Abstract
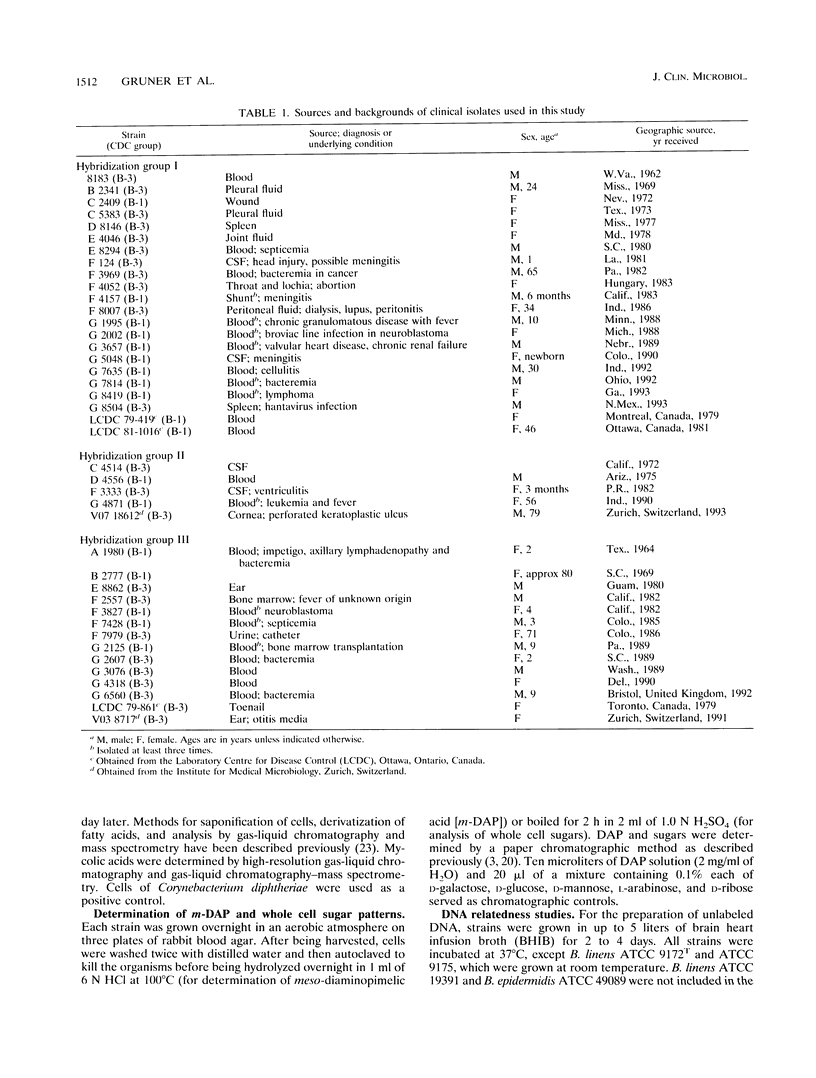
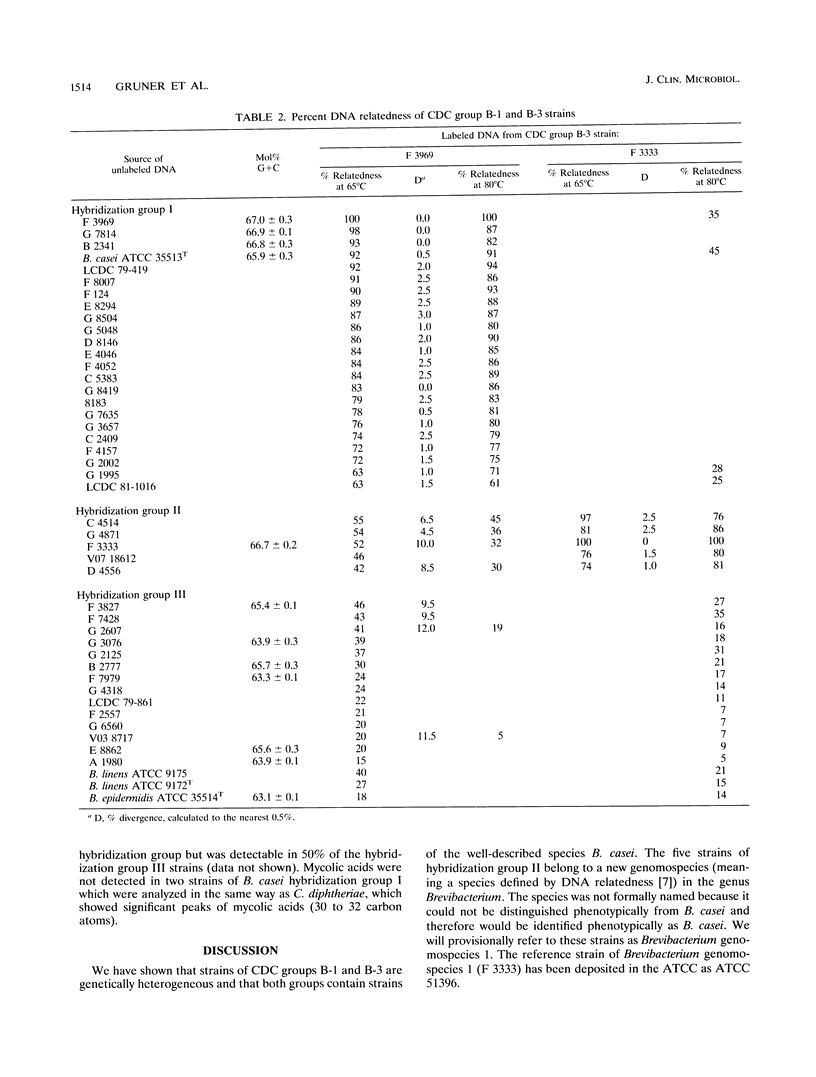
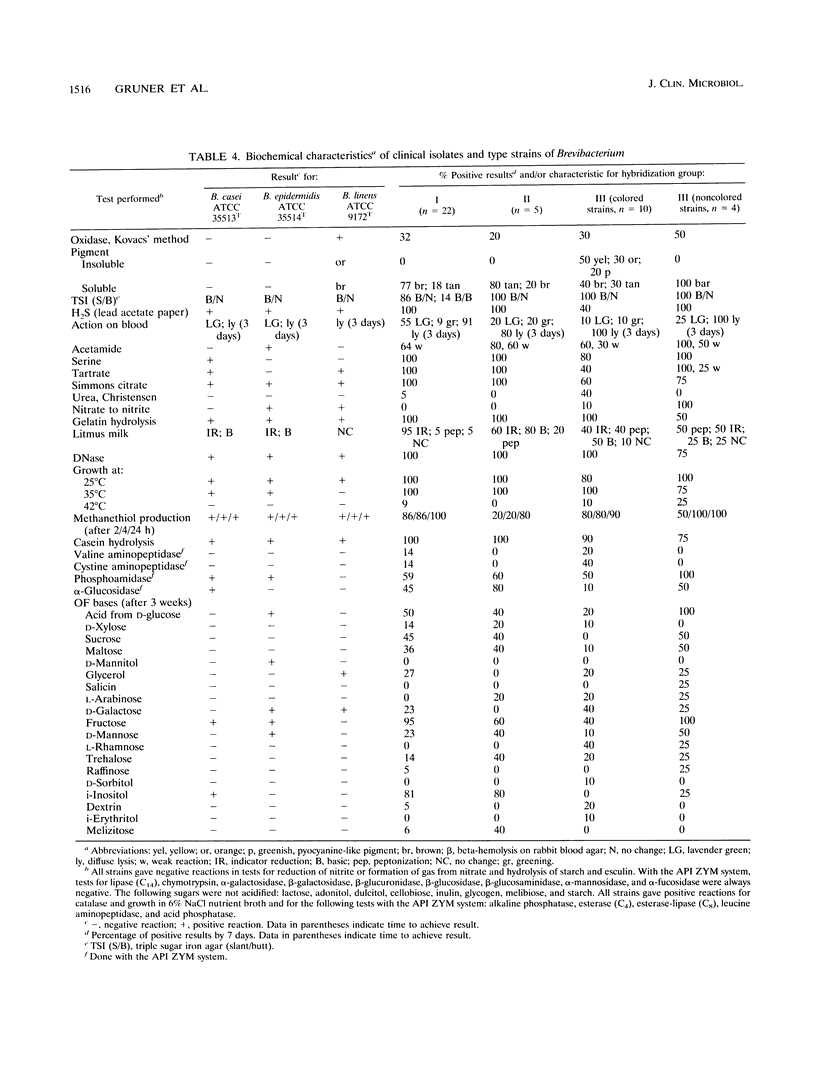
Forty-one clinical strains of CDC coryneform groups B-1 and B-3 were compared biochemically, by analysis of cell wall sugars, amino acids, and cellular fatty acids, and by DNA relatedness to the type strains of Brevibacterium casei, Brevibacterium epidermidis, and Brevibacterium linens. Twenty-two strains were shown to be B. casei, while five other strains formed a phenotypically inseparable genomospecies in the same genus. The remaining isolates were genetically heterogeneous, and most are probably members of the genus Brevibacterium. They were not further identified, but they were biochemically distinguishable from B. casei. Eleven of the clinical strains of B. casei were isolated from blood, and two each were isolated from cerebrospinal fluid and from pleural fluid. At least five isolates were from multiple blood or cerebrospinal fluid cultures. To our knowledge, these strains are the first described clinical isolates identified as B. casei, which was previously considered to be a nonpathogenic species.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony R. M., Noble W. C., Pitcher D. G. Characterization of aerobic non-lipophilic coryneforms from human feet. Clin Exp Dermatol. 1992 Mar;17(2):102–105. doi: 10.1111/j.1365-2230.1992.tb00174.x. [DOI] [PubMed] [Google Scholar]
- BECKER B., LECHEVALIER M. P., GORDON R. E., LECHEVALIER H. A. RAPID DIFFERENTIATION BETWEEN NOCARDIA AND STREPTOMYCES BY PAPER CHROMATOGRAPHY OF WHOLE-CELL HYDROLYSATES. Appl Microbiol. 1964 Sep;12:421–423. doi: 10.1128/am.12.5.421-423.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K. A., Bellefeuille M., Ewan E. P. Cellular fatty acid composition as an adjunct to the identification of asporogenous, aerobic gram-positive rods. J Clin Microbiol. 1991 Jan;29(1):83–89. doi: 10.1128/jcm.29.1.83-89.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Brenner D. J., Neufeld B. R., Britten R. J. Reduction in the rate of DNA reassociation by sequence divergence. J Mol Biol. 1973 Dec 5;81(2):123–135. doi: 10.1016/0022-2836(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Grimont P. A., Steigerwalt A. G., Fanning G. R., Ageron E., Riddle C. F. Classification of citrobacteria by DNA hybridization: designation of Citrobacter farmeri sp. nov., Citrobacter youngae sp. nov., Citrobacter braakii sp. nov., Citrobacter werkmanii sp. nov., Citrobacter sedlakii sp. nov., and three unnamed Citrobacter genomospecies. Int J Syst Bacteriol. 1993 Oct;43(4):645–658. doi: 10.1099/00207713-43-4-645. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., McWhorter A. C., Knutson J. K., Steigerwalt A. G. Escherichia vulneris: a new species of Enterobacteriaceae associated with human wounds. J Clin Microbiol. 1982 Jun;15(6):1133–1140. doi: 10.1128/jcm.15.6.1133-1140.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombach W. H. Morphology and physiology of coryneform bacteria. Antonie Van Leeuwenhoek. 1974;40(3):361–376. doi: 10.1007/BF00399348. [DOI] [PubMed] [Google Scholar]
- Crombach W. H. Relationships among coryneform bacteria from soil, cheese and sea fish. Antonie Van Leeuwenhoek. 1974;40(3):347–359. doi: 10.1007/BF00399346. [DOI] [PubMed] [Google Scholar]
- Gruner E., Pfyffer G. E., von Graevenitz A. Characterization of Brevibacterium spp. from clinical specimens. J Clin Microbiol. 1993 Jun;31(6):1408–1412. doi: 10.1128/jcm.31.6.1408-1412.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates S. G., Nordstrom K. M., McGinley K. J., Leyden J. J. Microbial ecology of interdigital infections of toe web spaces. J Am Acad Dermatol. 1990 Apr;22(4):578–582. doi: 10.1016/0190-9622(90)70075-s. [DOI] [PubMed] [Google Scholar]
- Keddie R. M., Cure G. L. The cell wall composition and distribution of free mycolic acids in named strains of coryneform bacteria and in isolates from various natural sources. J Appl Bacteriol. 1977 Apr;42(2):229–252. doi: 10.1111/j.1365-2672.1977.tb00689.x. [DOI] [PubMed] [Google Scholar]
- Lechevalier M. P. Identification of aerobic actinomycetes of clinical importance. J Lab Clin Med. 1968 Jun;71(6):934–944. [PubMed] [Google Scholar]
- Martin R., Riley P. S., Hollis D. G., Weaver R. E., Krichevsky M. I. Characterization of some groups of gram-negative nonfermentative bacteria by the carbon source alkalinization technique. J Clin Microbiol. 1981 Jul;14(1):39–47. doi: 10.1128/jcm.14.1.39-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughey C., Damani N. N. Central venous line infection caused by Brevibacterium epidermidis. J Infect. 1991 Sep;23(2):211–212. doi: 10.1016/0163-4453(91)92451-a. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Wallace P. L., Hollis D. G., Weaver R. E. Cultural and chemical characterization of CDC groups EO-2, M-5, and M-6, Moraxella (Moraxella) species, Oligella urethralis, Acinetobacter species, and Psychrobacter immobilis. J Clin Microbiol. 1988 Mar;26(3):484–492. doi: 10.1128/jcm.26.3.484-492.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na'Was T. E., Hollis D. G., Moss C. W., Weaver R. E. Comparison of biochemical, morphologic, and chemical characteristics of Centers for Disease Control fermentative coryneform groups 1, 2, and A-4. J Clin Microbiol. 1987 Aug;25(8):1354–1358. doi: 10.1128/jcm.25.8.1354-1358.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D. G., Malnick H. Identification of Brevibacterium from clinical sources. J Clin Pathol. 1984 Dec;37(12):1395–1398. doi: 10.1136/jcp.37.12.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D. G. Rapid identification of cell wall components as a guide to the classification of aerobic coryneform bacteria from human skin. J Med Microbiol. 1977 Nov;10(4):439–445. doi: 10.1099/00222615-10-4-439. [DOI] [PubMed] [Google Scholar]
- Riegel P., de Briel D., Prévost G., Jehl F., Monteil H., Minck R. Taxonomic study of Corynebacterium Group ANF-1 strains: Proposal of Corynebacterium afermentans sp. nov. containing the subspecies C. afermentans subsp. afermentans subsp. nov. and C. afermentans subsp. lipophilum subsp. nov. Int J Syst Bacteriol. 1993 Apr;43(2):287–292. doi: 10.1099/00207713-43-2-287. [DOI] [PubMed] [Google Scholar]
- Sharpe M. E., Law B. A., Phillips B. A., Pitcher D. G. Methanethiol production by coryneform bacteria: strains from dairy and human skin sources and Brevibacterium linens. J Gen Microbiol. 1977 Aug;101(2):345–349. doi: 10.1099/00221287-101-2-345. [DOI] [PubMed] [Google Scholar]
- Simonet M., De Briel D., Boucot I., Minck R., Veron M. Coryneform bacteria isolated from middle ear fluid. J Clin Microbiol. 1993 Jun;31(6):1667–1668. doi: 10.1128/jcm.31.6.1667-1668.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. B., Hancock G. A., Rhoden D. L. Improved medium for detecting deoxyribonuclease-producing bacteria. Appl Microbiol. 1969 Dec;18(6):991–993. doi: 10.1128/am.18.6.991-993.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



