Abstract
The neonatal period is characterized by rapid growth and elevated rates of synthesis and accretion of skeletal muscle proteins. The fractional rate of muscle protein synthesis is very high at birth and declines rapidly with age. The elevated capacity for muscle protein synthesis in the neonatal pig is driven by the high ribosome content and, together with an increased efficiency of the translation process, promotes accelerated protein synthesis rates. Feeding profoundly stimulates muscle protein synthesis in neonatal pigs and the response decreases with age. The feeding-induced stimulation of muscle protein synthesis is modulated by an enhanced sensitivity to the postprandial increase in insulin and amino acids. The developmental decline in the response to insulin and amino acids parallels a marked decrease in the feeding-induced activation of translation initiation factors that regulate the binding of mRNA to the 40S ribosomal complex. The abundance and activation of many known positive regulators of the nutrient- and insulin-signaling pathways that are involved in translation initiation are high, whereas those of many negative regulators are low in skeletal muscle of younger pigs. Thus, the activation and(or) abundance of the positive regulators, such as the insulin receptor, insulin receptor-substrate-1, phosphoinositide-3 kinase, phosphoinositide-dependent kinase-1, protein kinase B, mammalian target of rapamycin, raptor, ribosomal protein S6 kinase-1, eukaryotic initiation factor (eIF) 4E-binding protein 1, and eIF4E associated with eIF4G, are greater in 7-d-old pigs than in 26-d-old pigs. The activation of negative regulators, including protein tyrosine phosphatase-1B, phosphatase and tensin homologue deleted on chromosome 10, protein phosphatase 2A, and tuberous sclerosis complex 1/2, are lower in 7-d-old pigs than in 26-d-old pigs. Thus, the developmental decline in the stimulation of skeletal muscle protein synthesis by insulin and amino acids is due in part to the developmentally related decrease in the activation of the signaling pathways that lead to translation initiation.
Keywords: amino acid, insulin, muscle, neonate, pig, translation initiation
INTRODUCTION
Growth rate is greater during the neonatal period than at any other stage of postnatal life (Denne and Kalhan, 1987;Joung, 1970). Protein deposition is very rapid during this period of development and the gain in protein mass is more rapid in skeletal muscle than in other tissues in the body. Protein deposition is dependent on the rate of protein synthesis being greater than the rate of protein degradation. In the neonate, the high rate of protein deposition is driven by the high fractional rate of protein synthesis in skeletal muscle and this rate decreases sharply with age (Davis et al., 1989). Protein degradation is modestly elevated during early life and the changes with age are relatively small. Protein synthesis rates are dependent upon the number of ribosomes in a tissue and the efficiency with which the ribosomes translate mRNA into protein. The elevated capacity for muscle protein synthesis in the neonatal pig is driven by high ribosome content and an increased efficiency of the translation process (Davis et al., 2001).
POSTNATAL ONTOGENY OF THE FEEDING-INDUCED STIMULATION OF MUSCLE PROTEIN SYNTHESIS
During early postnatal life, the efficiency with which dietary amino acids are used for protein deposition is high and decreases with age (Davis et al., 1993). This high efficiency in the neonate is enabled by an elevated response of protein synthesis to stimulation by feeding (Denne et al., 1991; Burrin et al., 1992; Davis et al., 1996). Although feeding stimulates protein synthesis in all tissues of the neonatal pig, the greatest response occurs in skeletal muscle. In addition, the most profound developmental decline in the response to feeding also occurs in skeletal muscle (Burrin et al., 1995; Davis et al., 1996). The enhanced ability of protein synthesis in skeletal muscle of neonates to respond to the provision of nutrients is expected, because the rate of protein deposition during the postprandial period must be greater than the rate of protein loss during the postabsorptive period to permit growth.
Circulating insulin and amino acids increase rapidly after feeding. Because studies performed in vitro have shown a stimulatory effect of both insulin and amino acids on protein synthesis (Harmon et al., 1984), studies to identify the mechanism responsible for the stimulation of protein synthesis by feeding have focused on insulin and amino acids. In weaned rats, the response of protein synthesis in skeletal muscle to feeding can be blocked by anti-insulin serum and, in the fasting state insulin infusion stimulates muscle protein synthesis (Garlick et al., 1983; Preedy and Garlick, 1986). Insulin also stimulates whole-body protein synthesis in the fetal sheep and hindlimb protein synthesis in the lamb (Liechty et al., 1992; Wester et al., 2000). In contrast to studies performed in growing animals, most studies in adult animals and humans show little, if any, response of muscle protein synthesis to increases in circulating insulin (Gelfand and Barrett, 1987; Baillie and Garlick, 1992). This indicates that the response of muscle protein synthesis to insulin may be developmentally regulated. On the other hand, amino acids appear to have the ability to stimulate protein synthesis in skeletal muscle throughout the life cycle. Acute amino acid infusion stimulates protein synthesis in skeletal muscle of growing, adult, and older mammals (Preedy and Garlick, 1986; Bennet et al., 1990; Volpi et al., 1998); however, the magnitude of the response appears to decrease with age (Davis et al., 2002).
Circulating concentrations of amino acids decrease during experimentally induced hyperinsulinemia. Because a decrease in amino acids during the infusion of insulin could limit the ability of insulin to stimulate protein synthesis, we developed a novel technique to examine the role of insulin in the regulation of protein synthesis, independent of changes in circulating amino acids and glucose (Wray-Cahen et al., 1997). This technique, called the hyperinsulinemic-euglycemic-euaminoacidemic clamp, allows the maintenance of amino acids and glucose at fasting or other desired levels during the infusion of insulin. Using the hyperinsulinemic-euglycemic-euaminoacidemic clamp technique, we showed that insulin infusion increased amino acid utilization in the whole body of neonatal pigs when amino acids and glucose were maintained at fasting levels. Both the insulin sensitivity and responsiveness of amino acid disposal decrease with age. This developmental change in the insulin sensitivity of whole-body amino acid disposal likely plays a critical role in the developmental change in the efficiency of utilization of dietary amino acids for protein deposition.
Further study provided strong evidence that insulin mediates the stimulation of protein synthesis in skeletal muscle of the neonatal pig after feeding. When amino acids and glucose were maintained at fasting levels, infusion of insulin to achieve concentrations similar to those of the fed state increased the rate of muscle protein synthesis to concentrations similar to those of fed neonatal pigs (Wray-Cahen et al., 1998). The infusion of insulin, at doses within the physiological range, stimulated protein synthesis in skeletal muscle in a dose-dependent manner (O’Connor et al., 2003a). The response to insulin appears to be independent of amino acids because insulin stimulated protein synthesis even when amino acids were allowed to fall to half of the fasting levels. The response of protein synthesis to insulin is unique to skeletal muscle but decreases with age (Davis et al., 2001; O’Connor et al., 2004).
Amino acids are another mediator of the stimulation of protein synthesis by feeding in skeletal muscle of the neonatal pig. When a balanced mixture of amino acids was infused into fasted neonatal pigs to mimic levels present in the fed state, skeletal muscle protein synthesis rates increased to levels comparable to those of fed neonatal pigs (Davis et al., 2002). The stimulation of protein synthesis occurred in the presence of fasting levels of insulin, at insulin concentrations that were intermediate between the fasting and the fully fed state, and when insulin concentrations were reduced to undetectable levels using somatostatin to block insulin secretion (O’Connor et al., 2003a). The response of muscle protein synthesis to amino acids decreases with age. Thus, amino acids and insulin appear to act independently to stimulate protein synthesis in skeletal muscle of the neonatal pig after a meal. The ability of skeletal muscle to respond separately to both insulin and amino acids likely contributes to the more rapid gain in protein mass in skeletal muscle, compared with other tissues of the body, in the neonatal pig. The absence of an additive effect of insulin and amino acids on protein synthesis implies that their mechanism of action most likely involves a common final pathway.
POSTNATAL ONTOGENY OF THE INSULIN AND AMINO ACID SIGNALING PATHWAYS THAT LEAD TO TRANSLATION INITIATION
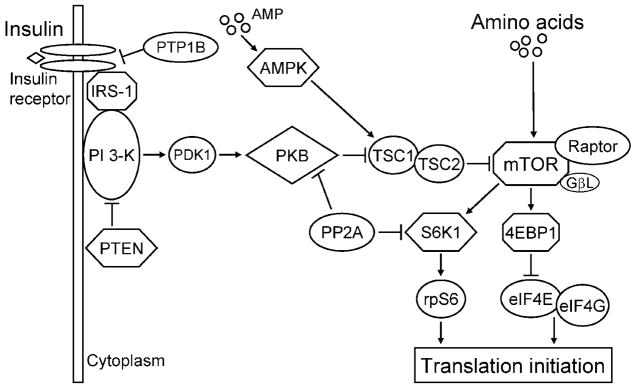
The molecular mechanism by which insulin stimulates protein synthesis has been studied extensively. Insulin initiates intracellular signaling upon binding to the insulin receptor and inducing the autophosphorylation of the receptor (Figure 1) on its tyrosine residues, followed by the activation of its tyrosine kinase activity (Di Guglielmo et al., 1998; Bevan, 2001). Insulin receptor substrate 1 (IRS-1), which functions as a docking protein, can then bind to the activated receptor and transmit the insulin signal to downstream proteins. The Src homology 2 domain of the p85 subunit of phosphoinositide 3 (PI3) kinase binds to activated IRS-1 and becomes activated (Harrington et al., 2005). Protein kinase B (PKB), a phospholipid-dependent Ser/Thr kinase, is then recruited to the cell membrane by a PI3 kinase-dependent mechanism. Phosphorylation of PKB is facilitated by phosphoinositide-dependent kinase 1 (PDK-1), resulting in its activation. The activation of mammalian target of rapamycin (mTOR), a master protein kinase, is induced by PKB (Asnaghi et al., 2004; Avruch et al., 2005). The complex of mTOR with raptor and G protein β-subunit-like protein (GβL; also referred to as mLST8) is referred to as TOR complex 1 (TORC1). Although raptor is a regulatory protein whose association with TOR appears to be essential for TOR signaling, its role is not clearly understood. Recent evidence indicates that raptor serves as a scaffold for the association of mTOR with ribosomal protein S6 kinase 1 (S6K1) and eukaryotic initiation factor (eIF) 4E-binding protein-1 (4EBP1). Activation of mTOR upregulates mRNA translation by enhancing the phosphorylation of S6K1 and 4EBP1 (Asnaghi et al., 2004). phorylated 4EBP1 releases eIF4E from the inactive eIF4E·4EBP1 complex, allowing the formation of the active eIF4G·eIF4E complex, which mediates the binding of mRNA to the 43S ribosomal complex (Niedzwiecka et al., 2002).
Figure 1.
Insulin and amino acid signaling pathway leading to the stimulation of translation initiation. Activation of the insulin signaling pathway is initiated by the binding of insulin to its receptor. This activates the insulin receptor and insulin receptor substrate-1 (IRS-1), followed by the activation of phosphoinositide-3 kinase (PI 3-K). Activated PI 3-K then stimulates the activation of phosphoinositide-dependent kinase 1 (PDK-1) and protein kinase B (PKB). Phosphorylation of PKB inactivates tuberous sclerosis complex 1/2 (TSC1/2), thereby inducing the activation of mammalian target of rapamycin (mTOR). Amino acids as well as insulin can activate mTOR, which exists in a complex with raptor and G protein β-subunit-like protein (GβL). Activated mTOR phosphorylates ribosomal protein S6 kinase 1 (S6K1) and eukaryotic initiation factor (eIF) 4E binding protein-1 (4EBP1). Phosphorylation of S6K1 enhances the activation of ribosomal subunit S6 (rpS6), which increases the translation of specific mRNA. Phosphorylated 4EBP1 releases eIF4E from an inactive eIF4E·4EBP1 complex, allowing the formation of the active eIF4G·eIF4E complex, which mediates the binding of mRNA to the 43S ribosomal complex. Insulin signaling can be attenuated by protein tyrosine phosphatase-1B (PTP-1B), which dephosphorylates the insulin receptor and IRS-1; phosphatase and tensin homologue deleted on chromosome 10 (PTEN), which inactivates PI 3-K; and protein phosphatase 2A (PP2A), which acts on PKB and S6K1. An increase in adenosine monophosphate (AMP) levels enhances AMP kinase activation, resulting in the activation of TSC1/2 complex and the decreased activation of mTOR.
The amino acid-induced signaling pathway that stimulates protein synthesis has not been well elucidated. Amino acid and insulin signals are integrated by mTOR via multiple mechanisms including phosphorylation of S6K1 and 4EBP1, resulting in modulation of protein synthesis and cell growth (Proud, 2004). Little is known about the mechanism by which amino acids modulate the activation of signaling components upstream of mTOR. Recent findings indicate that amino acids regulate the binding of raptor to mTOR, resulting in the activation of S6K1 and 4EBP1 (Hara et al., 2002).
Most of the studies that have examined the intracellular mechanisms by which insulin and amino acids stimulate protein synthesis were conducted in cell culture systems, and little was known about the role of the insulin and amino acid signaling components in the intact animal. Our studies in nursing piglets indicate that ontogenic changes in the abundance and activation of components of the insulin and amino acid intracellular signaling pathways in skeletal muscle contribute to the developmental changes in muscle protein synthesis. The abundance of the insulin receptor and the downstream signaling proteins PDK-1 and PKB decrease with development in skeletal muscle of the nursing pig (Suryawan et al., 2001; Suryawan and Davis, 2005). In addition, the activation of the insulin receptor, as well as IRS-1, PI3 kinase, and PKB, by feeding in skeletal muscle of the neonatal pig decrease with age in parallel with the developmental decline in muscle protein synthesis. Insulin infusion within the physiological range increases the activation of these early steps in the insulin signaling pathway in a dose-dependent manner (Suryawan et al., 2004). However, physiological changes in amino acid levels have no effect on the activation of the insulin signaling pathway upstream of PKB and do not alter the response to insulin in skeletal muscle of neonatal pigs.
We have demonstrated that ontogenic changes in the abundance and activation of translation initiation factors in skeletal muscle of the pig also contribute to developmental changes in muscle protein synthesis. In skeletal muscle of neonatal pigs, the abundance of mTOR and raptor and the association of mTOR with raptor decrease with age (Davis et al., 2000; Kimball et al., 2002; Suryawan et al., 2006). However, the abundance of the downstream effectors of mTOR (i.e., S6K1 and 4EBP1) does not change with age. Feeding increases the phosphorylation and, thus, activation of mTOR, but does not alter its association with raptor. Feeding also increases the phosphorylation of S6K1 and 4EBP1, resulting in a decrease in the abundance of the inactive 4E-BP1·eIF4E complex. This leads to an increase in the association of eIF4E with eIF4G in an active complex that regulates the binding of mRNA to the ribosomal complex, and promotes translation initiation. All of these responses to feeding decline with age in skeletal muscle of pigs. When either insulin or amino acids are infused within the physiological range, the phosphorylation of S6K1 and 4EBP1 are increased, whereas the binding of 4E-BP1 to eIF4E, decreases and promotes eIF4E binding to eIF4G (O’Connor et al., 2003b). Thus, the postprandial increase in insulin, but not amino acids, activates PKB and the signaling components that are upstream of PKB (i.e., the insulin receptor, IRS-1, and PI3 kinase). The increase in both insulin and amino acids after a meal activates signaling components that are downstream of PKB, including mTOR, S6K1, 4EBP1, and eIF4E·eIF4G.
Recent studies using in vitro and cell culture systems have identified new signaling components that are negative regulators of the insulin and amino acid signaling pathways leading to translation initiation. Recent evidence indicates that Ser/Thr phosphorylation of the insulin receptor and IRS-1 serves as a negative feedback control mechanism that inhibits the action of the insulin receptor and IRS-1, and that enhanced Ser/Thr phosphorylation of these proteins may play a role in the development of insulin resistance (Zick, 2004). Protein tyrosine phosphatase 1B, a negative regulator of tyrosine phosphorylation of the insulin receptor and IRS-1, is activated by tyrosine phosphorylation upon association with the insulin receptor, IRS-1, and growth factor receptor-bound protein 2 (Grb2; Egawa et al., 2001). Phosphatase and tensin homologue on chromosome 10 (PTEN) is a lipid phosphatase that antagonizes the activation of PKB by PI3 kinase (Goberdhan and Wilson, 2003). Protein phosphatase 2A (PP2A) is a phosphatase that negatively regulates insulin signaling by inhibiting the phosphorylation of PKB and S6K1 (Peterson et al., 1999; Ugi et al., 2004). Protein kinase B phosphorylates and inactivates an inhibitor of cell growth, tuberous sclerosis complex 2 (TSC2), thereby inactivating the function of the TSC1/2 complex, and enabling activation of mTOR (Krymskaya, 2003; Kwiatkowski and Manning, 2005). During conditions of energy insufficiency when there is an increase in adenosine monophosphate (AMP) levels within the cell, AMP kinase is activated (Carling, 2005). The energy sensor, AMP kinase, phosphorylates TSC2, resulting in the activation of TSC1/2 complex, thereby decreasing the activation of mTOR (Kwiatkowski and Manning, 2005).
Little is known about the negative regulators of the insulin and amino acid signaling pathways that lead to translation initiation in whole animals under physiologically relevant conditions. Therefore, we conducted a detailed study of the role of development in the activation and protein abundance of these signaling components. We determined that the activation of PTP1B, PTEN, PP2A, and TSC2, all negative regulators of insulin signaling, are low in muscle of neonatal pigs and increase with age, consistent with a developmental decrease in the activation of the pathways that promote translation initiation (Suryawan and Davis, 2003; Suryawan et al., 2006). However, we were able to detect an effect of feeding only on TSC2 in which feeding decreased the activation of TSC2 and this effect of feeding was attenuated with age. Interestingly, we did not detect any effects of feeding or age on the activation of AMP kinase, a negative regulator of the mTOR pathway and a sensor of cellular energy. Serine/threonine phosphorylation of the insulin receptor and IRS-1, which negatively regulate insulin signaling, were also unaffected by feeding or age. Together, the results indicate that the developmental changes in the activation of both positive and negative regulators of the insulin and amino acid signaling pathways contribute to the high rate of protein synthesis in neonatal pigs and the decrease in responsiveness that occurs as the muscles mature.
SUMMARY
The fractional rate of protein synthesis in skeletal muscle is high in neonatal pigs and declines with age. The elevated rate of muscle protein synthesis is driven by the high ribosome content and an increased efficiency of translation. Feeding profoundly stimulates muscle protein synthesis in neonatal pigs and the response is greater the younger the animal. The feeding-induced stimulation of muscle protein synthesis is modulated by enhanced sensitivity to the postprandial rise in insulin and amino acids. Insulin and amino acid signaling components have been identified that are involved in the feeding-induced stimulation of protein synthesis in skeletal muscle. Ontogenic changes in the activation of these signaling components contribute to the developmental changes in protein synthesis in skeletal muscle of piglets.
Footnotes
This work is a publication of the USDA-ARS Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children’s Hospital, Houston, Texas. This project has been funded, in part, by the NIH grants R01 AR 44474, R01 AR46308, and K08 AR51563-01A1, the USDA-ARS under Cooperative Agreement number 58-6250-6-001, and National Research Initiative Competitive Grant no. 2005-35206-15273 from the USDA Cooperative State Research, Education, and Extension Service. The contents of this publication do not necessarily reflect the views or policies of the US Department of Agriculture, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
Presented at the Nonruminant Nutrition symposium at the annual meeting of the American Society of Animal Science, San Antonio, TX, July 8 to 12, 2007.
LITERATURE CITED
- Asnaghi L, Bruno P, Priulla M, Nicolin A. mTOR: A protein kinase switching between life and death. Pharmacol Res. 2004;50:545–549. doi: 10.1016/j.phrs.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Avruch J, Lin Y, Long X, Murthy S, Ortiz-Vega S. Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr Opin Clin Nutr Metab Care. 2005;8:67–72. doi: 10.1097/00075197-200501000-00010. [DOI] [PubMed] [Google Scholar]
- Baillie AG, Garlick PJ. Attenuated responses of muscle protein synthesis to fasting and insulin in adult female rats. Am J Physiol. 1992;262:E1–E5. doi: 10.1152/ajpendo.1992.262.1.E1. [DOI] [PubMed] [Google Scholar]
- Bennet WM, Connacher AA, Scrimgeour CM, Rennie MJ. The effect of amino acid infusion on leg protein turnover assessed by L-[15N]phenylalanine and L-[1-13C]leucine exchange. Eur J Clin Invest. 1990;20:41–50. doi: 10.1111/j.1365-2362.1990.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Bevan P. Insulin signalling. J Cell Sci. 2001;114:1429–1430. doi: 10.1242/jcs.114.8.1429. [DOI] [PubMed] [Google Scholar]
- Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S. Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatr Res. 1995;37:593–599. doi: 10.1203/00006450-199505000-00006. [DOI] [PubMed] [Google Scholar]
- Burrin DG, Shulman RJ, Reeds PJ, Davis TA, Gravitt KR. Porcine colostrum and milk stimulate visceral organ and skeletal muscle protein synthesis in neonatal piglets. J Nutr. 1992;122:1205–1213. doi: 10.1093/jn/122.6.1205. [DOI] [PubMed] [Google Scholar]
- Carling D. AMP-activated protein kinase: Balancing the scales. Biochimie. 2005;87:87–91. doi: 10.1016/j.biochi.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than 26-day-old pigs. Am J Physiol. 1996;270:E802–E809. doi: 10.1152/ajpendo.1996.270.5.E802. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Beckett PR, Burrin DG, Reeds PJ, Wray-Cahen D, Nguyen HV. Differential effects of insulin on peripheral and visceral tissue protein synthesis in neonatal pigs. Am J Physiol. 2001;280:E770–E779. doi: 10.1152/ajpendo.2001.280.5.E770. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O’Connor PM. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol. 2002;282:E880–E890. doi: 10.1152/ajpendo.00517.2001. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of suckling rats. Am J Physiol Regul Integr Comp Physiol. 1989;257:R1141–R1146. doi: 10.1152/ajpregu.1989.257.5.R1141. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Reeds PJ. Amino acid compositions of body and milk protein change during the suckling period in rats. J Nutr. 1993;123:947–956. doi: 10.1093/jn/123.5.947. [DOI] [PubMed] [Google Scholar]
- Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol. 2000;279:E1226–E1234. doi: 10.1152/ajpendo.2000.279.6.E1226. [DOI] [PubMed] [Google Scholar]
- Denne SC, Kalhan SC. Leucine metabolism in human newborns. Am J Physiol. 1987;253:E608–E615. doi: 10.1152/ajpendo.1987.253.6.E608. [DOI] [PubMed] [Google Scholar]
- Denne SC, Rossi EM, Kalhan SC. Leucine kinetics during feeding in normal newborns. Pediatr Res. 1991;30:23–27. doi: 10.1203/00006450-199107000-00005. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo GM, Drake PG, Baass PC, Authier F, Posner BI, Bergeron JJ. Insulin receptor internalization and signalling. Mol Cell Biochem. 1998;182:59–63. [PubMed] [Google Scholar]
- Egawa K, Maegawa H, Shimizu S, Morino K, Nishio Y, BryerAsh M, Cheung AT, Kolls JK, Kikkawa R, Kashiwagi A. Protein-tyrosine phosphatase-1B negatively regulates insulin signaling in L6 myocytes and FaO hepatoma cells. J Biol Chem. 2001;276:10207–10211. doi: 10.1074/jbc.M009489200. [DOI] [PubMed] [Google Scholar]
- Garlick PJ, Fern M, Preedy VR. The effect of insulin infusion and food intake on muscle protein synthesis in postabsorptive rats. Biochem J. 1983;210:669–676. doi: 10.1042/bj2100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand RA, Barrett EJ. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest. 1987;80:1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberdhan DC, Wilson C. PTEN: Tumour suppressor, multifunctional growth regulator and more. Hum Mol Genet. 2003;12:R239–R248. doi: 10.1093/hmg/ddg288. [DOI] [PubMed] [Google Scholar]
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- Harmon CS, Proud CB, Pain VM. Effects of starvation, diabetes, and acute insulin treatment on the regulation of polypeptide-chain initiation in rat skeletal muscle. Biochem J. 1984;223:687–696. doi: 10.1042/bj2230687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci. 2005;30:35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Farrell PA, Nguyen HV, Jefferson LS, Davis TA. Developmental decline in components of signal transduction pathways regulating protein synthesis in pig muscle. Am J Physiol. 2002;282:E585–E592. doi: 10.1152/ajpendo.00269.2001. [DOI] [PubMed] [Google Scholar]
- Krymskaya VP. Tumour suppressors hamartin and tuberin: Intracellular signalling. Cell Signal. 2003;15:729–739. doi: 10.1016/s0898-6568(03)00040-8. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Manning BD. Tuberous sclerosis: A GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 2005;14:R251–R258. doi: 10.1093/hmg/ddi260. [DOI] [PubMed] [Google Scholar]
- Liechty EA, Boyle DW, Moorehead H, Liu YM, Denne SC. Effect of hyperinsulinemia on ovine fetal leucine kinetics during prolonged maternal fasting. Am J Physiol. 1992;263:E696–E702. doi: 10.1152/ajpendo.1992.263.4.E696. [DOI] [PubMed] [Google Scholar]
- Niedzwiecka A, Stepinski J, Darzynkiewicz E, Sonenberg N, Stolarski R. Positive heat capacity change upon specific binding of translation initiation factor eIF4E to mRNA 5′cap. Biochemistry. 2002;41:12140–12148. doi: 10.1021/bi0258142. [DOI] [PubMed] [Google Scholar]
- O’Connor PM, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol. 2003a;284:E110–E119. doi: 10.1152/ajpendo.00326.2002. [DOI] [PubMed] [Google Scholar]
- O’Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol. 2003b;285:E40–E53. doi: 10.1152/ajpendo.00563.2002. [DOI] [PubMed] [Google Scholar]
- O’Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of neonatal liver protein synthesis by insulin and amino acids in pigs. Am J Physiol. 2004;286:E994–E1003. doi: 10.1152/ajpendo.00391.2003. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Desai BN, Hardwick JS, Schreiber SL. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinas-sociated protein. Proc Natl Acad Sci USA. 1999;96:4438–4442. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preedy VR, Garlick PJ. The response of muscle protein synthesis to nutrient intake in postabsorptive rats: The role of insulin and amino acids. Biosci Rep. 1986;6:177–183. doi: 10.1007/BF01115004. [DOI] [PubMed] [Google Scholar]
- Proud GC. Role of mTOR signalling in the control of translation initiation and elongation by nutrients. Curr Top Microbiol Immunol. 2004;279:215–244. doi: 10.1007/978-3-642-18930-2_13. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Davis TA. Protein-tyrosine-phosphatase1B activation is regulated developmentally in muscle of neonatal pigs. Am J Physiol. 2003;284:E47–E54. doi: 10.1152/ajpendo.00210.2002. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Davis TA. Developmental regulation of protein kinase B activation is isoform specific in skeletal muscle of neonatal pigs. Pediatr Res. 2005;58:719–724. doi: 10.1203/01.PDR.0000180536.51032.AB. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Escobar J, Frank JW, Nguyen HV, Davis TA. Developmental regulation of the activation of signaling components leading to translation initiation in skeletal muscle of neonatal pigs. Am J Physiol. 2006;291:E849–E859. doi: 10.1152/ajpendo.00069.2006. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Nguyen HV, Bush JA, Davis TA. Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. Am J Physiol. 2001;281:E908–E915. doi: 10.1152/ajpendo.2001.281.5.E908. [DOI] [PubMed] [Google Scholar]
- Suryawan A, O’Connor PM, Kimball SR, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Amino acids do not alter the insulin-induced activation of the insulin signaling pathway in neonatal pigs. J Nutr. 2004;134:24–30. doi: 10.1093/jn/134.1.24. [DOI] [PubMed] [Google Scholar]
- Ugi S, Imamura T, Maegawa H, Egawa K, Yoshizaki T, Shi K, Obata T, Ebina Y, Kashiwagi A, Olefsky JM. Protein phosphatase 2A negatively regulates insulin’s metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol Cell Biol. 2004;24:8778–8789. doi: 10.1128/MCB.24.19.8778-8789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester TJ, Lobley GE, Birnie LM, Lomax MA. Insulin stimulates phenylalanine uptake across the hind limb in fed lambs. J Nutr. 2000;130:608–611. doi: 10.1093/jn/130.3.608. [DOI] [PubMed] [Google Scholar]
- Wray-Cahen D, Beckett PR, Nguyen HV, Davis TA. Insulin-stimulated amino acid utilization during glucose and amino acid clamps decreases with development. Am J Physiol. 1997;273:E305–E314. doi: 10.1152/ajpendo.1997.273.2.E305. [DOI] [PubMed] [Google Scholar]
- Wray-Cahen D, Nguyen HV, Burrin DG, Beckett PR, Fiorotto ML, Reeds PJ, Wester TJ, Davis TA. Response of skeletal muscle protein synthesis to insulin in suckling pigs decreases with development. Am J Physiol. 1998;275:E602–E609. doi: 10.1152/ajpendo.1998.275.4.E602. [DOI] [PubMed] [Google Scholar]
- Young VR. The role of skeletal and cardiac muscle in the regulation of protein metabolism. In: Munro HM, editor. Mammalian Protein Metabolism. Academic Press; New York, NY: 1970. pp. 585–674. [Google Scholar]
- Zick Y. Uncoupling insulin signalling by serine/threonine phosphorylation: A molecular basis for insulin resistance. Biochem Soc Trans. 2004;32:812–816. doi: 10.1042/BST0320812. [DOI] [PubMed] [Google Scholar]