyodysenteriae. Vet Rec. 1991 Oct 5;129(14):314–315. doi: 10.1136/vr.129.14.314. [DOI] [PubMed] [Google Scholar]
- Olive D. M. Detection of enterotoxigenic Escherichia coli after polymerase chain reaction amplification with a thermostable DNA polymerase. J Clin Microbiol. 1989 Feb;27(2):261–265. doi: 10.1128/jcm.27.2.261-265.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard B., Lariviere S., Saheb S. A. Etude comparative des caractères biochimiques de tréponèmes hémolytiques isolés du porc. Can J Microbiol. 1980 Aug;26(8):985–991. [PubMed] [Google Scholar]
- Ramanathan M., Duhamel G. E., Mathiesen M. R., Messier S. Identification and partial characterization of a group of weakly beta-hemolytic intestinal spirochetes of swine distinct from Serpulina innocens isolate B256. Vet Microbiol. 1993 Oct;37(1-2):53–64. doi: 10.1016/0378-1135(93)90182-7. [DOI] [PubMed] [Google Scholar]
- Smith S. C., Roddick F., Ling S., Gerraty N. L., Coloe P. J. Biochemical and immunochemical characterisation of strains of Treponema hyodysenteriae. Vet Microbiol. 1990 Jul;24(1):29–41. doi: 10.1016/0378-1135(90)90048-z. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos C., Smith S. C., Coloe P. J. Characterization of two DNA probes specific for Serpulina hyodysenteriae. J Clin Microbiol. 1993 Jul;31(7):1746–1752. doi: 10.1128/jcm.31.7.1746-1752.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanton T. B., Lebo D. F. Treponema hyodysenteriae growth under various culture conditions. Vet Microbiol. 1988 Oct;18(2):177–190. doi: 10.1016/0378-1135(88)90063-6. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Simmons J. R., Laird H. M. Production of diarrhoea and dysentery in pigs by feeding pure cultures of a spirochaete differing from Treponema hyodysenteriae. Vet Rec. 1980 Apr 12;106(15):326–332. doi: 10.1136/vr.106.15.326. [DOI] [PubMed] [Google Scholar]
- Thomas W., Sellwood R. Monoclonal antibodies to a 16-kDa antigen of Serpulina (Treponema) hyodysenteriae. J Med Microbiol. 1992 Sep;37(3):214–220. doi: 10.1099/00222615-37-3-214. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]