Abstract
Polymeric carriers of protein antigens have great potential for improving the efficacy of vaccines and immunotherapeutics for diseases such as cancer. We recently developed a carrier system based on polyacrylamide hydrogel microparticles crosslinked with acid-labile moieties. After being phagocytosed by antigen-presenting cells, the protein encapsulated within the carrier is released and processed for subsequent presentation of antigenic epitopes. To understand the impact of particle size on the activation of T-cells following uptake by antigen-presenting cells, particles with mean diameters of 3.5 μm and 35 nm encapsulating a model protein antigen were synthesized by emulsion and microemulsion based polymerization techniques, respectively. In vivo tests demonstrated that both sizes of particles were effective at stimulating the proliferation of T-cells, and were capable of generating an antigen specific cytotoxic T-cell response when co-administered with immunostimulatory DNA. Contrary to previous reports in the literature, our results suggest that there is no significant difference in the magnitude of T-cell activation for the two sizes of particles used in these experiments. This disparity in findings may be related to fundamental differences in material properties of the carriers used in these studies, such as the hydrophilicity of the polyacrylamide particles described here versus the hydrophobic nature of carriers investigated by other groups.
INTRODUCTION
The physical dimensions of a biomaterial are key parameters that may dictate the interaction of the material with its surrounding biological environment, particularly when those dimensions are on the micro- or the nano-scale. For example, while many cell types are capable of ingesting small size particulate matter (typically < 200 nm in diameter) by mechanisms such as pinocytosis, and receptor- and clathrin-mediated endocytosis (1), the uptake of particles with diameters up to several microns is generally restricted to the phagocytic antigen-presenting cells of the immune system such as macrophages and dendritic cells. This difference in uptake has been exploited in the development of protein antigen carrier systems, which often increase the efficacy of protein-based vaccines (2-4). Tuning the size of particulate antigen delivery systems can enable passive targeting of exogenous proteins to antigen-presenting cells for presentation of derived peptide fragments on major histocompatibility class I (MHC I) molecules, which can subsequently enhance the activation of cytotoxic T-lymphocyte (CTL) immune responses (5).
Several reports have highlighted the impact that particle size may have on interactions with antigen-presenting cells and the immune system in general. For example, while dendritic cells are capable of ingesting polystyrene particles ranging in size from tens of nanometers to microns in diameter, it appears that these cells may demonstrate preferential uptake for smaller particles with diameters less than 300 nm (6). Early studies on the functional application of particulate antigen carriers indicated that for proteins bound to the surface of polystyrene beads with diameters between 500 nm and 10 μm, carriers with diameters between 2 – 3 μm were most effective at inducing cross-presentation of antigenic peptides in macrophages in an ovalbumin-based model system in vitro (7). However, in vivo studies using a similar polystyrene-based system with diameters ranging from 20 nm to 2 μm, suggested that particles less than 200 nm (8) or 100 nm (9) in diameter were most effective at generating CTL responses. Additional work showed that among those particles with diameters less than 100 nm, 40 – 50 nm particles were potentially more effective at biasing the activation of the adaptive immune system towards a CTL-dominated response (10).
Carrier size has also been shown to influence in vivo behavior at the systemic level. Poly(propylene sulfide) particles with 20 and 45 nm diameters were shown to traffic to draining lymph nodes via convective transport through the lymphatic capillaries after subcutaneous injection, with the potential for targeting lymph-node-resident dendritic cells. In contrast, 100 nm particles were not observed to drain to the lymph nodes to any significant extent (11, 12). These observations are in agreement with an earlier report demonstrating that 0.5 – 1 μm polystyrene particles were generally found to reside at the site of injection after several days where they were capable of targeting peripheral antigen-presenting cells (13).
With these studies in mind, we aimed to investigate the effect of particle size on T-cell activation in a microparticulate carrier system we recently developed based on polyacrylamide networks containing pH-sensitive crosslinks. This system has been shown to be effective at delivering protein antigens to cells of the immune system both in vitro and in vivo using particles typically greater than 1 μm in diameter (14-17). Compared to the particles used in the studies described above, which primarily utilized polystyrene or other hydrophobic-core carriers, this polyacrylamide-based system is inherently hydrophilic, mechanically compliant, and the protein payload is physically encapsulated, which should offer protection from proteolytic degradation prior to reaching the target antigen-presenting cells. The particles are relatively stable at physiological pH but degrade rapidly when they encounter the lower pH environment present in the lysosomes of cells. When ingested by antigen-presenting cells, the particles rapidly break down into smaller fragments, releasing their contents in a manner that enhances MHC I presentation of antigen-derived peptides (14, 15, 17).
To investigate the effect of particle size on T-cell activation using our polyacrylamide-based system, we first had to synthesize submicron-sized particles. To this end, we optimized the inverse emulsion conditions of free-radical polymerization to generate acid-labile polyacrylamide nanogels. After characterizing these nanoparticles, we investigated their ability to induce T-cell proliferation and an antigen-specific cytotoxic T-cell response in vivo in a direct comparison with the performance of analogous but larger acid-degradable particles.
EXPERIMENTAL PROCEDURES
General Procedures and Materials
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and used without additional purification. Acrylamide, N,N’-methylene-bis-acrylamide, ammonium persulfate, and N,N,N’,N’-tetramethylethylenediamine (TMEDA) were purchased from Bio-Rad (Hercules, CA). Hexane (OmniSolv grade) was purchased from EMD (Gibbstown, NJ). UltraPure 0.1-μm filtered water and phosphate buffered saline (pH 7.4, PBS) were purchased from Invitrogen–Gibco (Carlsbad, CA). The pH of PBS solutions was adjusted using either HCl or NaOH. Unless otherwise noted, dried particles were suspended in buffer by alternately vortexing the solution and sonicating in a VWR Model 75 water bath for 1-3 minutes until particle aggregates were no longer visible.
Crosslinker Synthesis
Synthesis of the benzylidene acetal crosslinker 1 (Scheme 1) was performed as previously reported (17).
Scheme 1.

(a) Acid-labile benzylidene acetal crosslinking monomer (1) used to prepare nanoparticles. (b) Hydrolysis of crosslinker in polymerized particles generates linear polyacrylamide chains with pendant alcohols along with the release of the corresponding benzaldehyde.
Preparation of 35 nm Diameter Particles
To prepare the organic continuous phase, AOT (sodium bis(2-ethylhexyl) sulfosuccinate) (178 mg, 0.40 mmol) and Brij 30 (polyethylene glycol dodecyl ether with Mn ~ 362) (344 mg, 0.95 mmol) were added to a 20 mL borosilicate glass vial to which a stir bar was added. The vial was sealed with a Teflon-lined septum cap and purged with dry nitrogen for 10 min. Hexane (5 mL) was deoxygenated by bubbling dry nitrogen through the solvent for 10 min and was added to the vial. The vial was placed in an oil bath with the temperature set to 23.5 °C, and stirring was started at 600 rpm to dissolve the surfactants. For the aqueous phase, ovalbumin (7.0 mg) was dissolved in PBS (pH 9, 203 μL), to which acrylamide (48.0 mg, 0.68 mmol) and crosslinker 1 (39.0 mg, 0.07 mmol) were added and dissolved. A 20% (w/v) solution of ammonium persulfate in PBS (pH 9, 30 μL) was added to the monomer/protein solution. The mixture was sonicated for 30 s in a Branson 1210 water bath, and added to the organic continuous phase dropwise via syringe over approximately 3 min. After 10 min, polymerization was initiated by the addition of TMEDA (20 μL) via syringe over the course of 2 min, and the mixture was stirred for 2 h. Solvent was removed by rotary evaporation, and absolute ethanol (5 mL) was added, resulting in a milky-white suspension. The particles were isolated by centrifugation for 10 min at 4000 × g at rt, and washed with absolute ethanol (4 × 5 mL), centrifuging as before. The particles were suspended with absolute ethanol (2 mL) and sonicated for 30 s in a Branson 1210 water bath. The ethanol was removed under vacuum, and the particles dried overnight to yield a fine white powder (54.0 mg).
Non-degradable 35 nm Diameter Particles
Non-degradable particles used in light scattering studies were prepared as described above with the exception that N,N’-methylene-bis-acrylamide (10.8 mg, 0.07 mmol) was substituted for crosslinker 1 in the monomer mixture.
Fluorescently-labeled 35 nm Diameter Particles
Particles encapsulating an ovalbumin – AlexaFluor 488 conjugate (OVA-AF488, Invitrogen-Molecular Probes, Carlsbad, CA) were prepared as described above with the exception that the monomers were dissolved in a PBS solution containing 2 mg/mL of OVA-AF488 in lieu of using unlabeled ovalbumin.
Preparation of 3.5 μm Diameter Particles
The 3.5 μm diameter particles were synthesized according to a previously reported procedure (16). Briefly, ovalbumin (7.0 mg) was dissolved in 300 mM phosphate buffer (pH 8, 250 μL), to which acrylamide (63.0 mg, 0.89 mmol) and crosslinker 1 (63.0 mg, 0.12 mmol) were then added and dissolved. Ammonium persulfate (7.0 mg, 0.03 mmol) was added to the monomer/protein solution and the mixture was added to the organic continuous phase (2.5 mL), consisting of a 3 wt. % surfactant solution in hexane, using a 3:1 weight ratio of Span 80 (sorbitan monooleate) to Tween 80 (polyethylene glycol - sorbitan monooleate). The two phases were emulsified by sonicating for 30 s using a Branson Sonifier 450 with a 0.5 inch flat tip, an output setting of 2, and a duty cycle of 40%. Polymerization was initiated by the addition of TMEDA (25 μL), and the mixture was stirred at rt for 10 min. The particles were isolated by centrifugation for 10 min at 1380 × g at rt, and washed with hexanes (3 × 2 mL) and acetone (4 × 2 mL), centrifuging as before. The solvent was decanted and the particles dried overnight under vacuum to yield a fine white powder (53.7 mg).
Fluorescently-labeled 3.5 μm Diameter Particles
Particles encapsulating OVA-AF488 were prepared as described above with the exception that the monomers were dissolved in a PBS solution containing 10 mg/mL of OVA-AF488 in lieu of using unlabeled ovalbumin.
Ovalbumin-free Particles
As background controls for determining protein loading, particles of each size were prepared as described above with the exception that no ovalbumin was included in the aqueous phase.
Scanning Electron Microscopy (SEM)
A sample of 35 nm particles was suspended at 20 mg/mL in 1 mM NH4OH (in UltraPure water) and deposited onto a silicon wafer. After 5 min, the residual solution was wicked away using filter paper and the residue was dried overnight under vacuum. The sample was sputter coated with 1 nm film of gold/palladium and visualized using a Hitachi S-5000 SEM at 10 kV.
Particle Size Measurements
The size distribution of the 35 nm particles was determined by dynamic light scattering using a Zetasizer Nano-ZS instrument (Malvern Instruments, Malvern, UK) after suspending the particles at 2 mg/mL in PBS (pH 10). Particle samples were maintained at 25 °C and the instrument settings were determined automatically. The reported results represent the average particle size ± half peak width at half-maximal height of fifteen sequential measurements (to ensure measurement stability) from a multimodal data fit using the manufacturer’s software package.
The size distribution of the 3.5 μm particles was determined using a Horiba Partica LA-950 laser scattering particle size distribution analyzer after suspending the particles at 5 mg/mL in PBS. The reported results represent the geometric mean ± standard deviation of the size distribution.
The refractive indices of PBS and polyacrylamide used in the size distribution calculations were 1.33 and 1.47 respectively (18), and the viscosity of PBS was 0.888 cP. Particle size distributions for both measurement techniques were volume-weighted.
Determination of Particle Loading
Particles containing ovalbumin were suspended at a concentration of 5 mg/mL in PBS (pH 5). The solutions were incubated overnight at room temperature while vortexing at 1000 rpm to completely degrade the particles. The amount of protein released from the particles was quantified using a bicinchoninic acid (BCA) assay kit according to the manufacturer’s instructions (Micro BCA Protein Assay Kit, Pierce, Rockford, IL). Dilutions of free ovalbumin were used as a standard. Absorbances were recorded using a SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, CA). Particle loading was corrected for background absorbance from ovalbumin-free particles degraded in the same manner.
1H NMR
NMR samples were prepared in deuterated phosphate-buffered saline consisting of potassium phosphate monobasic (0.144 mg/mL), sodium phosphate dibasic heptahydrate (0.795 mg/mL), and sodium chloride (9 mg/mL) in D2O. As necessary, this buffer was adjusted to pH 5 using 0.5 M DCl in D2O. Particles were suspended at 3 mg/mL. Spectra were acquired using a 400 MHz Bruker spectrometer. Chemical shifts are reported relative to 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid (sodium salt) as a standard.
To monitor crosslink hydrolysis at pH 7.4, a 35 nm particle sample was maintained at 37 °C and spectra were acquired at the indicated time points. To monitor crosslink hydrolysis at pH 5, a particle suspension was maintained at 37 °C and at the indicated time points, 0.6 mL aliquots were removed, hydrolysis was quenched by adding 2.4 μL of 1 M NaOD in D2O, and the samples were transferred to sealed NMR tubes. Spectra were acquired within 1 h after samples were quenched.
To determine the crosslinker hydrolysis rate, the ratio of the integration of the benzaldehyde proton signal (δ 9.8 ppm) to the integration of the standard signal was calculated. The ratios were normalized to the ratio obtained from a completely hydrolyzed particle spectrum. To obtain the half-life with respect to hydrolysis, the normalized ratio was plotted versus time and initial data points were fit with an exponential function. All peak areas were determined by numerical integration after background subtraction using Mathematica 5.0 (Wolfram Research).
Particle Degradation Measurements
To monitor degradation of the 35 nm particles by light scattering (19), samples were suspended at a concentration of 2 mg/mL in PBS at the indicated pH values. Samples (1 mL) were added to a cuvette sealed with parafilm to prevent evaporation and vortexed at 600 rpm at 37 °C between readings. Scattering intensity was measured using a Zetasizer Nano-ZS instrument with the temperature set to 37 °C, with the instrument settings held constant from run to run. Reported values represent the mean unadjusted scattering count rate.
Cell Lines and Culture
RAW 309 Cr.1 macrophages (#TIB-69) and HeLa cells (CCL-2), were purchased from ATCC (Manassas, VA) and were cultured in Dulbecco’s Modified Eagle Medium containing 4.5 g/L D-glucose, and supplemented with 10% fetal bovine serum, 100 units/mL of penicillin, 100 μg/mL streptomycin, and 2 mM GlutaMAX (DMEM). All culture media components were from Invitrogen-Gibco with the exception of the serum, which was from Hyclone (Logan, UT). Cell incubations were performed in a water-jacketed 37 °C/5% CO2 incubator.
Nanoparticle Cytotoxicity
RAW 309 and HeLa cells were added to the wells of a 96-well tissue culture plate at 5 × 103 cells/well (100 μL/well in DMEM). After overnight incubation, the medium was exchanged for dilutions of ovalbumin-loaded 35 nm particles prepared in DMEM at the indicated concentrations in triplicate (100 μL/well). After 7 h, the wells were washed 3 × 150 μL with DMEM, and 100 μL of DMEM was added to each well. After 24 h, the medium was exchanged for 120 μL of a 0.83 mg/mL solution of MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) in DMEM. After 3 h, the medium was exchanged for 200 μL of DMSO, and the plates were vortexed to dissolve the purple formazan crystals present. On a separate plate, 100 μL from each well was mixed with 100 μL of DMSO and 25 μL of a glycine buffer (0.1 M glycine, 0.1 M NaCl, pH 10.5), and the absorbance of each well was read at 570 nm using a SpectraMax 190 microplate reader. After background subtraction, each well was normalized to the average absorbance of wells containing cells not treated with particles.
Particle Uptake
RAW 309 and HeLa cells were added to the wells of a 12-well tissue culture plate at 1.5 × 105 cells/well (2 mL/well in DMEM). After overnight incubation, the medium was exchanged for particles encapsulating OVA-AF488 suspended at 0.25 mg/mL in DMEM (1 mL/well). At the indicated time points cells were washed 3 × 1 mL with PBS, harvested by trypsinization, and centrifuged for 10 seconds at 13,000 × g at rt. The cells were then washed with 1 mL of PBS and centrifuged as before. The cell pellet was resuspended in 300 μL of a 4% paraformaldehyde solution (Electron Microscopy Sciences, Hatfield, PA) in PBS. Cells were analyzed for particle uptake using a Becton-Dickinson Biosciences FACSCalibur flow cytometer (San Jose, CA) with 488 nm excitation.
Confocal Microscopy
RAW 309 cells were seeded in the wells of a four-chamber glass Lab-Tek chamber slide (Nunc, Rochester, NY) at 2 × 104 cells/well (0.75 mL/well) in DMEM. After overnight incubation, the medium was aspirated from each well and 35 nm particles (suspended at 0.25 mg/mL in DMEM) encapsulating OVA-AF488 were added to the wells (0.5 mL/well). After 8 h, the wells were washed 3 × 0.75 mL with DMEM, and 0.5 mL of DMEM was added to each well. After 16 h, the wells were washed 3 × 0.75 mL with PBS, and incubated in a 4% paraformaldehyde solution in PBS for 15 min at rt. The cells were then washed 3 × 0.75 mL with PBS, and incubated with 410 μL of a 1:40 dilution of phalloidin – AlexaFluor 546 (Invitrogen-Molecular Probes, Carlsbad, CA) in PBS for 30 min in the dark. After washing 3 × 0.75 mL with PBS and 1 × 0.75 mL with distilled water, mounting medium containing DAPI (4’,6 diamidino-2-phenylindole) (Vectashield, Vector Labs, Burlingame, CA) was added and the slide was coverslipped and sealed with Cytoseal 60 (Richard-Allan Scientific, Kalamazoo, MI). Imaging was performed using a Zeiss LSM 510 Meta confocal microscope with a 63× objective (Carl Zeiss, Germany) using excitation wavelengths of 361 nm for DAPI, 488 nm for OVA-AF488, and 543 nm for phalloidin – AlexaFluor 546.
Animals
Male C57BL/6 mice and CD45.1 congenic C57BL/6 mice, 6 to 8 weeks of age, were purchased from the Jackson Laboratory (Bar Harbor, ME). The 1100Mjb mice (OT-I mice) express an H-2Kb restricted T-cell receptor specific for the octapeptide SIINFEKL (OVA257-264) (20). All mice were housed in the Stanford Animal Facility in accordance with NIH guidelines, and all procedures were approved by the Stanford Institution for Animal Care and Use (IACUC).
In Vivo T-Cell Proliferation Assay
Vaccinations with each particle type were normalized to 5 μg of ovalbumin per mouse. C57BL/6 mice (n=3) received subcutaneous flank injections of particles in 200 μL of PBS followed by intravenous adoptive transfer of 7.5 × 106 congenic OT-I T-cells 24 h later. The OT-I T-cells were labeled with 10 μM 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen, Carlsbad, CA) prior to adoptive transfer in order to follow their proliferation by CFSE dilution. Mice vaccinated with 5 μg of free ovalbumin or PBS alone served as controls. Three days after adoptive transfer, draining inguinal lymph nodes were harvested from vaccinated mice and single cell suspensions were stained with fluorochrome-conjugated antibodies against CD3 (BD Biosciences, San Jose, CA) and CD8 (BioLegend, San Diego, CA). The CFSE proliferation profile of gated CD3+ CD8+ T-cells was determined by flow cytometry.
In Vivo Cytotoxicity Assay
C57BL/6 mice (n=4, minimum) were vaccinated with each particle type normalized to 50 μg ovalbumin, with or without 10 μg CpG ssDNA (TCCATGACGTTCCTGACGTT, phosphorothioate backbone, Oligos, Etc., Wilsonville, OR), in a final volume of 200 μL PBS. At day 7 after vaccination, mice were injected intravenously with 1 × 107 CFSE-labeled target cells consisting of 50% SIINFEKL-pulsed splenocytes labeled with 5 μM CFSE and 50% unpulsed splenocytes labeled with 0.5 μM CFSE. Mice were tail vein bled 18 h after adoptive transfer and the CFSE profile of transferred cells was determined by flow cytometry for analysis of ovalbumin-specific cytotoxicity.
Endotoxin Testing
Particles were suspended at 5 mg/mL in PBS (pH 5) and degraded overnight at 37 °C while vortexing at 1200 rpm. The degraded samples were diluted to 1 mg/mL and 0.5 mg/mL in PBS (pH 7.4). The presence of endotoxin in the sample was quantified using an FDA-licensed colorimetric limulus amebocyte lysate assay cartridge with an Endosafe-PTS test system (Charles River Laboratories, Charleston, SC) with a detection range of 0.05 – 5 endotoxin units per mL (EU/mL). Endotoxin levels were measured at 0.14 EU/mg and less than 0.05 EU/mg (below the detection range of the assay) for the 3.5 μm and 35 nm particles, respectively. The presence of these levels of endotoxin have been found to result in minimal stimulation of immune cells (21, 22).
Statistical Data Analysis
In experiments where replicate samples were analyzed, the results are presented as the mean ± standard deviation unless otherwise noted. Comparisons of statistical significance were made using one-way analysis of variance combined with paired Student’s t-tests at a significance level of p < 0.05 after confirming equal variances with the Bartlett test.
RESULTS AND DISCUSSION
Nanoparticle synthesis and characterization
Reports in the literature have suggested that the size of particulate antigen carriers may influence vaccine efficacy in vivo, however, to the best of our knowledge there are no studies investigating this phenomenon in hydrogel systems. To this end, we sought to produce acid-labile polyacrylamide particles with diameters smaller than 100 nm. To achieve this goal, we investigated the possibility of polymerizing aqueous monomer-containing droplets formed in inverse microemulsions. The stable droplets formed under these conditions are typically less than 50 nm in diameter and upon polymerization yield swollen hydrogels less than 100 nm in diameter with relatively narrow size distributions. The synthesis of crosslinked polyacrylamide and polyacrylate hydrogel particles in this size range utilizing inverse microemulsions has been reported in the literature (23, 24), and the encapsulation of enzymes (25-27) in particles prepared by this technique has been demonstrated. However, in most cases the encapsulated species was intended to be retained within the particle structure, thus non-degradable crosslinking monomers such as N,N’-methylene-bis-acrylamide were generally used. In this report, we synthesized particles incorporating an acid-labile acetal crosslinker (1, Scheme 1) to afford release of the encapsulated protein at pH values encountered in the endolysosomal vesicles of antigen-presenting cells (~ pH 5) (17).
In the present study, we utilized this polymerization method to afford a good yield (ca. 60%) of particles approximately 20 nm in diameter in the dry state (Figure 1) and showing a size distribution of 32.6 ± 6.8 nm as measured by dynamic light scattering when suspended in PBS. Throughout the remainder of this report these will be referred to as 35 nm particles. A small population of particles (approximately 10%) was also observed with a size centered around 310 nm, likely due to the formation of aggregates. To determine the amount of the model protein (ovalbumin) loaded in the particles, samples were degraded in PBS (pH 5) overnight and the released protein was quantified using a bicinchoninic acid assay (28). The particles were found to contain 5.4 ± 0.1 % of ovalbumin by weight.
Figure 1.
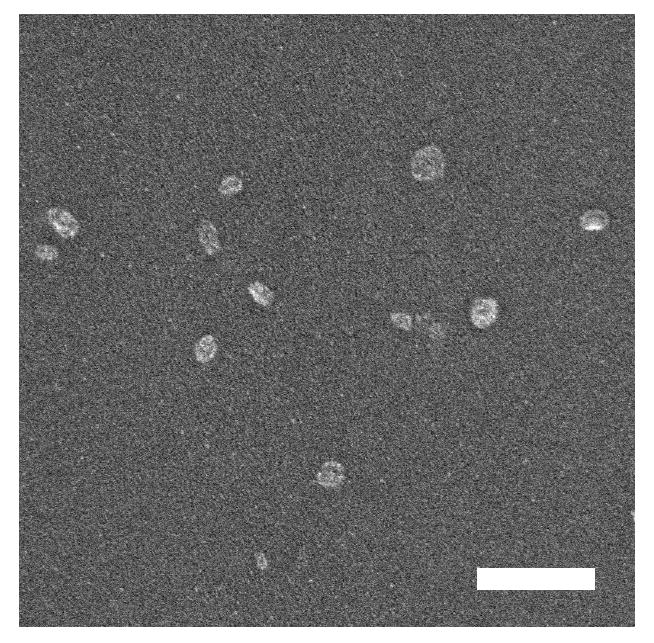
Representative SEM image of dried (unswollen), ovalbumin-loaded, 35 nm particles. The scale bar is equal to 200 nm.
Crosslinker Hydrolysis and Particle Degradation
To characterize the rate of crosslinker hydrolysis in the carrier particles, samples of 35 nm particles were suspended in deuterated phosphate buffers at pH 7.4 and pH 5 and 1H NMR spectra were acquired after various times. Figure 2a illustrates the general behavior of the particles upon exposure to acidic conditions. The resonance attributed to the benzylidene acetal proton of the crosslinker within the particles appears as a broad peak at 5.6 ppm. As the incubation time increases, the intensity of this peak diminishes along with the concomitant appearance of a sharp peak at 9.8 ppm corresponding to the proton of the benzaldehyde liberated during hydrolysis. The growth rate of the latter peak can be modeled using pseudo-first-order kinetics with respect to the crosslinker concentration (29) to yield a half-life of particle crosslink hydrolysis of 40 h at pH 7.4, compared to 10 min at pH 5 (Figure 2b). These values are roughly equivalent to hydrolysis rates reported for a similar crosslinker in monomeric form (30). Within 1 h, hydrolysis was essentially complete at pH 5, while at pH 7.4, hydrolysis progressed slowly over the course of 10 days (data not shown).
Figure 2.
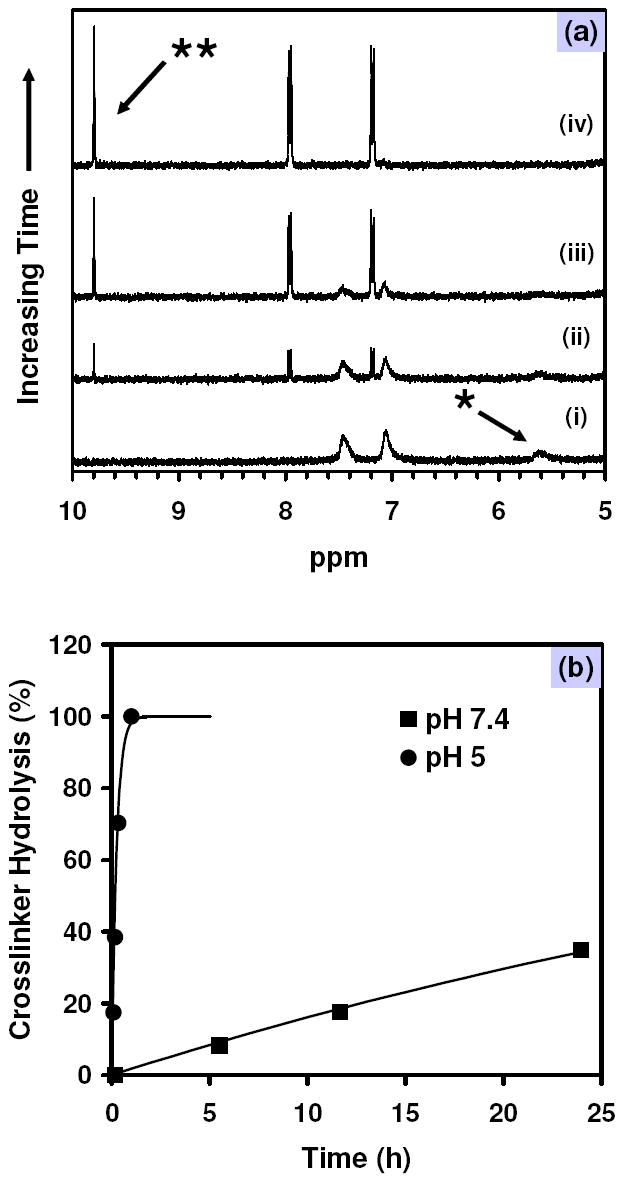
(a) 1H NMR spectra demonstrating the time-dependent hydrolysis of benzylidene acetal crosslinker (*) and release of the resultant aldehyde (**) from ovalbumin-loaded nanoparticles at pH 5 at (i) 0, (ii) 5, (iii) 20, and (iv) 60 min. (b) Crosslinker hydrolysis kinetics for particles hydrolyzed at pH 7.4 (■) and pH 5 (●). Solid lines represent pseudo-first-order hydrolsis models fit using the initial data points at each pH value.
A complementary view of particle stability as a function of pH was obtained using measurements of light scattering intensity for suspensions of the particles (Figure 3). The time required for the particles to fragment into linear polyacrylamide chains following crosslinker hydrolysis at pH 5 was approximately 3 h, as determined by a 90% decrease in scattering intensity. On the other hand, over the course of 8 h, no observable decrease in scattering intensity occurred at pH 7.4, and it took over 9 days for the scattering intensity to decrease 90% from its original value (data not shown). We confirmed that the decrease in scattering intensity observed in the protein-loaded particles at acidic pH was dependent on the presence of acid-labile crosslinks, as the scattering intensity of particles crosslinked with N,N’-methylene-bis-acrylamide showed no change in intensity at pH 5 over the same length of time (data not shown). Together, the NMR and light scattering data suggest that while the particle crosslinks hydrolyze relatively rapidly, overall particle degradation appears to be limited by disentanglement of the linear polymers that are formed as the result of crosslinker hydrolysis.
Figure 3.
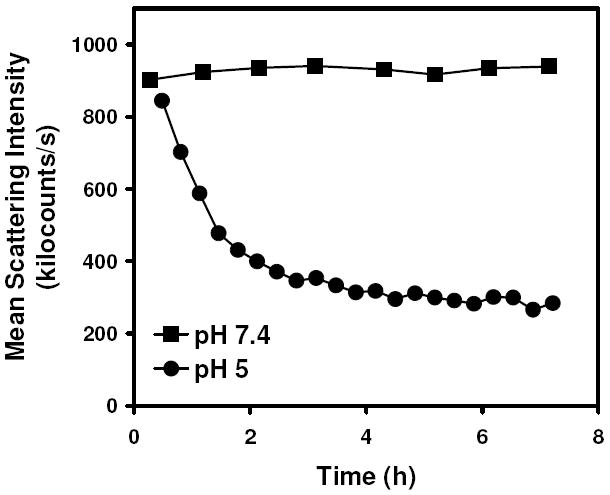
Fragmentation of ovalbumin-loaded nanoparticles as a function of time at pH 7.4 (■) and pH 5 (●) as observed by scattered light intensity measurements.
In Vitro Cytotoxicity and Uptake of 35 nm Diameter Particles
In order to assess the cellular toxicity of the 35 nm particles, MTT-based assays were performed using cell lines with both phagocytic (RAW 309 macrophages) and non-phagocytic (HeLa) phenotypes. Cells incubated with 35 nm particles demonstrated the very low toxicity of the particles (Figure 4), with approximately 75% and 85% viability retained at doses up to 2.5 mg/mL in RAW 309 and HeLa cells, respectively.
Figure 4.
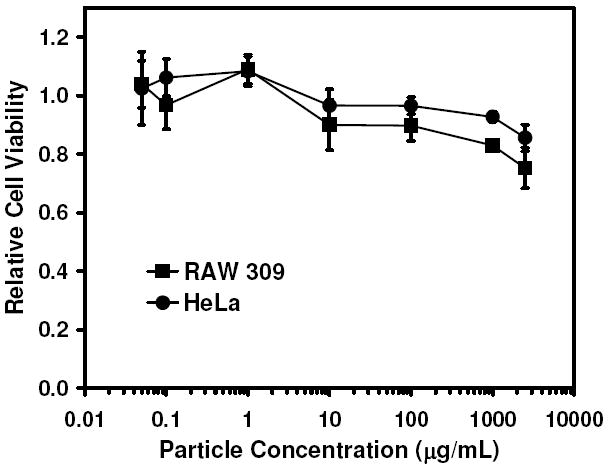
Evaluation of the cytotoxicity of ovalbumin-loaded 35 nm particles in phagocytic RAW 309 macrophages and non-phagocytic HeLa cells using an MTT-based assay.
As many non-antigen-presenting cells are capable of ingesting particulate material less than 200 nm in diameter, we compared the relative uptake of the 35 nm particles for both phagocytic (i.e., antigen-presenting cells) and non-phagocytic cells. The kinetics of particle uptake in RAW 309 macrophages was monitored in vitro by flow cytometry using acid-labile fluorescently-labeled 35 nm particles. A rapid increase in fluorescence intensity was initially observed, indicating cellular uptake of nanoparticles, with a plateau occurring at approximately 9 h (Figure 5). In contrast, non-phagocytic HeLa cells showed minimal uptake of the 35 nm particles during the same time frame with a 200-fold difference observed between the maximum uptake values for each cell type. The neutral, hydrophilic nature of the polyacrylamide-based particles may limit uptake of the particles by non-phagocytic mechanisms.
Figure 5.
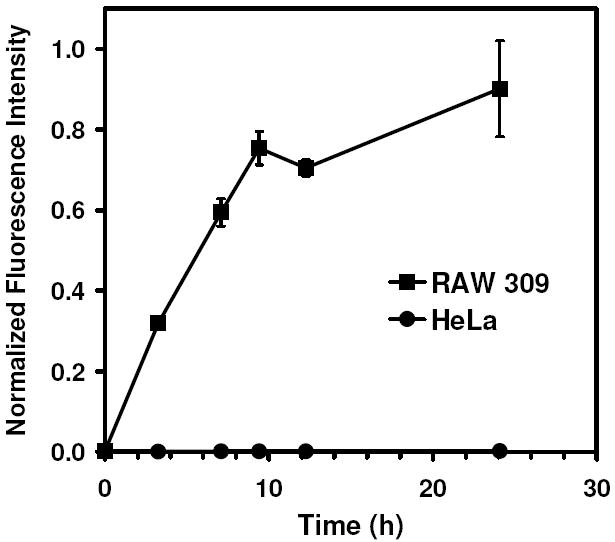
Comparison of the time-dependent in vitro cellular uptake of 35 nm particles encapsulating OVA-AF488 by phagocytic RAW 309 macrophages (■) and non-phagocytic HeLa cells (●) as measured by flow cytometry. Mean fluorescence channel values were averaged and normalized to the maximum observed value.
Particle uptake was also confirmed by confocal microscopy. After 8 h of incubation with 35 nm particles containing fluorescently-labeled ovalbumin, RAW 309 macrophages were fixed, stained, and imaged. As shown in Figure 6, fluorescence was observed throughout their cytoplasm. To ensure that the fluorescence was coming from within the cells, the focal plane was varied throughout the thickness of the cells. Fluorescence was observed as both punctate and diffuse signal. Although a definitive conclusion regarding protein release could not be reached from these data alone, the images may provide an indication that the diffuse signal may result from protein released from particles, while the punctate signal may result from protein entrapped in particles in the early stages of endocytosis prior to degradation (31). No fluorescence from within HeLa cells was observed when using the same microscope settings (data not shown), which is complimentary to the minimal uptake observed by flow cytometry.
Figure 6.

Confocal microscopy images of RAW 309 macrophages incubated with OVA-AF488-loaded 35 nm particles for 8 h followed by fresh medium for 16 h. (a) OVA-AF488 emission (green) and (b) merged with emission from actin stained with phalloidin-Alexa Fluor 546 (red), and nuclear DNA stained with DAPI (blue). The scale bar is equal to 10 μm.
Effect of Particle Size on T-cell Proliferation and Generation of Antigen-specific Effector T-cells
To assess the effect of particle size on the activation of T-cells in vivo, we prepared larger analogous acid-labile hydrogel particles by an inverse suspension polymerization method (14-17). Particles synthesized in this manner had a mean diameter of 3.67 ± 1.18 μm when suspended in PBS and are referred to as 3.5 μm particles in this report. These particles incorporated approximately the same mole percent of crosslinker (10%) and ovalbumin (7.3 ± 0.2 %), and were produced in ca. 40% yield. The 3.5 μm particles were comparable to the 35 nm particles in terms of their morphology as seen by SEM (Figure S1), their degradation characteristics, and their in vitro cytotoxicity (14). Additional experiments performed using particles containing fluorescently-labeled ovalbumin showed similar in vitro uptake of both sizes of particles in RAW 309 macrophages as confirmed by flow cytometry (Figure S2). Using confocal microscopy, we found that the intracellular appearance and distribution of both types of particles in macrophages were also similar (Figure S3). We therefore expected that differences in T-cell activation induced by these two types of particles would primarily be due to the effect of particle size.
The purpose of encapsulating ovalbumin within the particles was to exploit existing in vivo experimental models that rely on the presentation of the dominant, ovalbumin-derived, SIINFEKL epitope (OVA257-264) in the context of MHC I molecules (H-2Kb) on the surface of antigen-presenting cells. The first experiment we used to assess the activation of T-cells by the two sizes of particles examined the ability of each type of particle to induce T-cell proliferation. We have previously shown that ovalbumin-loaded pH-sensitive particles are capable of stimulating the proliferation of T-cells in vivo, even at relatively low ovalbumin concentrations (16). In the present study, C57BL/6 mice were injected with either 3.5 μm or 35 nm particles in quantities normalized to deliver 5 μg of ovalbumin. After 24 h, CFSE-labeled naïve OT-I T-cells, capable of specifically recognizing SIINFEKL/MHC I complexes, were adoptively transferred to each mouse. Three days later, draining lymph nodes were assessed for the fraction of OT-I cells that had undergone division in the interim as seen by a dilution of the CFSE signal present in the initially labeled OT-I cells. Figure 7 shows that both the 3.5 μm and the 35 nm particles were capable of stimulating OT-I cell proliferation with 57% and 68% of T-cells undergoing division, respectively, compared to mice injected with free ovalbumin, where only 41% of T-cells were found to be dividing. The level of T-cell division stimulated by 35 nm particles was greater than ovalbumin (p < 0.018), while 3.5 μm particles were not found to be more stimulatory than ovalbumin alone (p < 0.12). In addition, while the 35 nm particles appeared to be slightly more stimulatory than the 3.5 μm particles, the difference was not found to be statistically significant.
Figure 7.
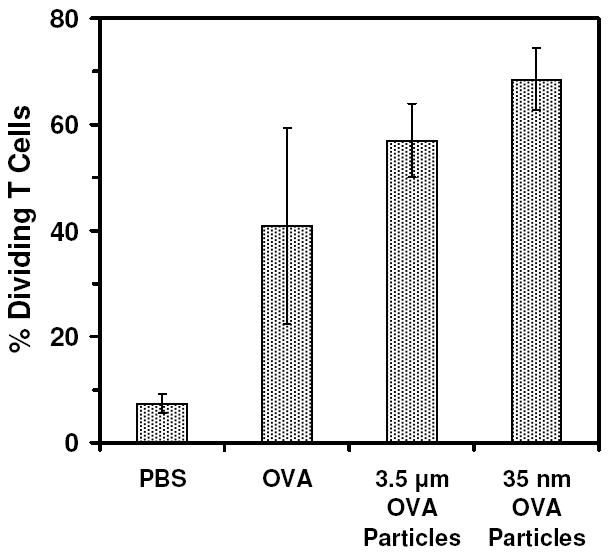
In vivo proliferation of SIINFEKL (OVA257-264)/H-2Kb specific CD8 T-cells in mice immunized with 3.5 μm and 35 nm ovalbumin-loaded particles. Data derived from CFSE profiles of gated CD3+ CD8+ T-cells isolated from lymph nodes of vaccinated mice 3 d after adoptive transfer.
The proliferation of T-cells stimulated by these acid-labile carriers, however, only provides a general perspective on the activation capacity of this particle system. More importantly, we wanted to determine if antigen-specific effector T-cells were being generated in vivo, as this would indicate whether or not the particle formulations would be capable of stimulating an effective CTL response necessary for vaccine-related applications. For this experiment, C57BL/6 mice were injected with either 3.5 μm or 35 nm particles in quantities normalized to deliver 50 μg of ovalbumin. After 7 days, a population of cells consisting of equal amounts of C57BL/6 mouse splenocytes either pulsed or not pulsed with SIINFEKL were injected into the mice. Prior to injection, the two cell types were labeled with either high (SIINFEKL-pulsed) or low (non-SIINFEKL-pulsed) concentrations of CFSE to enable tracking of each set of cells. After 18 h, the quantities of circulating CFSE-labeled cells were analyzed by flow cytometry (Figure 8). A decrease in the fraction of SIINFEKL-pulsed cells relative to the fraction of non-pulsed cells was taken to indicate the presence of antigen-specific effector T-cell cytotoxicity. Without co-administration of additional immunostimulatory compounds, mice injected with 3.5 μm particles alone were found to generate a greater cytotoxic response (46% specific target cell lysis) than ovalbumin alone (22% specific target cell lysis). On the other hand, the 35 nm particles alone did not seem to generate a significant cytotoxic response, with specific cell lysis roughly equivalent to both ovalbumin and PBS controls. It may be possible that the decreased response generated by the 35 nm particles relative to the 3.5 μm particles may be associated with a dilution of the injected dose that could occur if the 35 nm particles are indeed small enough to be convectively transported through the lymphatic capillaries draining the injection site. In contrast, due to their larger size, the 3.5 μm particles likely reside at relatively high concentration at the injection site. Elucidating the actual reason for the disparity between the cytotoxicity stimulated by the 3.5 μm and 35 nm particles requires additional study.
Figure 8.
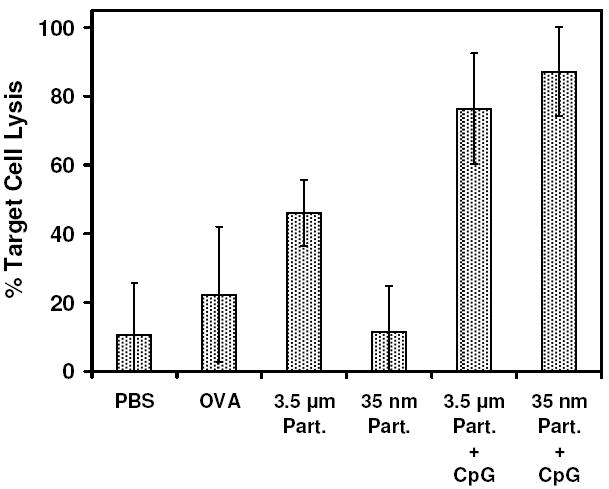
In vivo induction of T-cells capable of specific lysis of adoptively transferred SIINFEKL-pulsed splenocytes by 3.5 μm and 35 nm ovalbumin-loaded particles, both without and with co-administration of CpG oligonucleotide. The percentage of specific target cell lysis was determined by comparing remaining numbers of CFSE-labeled splenocytes pulsed with either SIINFEKL peptide or no peptide after adoptive transfer. Average and standard deviation of replicates (n=4 for PBS, n=5 for all others) is presented.
We have previously demonstrated that incorporating short, single-stranded oligonucleotide sequences containing unmethylated CpG dinucleotides into the structure of our polyacrylamide-based hydrogel system greatly enhances the activation of antigen-presenting cells and improves the overall immune response (16). In addition, it was demonstrated that a similar effect could be observed if the CpG-containing oligonucleotide was simply co-administered along with ovalbumin-loaded particles. As a result, these types of particle formulations represent a more relevant context for antigen delivery than using particles that encapsulate antigen alone. Thus, we sought to investigate the impact of particle size on T-cell activation in formulations where particles were combined with immunostimulatory DNA. For ease of manipulating the experimental conditions in this experiment such that particle size was the only factor varied, this latter method of co-administering the CpG oligonucleotide was chosen, and additional mice were administered with 10 μg of CpG DNA along with the 50 μg ovalbumin-equivalent of particles. Under these conditions, we found that both 3.5 μm and 35 nm particles were significantly more effective at generating antigen-specific cytotoxic T-cells compared to 3.5 μm particles administered without CpG (p < 0.0036 and p < 0.0002, respectively), with 76% and 87% specific target cell lysis, respectively (Figure 8). The small difference in target cell lysis between these two treatments was not found to be statistically significant.
Overall, the results of the in vivo tests of T-cell activation suggest that for our acid-labile polyacrylamide-based hydrogel carrier system, particle size does not appear to play a significant role in the generation of CTL responses. Both sizes of particles tested in this study demonstrated a high capacity for stimulating populations of antigen-specific cytotoxic T-cells. Why the behavior we observed differs from reports in the literature for hydrophobic carriers suggesting that smaller particles are more effective in this application requires additional study. In all of the experiments described in this study, we were unable to ascribe the immunostimulatory activity observed using either of the particle types to the presence of particle-associated endotoxin. It is possible that these differences in behavior to previously studied polystyrene and poly(propylene sulfide) systems may be related to the overall hydrophilicity of the carrier system or the site of immobilization of the protein antigen being delivered (i.e., encapsulated within the bulk of the carrier, or bound to the carrier surface), which may influence interactions of the particle system with phagosomal receptors in antigen-presenting cells. To this end, we plan to compare our polyacrylamide-based particle system side-by-side with polystyrene-based carriers to determine their relative stimulatory capacities.
CONCLUSION
In this study, we successfully synthesized and characterized acid-labile and protein-loaded polyacrylamide particles with sub – 100 nm diameters by utilizing a microemulsion-based polymerization technique. These nanoparticles were analyzed to determine their degradation characteristics and were found to be readily taken up by antigen-presenting cells of the immune system. We were able to investigate the effect of particle size on T-cell activation in vivo using this hydrogel system in comparison to larger analogous particles. In comparison to 3.5 μm diameter particles, 35 nm particles were found to be equivalent at inducing T-cell proliferation and antigen-specific cytotoxicity in vivo when co-administered with immunostimulatory DNA. These results suggest that for hydrophilic carrier systems, size may not be as critical of a factor in stimulatory capacity as in hydrophobic, surface-conjugated systems currently under evaluation. While the present work focused on the use of microemulsion-based hydrogels in immunological applications, we anticipate that these pH-sensitive particles with diameters less than 100 nm may also find use in other in vivo applications where their small size and inherently hydrophilic character could be advantageous. For example, we are currently evaluating these nanoparticles as targeted carriers of imaging and therapeutic agents for intravenous or pulmonary administration.
Supplementary Material
Comparison of particle morphology by SEM and comparison of in vitro particle uptake by flow cytometry and confocal microscopy. This material is available free of charge via the Internet at http://pubs.acs.org/BC.
Acknowledgments
We thank the National Institutes of Health (R01 EB005824) for funding of this research; additional NIH support (PEN Grant 1 U01 HL080729-01) led to the development of the 35 nm particles used in this study. We thank Ann Fischer and Michelle Yasukawa for help with cell culture studies and Paul Hudson for assistance with analysis of NMR spectra.
LITERATURE CITED
- 1.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bramwell VW, Perrie Y. Particulate delivery systems for vaccines. Crit Rev Ther Drug Carrier Syst. 2005;22:151–214. doi: 10.1615/critrevtherdrugcarriersyst.v22.i2.20. [DOI] [PubMed] [Google Scholar]
- 3.Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Delivery Rev. 2005;57:391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Reddy ST, Swartz MA, Hubbell JA. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 2006;27:573–579. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Heath WR, Belz GT, Behrens GMN, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 6.Reece JC, Vardaxis NJ, Marshall JA, Crowe SM, Cameron PU. Uptake of HIV and latex particles by fresh and cultured dendritic cells and monocytes. Immunol Cell Biol. 2001;79:255–263. doi: 10.1046/j.1440-1711.2001.01011.x. [DOI] [PubMed] [Google Scholar]
- 7.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. Efficient Major Histocompatibility Complex Class I Presentation of Exogenous Antigen Upon Phagocytosis by Macrophages. Proc Natl Acad Sci U S A. 1993;90:4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–4933. [PubMed] [Google Scholar]
- 9.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IFC, Plebanski M. Size-Dependent Immunogenicity: Therapeutic and Protective Properties of Nano-Vaccines against Tumors. J Immunol. 2004;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 10.Mottram PL, Leong D, Crimeen-Irwin B, Gloster S, Xiang SD, Meanger J, Ghildyal R, Vardaxis N, Plebanski M. Type 1 and 2 Immunity Following Vaccination Is Influenced by Nanoparticle Size: Formulation of a Model Vaccine for Respiratory Syncytial Virus. Mol Pharmaceutics. 2007;4:73–84. doi: 10.1021/mp060096p. [DOI] [PubMed] [Google Scholar]
- 11.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Controlled Release. 2006;112:26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 13.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of Phagocytic Monocytes into Lymph Node Dendritic Cells In Vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 14.Murthy N, Xu M, Schuck S, Kunisawa J, Shastri N, Frechet JMJ. A macromolecular delivery vehicle for protein-based vaccines: Acid-degradable protein-loaded microgels. Proc Natl Acad Sci U S A. 2003;100:4995–5000. doi: 10.1073/pnas.0930644100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon YJ, Standley SM, Goodwin AP, Gillies ER, Frechet JMJ. Directed Antigen Presentation Using Polymeric Microparticulate Carriers Degradable at Lysosomal pH for Controlled Immune Responses. Mol Pharmaceutics. 2005;2:83–91. doi: 10.1021/mp0498953. [DOI] [PubMed] [Google Scholar]
- 16.Standley SM, Mende I, Goh SL, Kwon YJ, Beaudette TT, Engleman EG, Frechet JMJ. Incorporation of CpG Oligonucleotide Ligand into Protein-Loaded Particle Vaccines Promotes Antigen-Specific CD8 T-Cell Immunity. Bioconjugate Chem. 2007;18:77–83. doi: 10.1021/bc060165i. [DOI] [PubMed] [Google Scholar]
- 17.Standley SM, Kwon YJ, Murthy N, Kunisawa J, Shastri N, Guillaudeu SJ, Lau L, Frechet JMJ. Acid-Degradable Particles for Protein-Based Vaccines: Enhanced Survival Rate for Tumor-Challenged Mice Using Ovalbumin Model. Bioconjugate Chem. 2004;15:1281–1288. doi: 10.1021/bc049956f. [DOI] [PubMed] [Google Scholar]
- 18.Franklin J, Wang ZY. Refractive Index Matching: A General Method for Enhancing the Optical Clarity of a Hydrogel Matrix. Chem Mater. 2002;14:4487–4489. [Google Scholar]
- 19.Hrubý M, Koňák Č, Ulbrich K. Poly(ethylene oxide)-coated polyamide nanoparticles degradable by glutathione. Colloid Polym Sci. 2007;285:569–574. [Google Scholar]
- 20.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T-Cell Receptor Antagonist Peptides Induce Positive Selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 21.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting Edge: Toll-Like Receptor 4 (TLR4)-Deficient Mice Are Hyporesponsive to Lipopolysaccharide: Evidence for TLR4 as the Lps Gene Product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 22.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaskova V, Stillhammerova M, Barton J. Inverse Microemulsion Polymerization of Acrylamide in the Presence of Bi-Unsaturated Vinyl Monomers. Chem Pap. 1994;48:355–361. [Google Scholar]
- 24.McAllister K, Sazani P, Adam M, Cho MJ, Rubinstein M, Samulski RJ, DeSimone JM. Polymeric Nanogels Produced via Inverse Microemulsion Polymerization as Potential Gene and Antisense Delivery Agents. J Am Chem Soc. 2002;124:15198–15207. doi: 10.1021/ja027759q. [DOI] [PubMed] [Google Scholar]
- 25.Birrenbach G, Speiser PP. Polymerized Micelles and Their Use as Adjuvants in Immunology. J Pharm Sci. 1976;65:1763–1766. doi: 10.1002/jps.2600651217. [DOI] [PubMed] [Google Scholar]
- 26.Daubresse C, Grandfils C, Jerome R, Teyssie P. Enzyme Immobilization in Nanoparticles Produced by Inverse Microemulsion Polymerization. J Colloid Interface Sci. 1994;168:222–229. [Google Scholar]
- 27.Munshi N, Chakarvorty K, De TK, Maitra AN. Activity and stability studies of ultrafine nanoencapsulated catalase and penicillinase. Colloid Polym Sci. 1995;273:464–472. [Google Scholar]
- 28.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 29.Kreevoy MM, Taft RW. The Evaluation of Inductive and Resonance Effects on Reactivity. I. Hydrolysis Rates of Acetals of Non-conjugated Aldehydes and Ketones. J Am Chem Soc. 1955;77:5590–5595. [Google Scholar]
- 30.Murthy N, Thng YX, Schuck S, Xu MC, Frechet JMJ. A Novel Strategy for Encapsulation and Release of Proteins: Hydrogels and Microgels with Acid-Labile Acetal Cross-Linkers. J Am Chem Soc. 2002;124:12398–12399. doi: 10.1021/ja026925r. [DOI] [PubMed] [Google Scholar]
- 31.Cohen JL, Almutairi A, Cohen JA, Bernstein M, Brody SL, Schuster DP, Fr, xe, and chet JMJ. Enhanced Cell Penetration of Acid-Degradable Particles Functionalized with Cell-Penetrating Peptides. Bioconjugate Chem. 2008;19:876–881. doi: 10.1021/bc700414j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of particle morphology by SEM and comparison of in vitro particle uptake by flow cytometry and confocal microscopy. This material is available free of charge via the Internet at http://pubs.acs.org/BC.


