Abstract
Photobiomodulation with near infrared light (NIR) provides cellular protection in various disease models. Previously, infrared light emitted by a low-energy laser has been shown to significantly improve recovery from ischemic injury of the canine heart. The goal of this investigation was to test the hypothesis that NIR (670 nm) from light emitting diodes produces cellular protection against hypoxia and reoxygenation-induced cardiomyocyte injury. Additionally, nitric oxide (NO) was investigated as a potential cellular mediator of NIR. Our results demonstrate that exposure to NIR at the time of reoxygenation protects neonatal rat cardiomyocytes and HL-1 cells from injury, as assessed by lactate dehydrogenase release and MTT assay. Similarly, indices of apoptosis, including caspase 3 activity, annexin binding and the release of cytochrome c from mitochondria into the cytosol, were decreased after NIR treatment. NIR increased NO in cardiomyocytes, and the protective effect of NIR was completely reversed by the NO scavengers carboxy-PTIO and oxyhemoglobin, but only partially blocked by the NO synthase (NOS) inhibitor L-NMMA. Mitochondrial metabolism, measured by ATP synthase activity, was increased by NIR, and NO-induced inhibition of oxygen consumption with substrates for complex I or complex IV was reversed by exposure to NIR. Taken together these data provide evidence for protection against hypoxia and reoxygenation injury in cardiomyocytes by NIR in a manner that is dependent upon NO derived from NOS and non-NOS sources.
INTRODUCTION
Light in the red to near infrared (NIR) range (630–1,000 nm) generated by using low energy laser or light-emitting diode (LED) arrays has been reported to have beneficial biological effects in many injury models. Such photobiomodulation has been observed to increase mitochondrial metabolism [1–4], facilitate wound healing [5–7] and promote angiogenesis in skin [5], bone [8], nerve [9] and skeletal muscle [10–13]. Despite its widespread therapeutic potential, the mechanisms responsible for the therapeutic actions of photobiomodulation by NIR have not been elucidated in detail.
Interestingly, beneficial effects of NIR were frequently observed in injuries caused after metabolically challenging mitochondria. NIR reversed the toxic effects of tetrodotoxin (TTX), a voltage-dependent sodium channel blocker and down regulator of cytochrome c oxidase (COX), and potassium cyanide (KCN), an irreversible inhibitor of COX, in primary neurons [6, 14]. NIR improved retinal function in an animal model of mitochondrial dysfunction caused by methanol-induced formate, a reversible COX inhibitor [15]. These in vitro and in vivo studies suggest that modulation of mitochondrial proteins having chromophore-containing groups such as COX plays an important role in photobiomodulation, particularly under conditions when mitochondria are metabolically challenged. On one hand, mitochondria have been recognized central to the development of ischemic injury. They play a role in apoptotic cell death, by releasing pro-apoptotic factors into the cytoplasm which activate caspases [16]. Apoptosis as well as necrosis contribute to tissue injury in myocardium following ischemia and reperfusion. Between 5 and 30% of cardiomyocytes undergo apoptosis in the rodent and human heart within 16 hours of reperfusion [17–19] and this trend persists for months [19–20]. On the other hand, mitochondria are potential sites for protection of the heart and other organs, as evidenced by their importance in ischemic or pharmacologic preconditioning and postconditioning [21–23].
In vivo studies have demonstrated protection of myocardium from ischemic injury by NIR. In an experimental model of myocardial infarction, Oron et al showed a profound effect of repetitive exposure of chronic infarcted myocardium in rats and dogs to low energy lasers (803 nm), resulting in a 50–70% reduction in infarct size 4–6 weeks after left descending coronary artery occlusion [24]. An upregulation in the expression of inducible nitric oxide synthase (NOS) and vascular endothelial growth factor was associated with cardioprotection and enhanced angiogenesis [25]. In most previous investigations with NIR treatments, light exposure was applied repeatedly over a relatively long time frame before and/or after stress causing injury. Similarities observed between the effects of postconditioning and NIR exposure encouraged us to test NIR treatments using protocols designed for postconditioning experiments. Interestingly, in an open-chest rabbit model, NIR (670 nm) provided powerful cardioprotection against ischemia and reperfusion injury when myocardium was exposed for 5 min at the onset of reperfusion. (P. Pratt, personal communication, a manuscript on these results is under submission). The goal of the present investigation was to characterize the effect of NIR on cultured rat and mouse cardiomyocytes undergoing hypoxia and reoxygenation, and establish the mechanism for protection of NIR against injury without confounding factors present in vivo.
MATERIALS AND METHODS
All experimental procedures and protocols used in this investigation were reviewed and approved by the Animal Care and Use Committee of the Medical College of Wisconsin, Milwaukee, Wisconsin. All conformed to the Guiding Principles in the Care and Use of Animals of the American Physiologic Society [26] and were in accordance with the Guide for the Care and Use of Laboratory Animals [27].
Culture of Neonatal Rat Ventricular Myocytes
Hearts from one-day-old Sprague-Dawley rats were excised, and the ventricular myocardium was cut into small pieces (~2 mm3) in isolation buffer (116 mM NaCl, 20 mM HEPES, 1 mM NaH2PO4, 5.5 mM glucose, 5.4 mM KCl, 0.8 mM MgSO4, 3 mg/ml phenol red, pH 7.35) with 0.15 mg/ml collagenase II (Worthington, Lakewood, NJ), 0.52 mg/ml pancreatin (Invitrogen, Carlsbad, CA) and incubated on a shaker at 37 °C for 20 min at 100 rpm. Tissue pieces were allowed to settle, and the supernatant was collected, suspended in 1 ml newborn calf serum (Invitrogen), and centrifuged at 1,000 rpm for 6 min. The cell pellet was resuspended in 1 ml newborn calf serum and stored at 37 °C. This procedure was repeated until all tissue was digested. The cells were then resuspended in plating medium consisting of Dulbecco’s modified essential medium (DMEM) supplemented with 17% medium 199, 10% horse serum, 5% fetal bovine serum (FBS), 0.5% penicillin–streptomycin, and 20 mm HEPES (pH 7.2) and incubated for 2 h on cell culture dishes to separate ventricular myocytes from the faster attaching non-myocytes. The ventricular myocytes in the supernatant were then collected and plated on gelatin-coated dishes and cultured in plating medium containing 5-bromo-2-deoxyuridine at a final concentration of 0.1 mM. Cells were used for experiments 48 to 72 h after isolation when demonstrating rhythmic contractions.
HL-1 Cells
HL-1 cells, a cardiac muscle cell line derived from the AT-1 mouse atrial myocyte tumor lineage, were a gift from William C. Claycomb, Ph.D. (Professor of Biochemistry and Molecular Biology, Louisiana State University Health Sciences Center, New Orleans, Louisiana), and maintained according to described protocols [28]. They where used for experiments when approximately 70 to 80% confluent.
Treatment of Cells
Rat neonatal myocytes or HL-1 cells were serum starved in DMEM for 12 h before experiments. In separate experimental groups, cells received no intervention (normoxia control, 95% air and 5% CO2) or were exposed to hypoxia and reoxygenation (HR) with or without NIR treatment. Hypoxia was produced by exposure to 5% CO2 and 95% N2 in an airtight chamber for 6 h in the presence of serum- and glucose-free DMEM containing 10 mM deoxyglucose to inhibit glycolysis. The O2 content during hypoxia was continuously monitored (Pro-Ox 110, Biospherix Ltd, Redfield, NY) and was not allowed to exceed 2% at any time. Reoxygenation was performed for 2 h in DMEM+10% FBS except if otherwise mentioned (analyses of cytochrome c release). For NIR treated groups, a LED array (25 cm×10 cm) with a peak wavelength of 670 nm (Quantum Devices Inc, Barneveld, WI) was placed beneath the culture dish. The full-width half-maximum of the emission spectrum of the LED array is 28 nm. NIR at 670 nm was administered for 5 min with incremental dose applications including 5, 25, and 50 mW/cm2 corresponding to a total energy density of 1.5, 7.5, and 15 J/cm2 [for lactate dehydrogenase (LDH) release and caspase 3 experiments] or with a single dose application of 25 mW/cm2 at a total energy density of 7.5 J/cm2 (in the other experiments). Exposure to NIR was performed immediately after hypoxia coincident with reoxygenation. One group of cells maintained in a normoxic environment (FiO2 0.21, 5% CO2) was also exposed to NIR. In some experiments, NO scavengers or a NOS inhibitor were applied before exposure to hypoxia. NO scavengers included 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxid (carboxy-PTIO, 100 μM, Calbiochem, Gibbstown, NJ) and oxyhemoglobin (oxyHb, 10 μM, prepared from fresh human blood according to a published method [29]) and as NOS inhibitor NG-monomethyl-L-arginine (L-NMMA, 1 mM, Alexis Biochemicals, San Diego, CA) was used. None of the NO scavenger exhibits any significant absorption at the applied wavelength of 670 nm. Sodium nitrite (150 nM) was added in the presence or absence of L-NMMA to test for a role of nitrite reductase activity of myoglobin.
Cell Viability
Cell viability was determined by LDH-release and MTT assays. LDH release into the cell culture medium was used as an indicator of membrane damage and measured spectrophotometrically according to the manufacturer’s instructions (Diagnostic Chemicals Limited, Oxford, CT) at a wavelength of 340 nm. Enzymatic activity was expressed as percent change from control. Cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay following the manufacturer’s protocol (Molecular Probes, Eugene, OR) [30].
Caspase 3 Assay
Cells were processed as directed for the caspase-3 colorimetric assay (R&D Systems, Minneapolis, MN) by harvesting in the lysis buffer provided and centrifugation at 14,000 g for 10 min. Caspase 3 activity was assayed in the supernatant using peptide based DEVD-pNA as substrate. The absorbance at 405 nm of the released pNA was monitored spectrophotometrically.
Annexin V Binding
Annexin V binds to phosphatidyl serine (PS), which appears in the outer leaflet of the plasma membrane in early apoptotic cells. Cells were washed with PBS and treated with FITC-labeled annexin V (0.2 μg/ml) for 20 min on ice according to the manufacturer’s protocol (BD Biosciences, San Diego, CA) and as previously described [31]. The labeled cells (10,000/sample) were analyzed by measuring fluorescence intensity using a FACScan flow cytometer (BD Bioscience, San Jose, CA) in conjunction with CellQuest software (BD Biosciences).
Cytochrome c Release
Release of mitochondrial cytochrome c into the cytosol was assessed as described previously [32]. For these experiments, reoxygenation time was increased to 6 h. Cells were lifted from incubation plates at the conclusion of each experiment, and the mitochondrial and cytosolic fractions were extracted and differentially centrifuged. Protein concentration was determined by the Biorad DC Protein Assay (Biorad, Hercules, CA). Equal amounts of cytosolic or mitochondrial protein (15 μg) were loaded on a polyacrylamide gel (Biorad Readygel Tris-HCl 4–20%). The lysates were resolved by electrophoresis (100 V for 1 h) and transferred onto a PVDF membrane (Biorad) as described. The membranes were treated with primary antibody for purified mouse anti-cytochrome c (BD Biosciences) overnight at 4 °C and washed 5 times before incubating with secondary antibody (1:2000) for 1 h. The protein bands were developed with chemiluminescence reagents (ECL, GE Healthcare, Piscataway, NJ). After stripping, protein loading was confirmed using antibodies against β–actin (1:2000, Sigma, Saint Louis, MO) for cytosol and cytochrome oxidase (COX) subunit I (Invitrogen) for mitochondria.
Measurement of Intracellular NO
These experiments examined whether exposure to NIR alters the generation of NO in neonatal myocytes and were performed without hypoxia and reoxygenation. After reaching 80% confluency, cells were washed and incubated with PSS (mM: NaCl 141, KCl 4.7, CaCl2 2.5, MgCl2 0.72, KH2PO4 1.7, NaHCO3 10, glucose 11, HEPES 10) for 2 h. Cells were then loaded with a fluorescent NO indicator, 4-amino-5 methylamino-2′,7′-difluorofluorescein diacetate (DAF, Invitrogen) [33] in PSS for 20 min at 37 °C. After loading, cells were washed with PSS three times before imaging. Images were acquired and captured with a Nikon Eclipse TE200 microscope equipped with a fluorescence attachment (Lambda DG-4, Sutter Instrument Corp, Novato, CA), using a Hamamatsu digital camera C4742-95. Fluorescence was monitored with excitation at 475 nm and emission at 510 nm using an FITC filter. The values of fluorescence intensity were quantified using the Metamorph version 6.2 software. Background fluorescence was measured in an area free of cells and subtracted from the fluorescence intensity of cells on the same image. Cells were subdivided into 6 groups: 1) Cells without treatment; 2) cells exposed to NIR as described above; 3) cells treated with carboxy-PTIO, 4) oxyHB, or 5) L-NMMA 10 min before loading DAF and exposure to NIR; 6) cells treated with NO donor DETA NONOate (1 mM, Alexis Biochemicals) as a positive control.
Oxygen Consumption
To assess the effect of NIR on mitochondrial function, mitochondria were isolated from rat heart as described previously [34]. Mitochondrial respiration was monitored with an oxygen electrode (Hansatech Instruments Ltd., Norfolk, United Kingdom) in respiration buffer containing (in mM): KCl 130, K2HPO4 5, MOPS 20, EGTA 2.5, Na4P2O7 0.001, 0.1% BSA (pH 7.2), and 1 g/L mitochondria. State 2 respiration was stimulated with the combination of pyruvate and malate (5 mM each) as substrates for complex I of the electron transport chain, or ascorbate (2.5 mM) and TMPD (0.25 mM) for complex IV. ADP-stimulated state 3 respiration was measured in the presence of 250 μM ADP, and state 4 respiration after added ADP was consumed. NIR exposure (50 mW/cm2 for 1 min corresponding to a total energy density of 3 J/cm2) was performed during state 4 respiration in the presence and absence of the NO-donor PAPA-NONOate (0.5 mM, corresponding to a final concentration of 4.6 μM detected electrochemically).
ATP Synthesis
Isolated mitochondria and permeabilized (1 μg digitonin/1×106 cells for 5 min) HL-1 cells were divided into two groups, one of which was exposed to NIR at 670 nm for 5 min (25 mW/cm2 corresponding to a total energy density of 7.5 J/cm2) and the other served as a control group without NIR exposure. Two additional HL-1 cell groups were exposed to HR with and without NIR exposure during reperfusion (as described above) before permeabilization and ATP production measurement. Mitochondrial ATP production rate was determined with a chemiluminescence-based method utilizing firefly luciferase and luciferin (Invitrogen), as described [35]. Reaction solution contained respiration buffer (as described above), diadenosine pentaphosphate (0.2 μM), pyruvate and malate (each 5 mM), 0.2 g/L protein, 0.1 g/L luciferine and 1.25 mg/L luciferase. The reaction was initiated by the addition of 30 μM ADP (made ATP-free by hexokinase treatment). Measurements in the presence of oligomycin (1 μg/ml) served as blank controls. Chemiluminescence was monitored in a Modulus Luminometer (Turner Biosystems, Sunnyvale, CA) at room temperature for 2 min. The standard curve was obtained with defined ATP concentrations, from which the rate of mitochondrial ATP production was calculated.
Statistical Analysis
All values are expressed as the means ± SEM. Comparisons between controls and treatments were analyzed by ANOVA followed by Tukey’s test. Comparisons between 2 groups for NO measurement were performed using a t-test. Values for p<0.05 were considered significant.
RESULTS
NIR decreases HR-induced cell death in rat neonatal cardiomyocytes and HL-1 cells
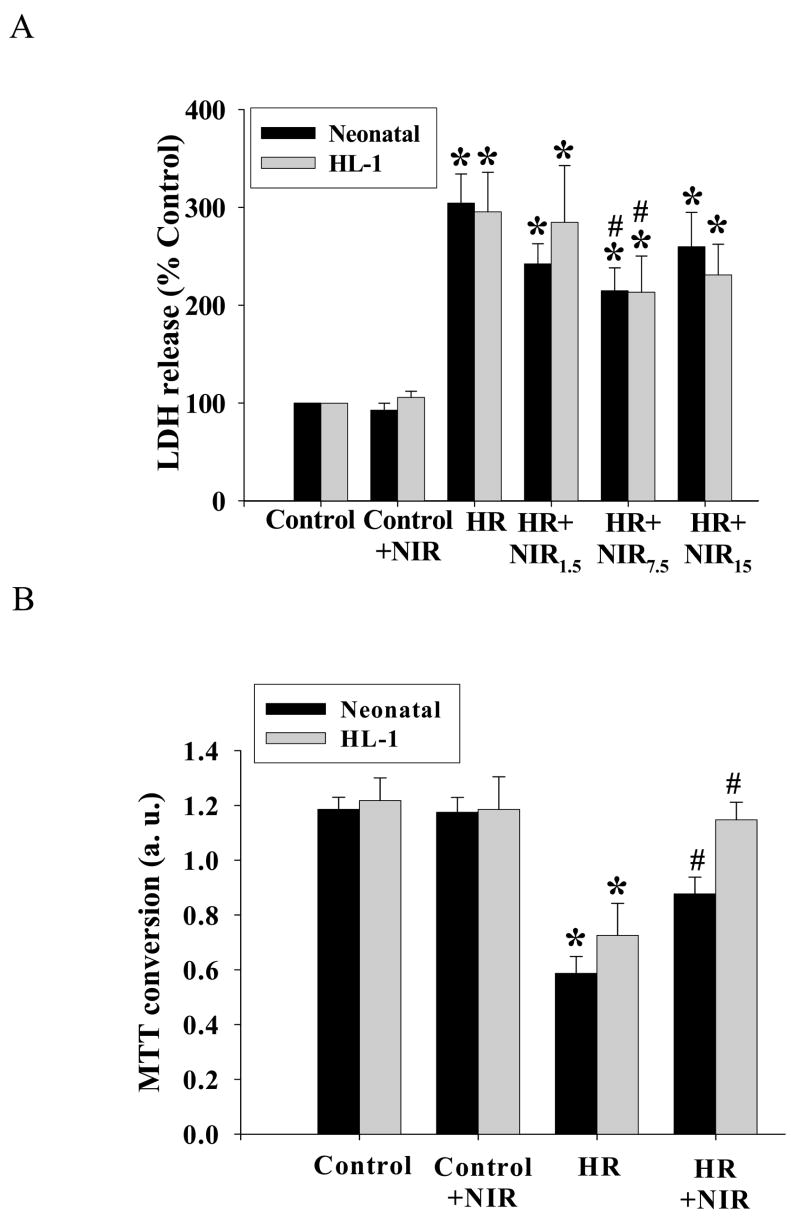
The release of cytosolic LDH is widely used as a marker of cell membrane injury. In rat neonatal cardiomyocytes NIR was administered after hypoxia in three incremental doses. HR produced a significant increase of LDH release into the cell culture medium (304±20% as compared to the normoxic control values). Following the treatment with NIR, there was a significant reduction in LDH release at the power intensity of 25 mW/cm2 (total energy density of 7.5 J/cm2; 215±23%), while energy densities of 1.5 J/cm2 and 15 J/cm2 were less effective (no significant difference from HR group). Similarly, in HL-1 cells, exposure to NIR (7.5 J/cm2) after hypoxia decreased LDH release as compared to untreated cells (214±37% vs. 296±40%), while lower or higher energy densities showed no significant effect (Figure 1A). NIR administered to the normoxic kept control cells at an energy density of 7.5 J/cm2 had no effect on the release of LDH in either cell type. These results indicated that the dose of 7.5 J/cm2 was optimal and was therefore used in further experiments unless otherwise indicated. The results obtained from the LDH release assay were confirmed with the MTT cell survival assay. Neonatal cardiomyocytes and HL-1 cells demonstrated increased survival rates [0.88±0.06 absorption units (a. u.) and 1.15±0.06 a.u.], close to normoxic controls (1.19±0.05 a.u. and 1.21±0.09 a.u.), when exposed to NIR (7.5 J/cm2) after hypoxia. This was in contrast to cells subjected to HR without NIR exposure (0.58±0.06 a.u. and 0.72±0.11 a.u. for neonatal cardiomyocytes and for HL-1 cells, respectively, Figure 1B).
Figure 1. NIR mitigates HR-induced cell injury.
Neonatal myocytes and HL-1 cells were maintained under normoxia (Control) or exposed to hypoxia and reoxygenation (HR) with or without NIR (1.5, 7.5, and 15 J/cm2 for LDH release, 7.5 J/cm2 for MTT assay) as described under Material and Methods section. NIR was administered for 5 min at the beginning of reoxygenation. LDH activity in medium (A) and MTT conversion to formazan (B) were assessed. Values for LDH activity were expressed as % control which was set as 100%, and for MTT assay as absorption units at 540 nm. * p<0.05 vs. control, # p<0.05 vs. HR, n=6 for LDH activity and n=4 for MTT assay.
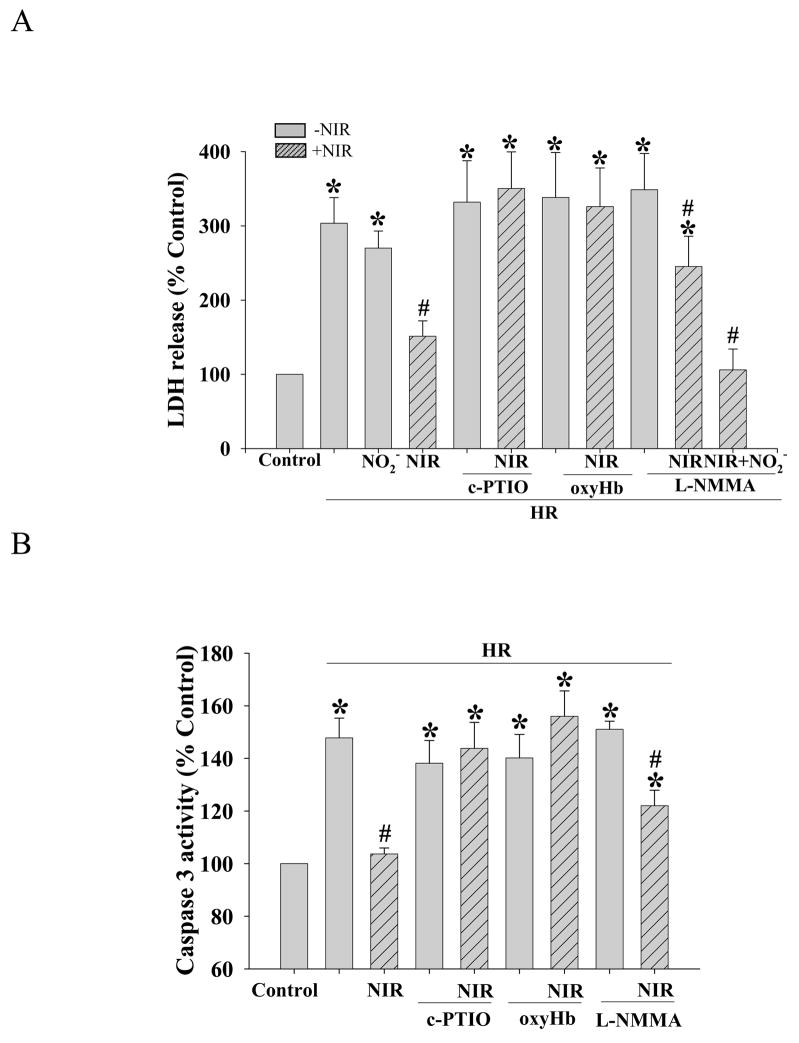
NIR protects rat neonatal cardiomyocytes and HL-1 cells against HR-induced apoptosis
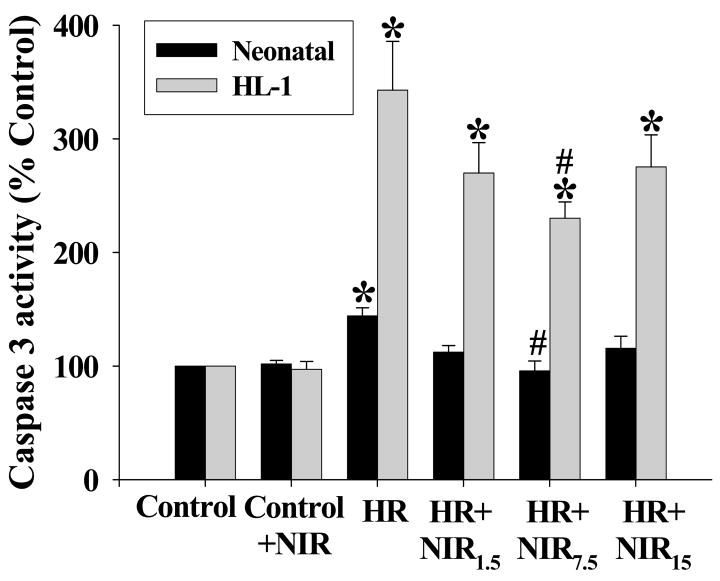
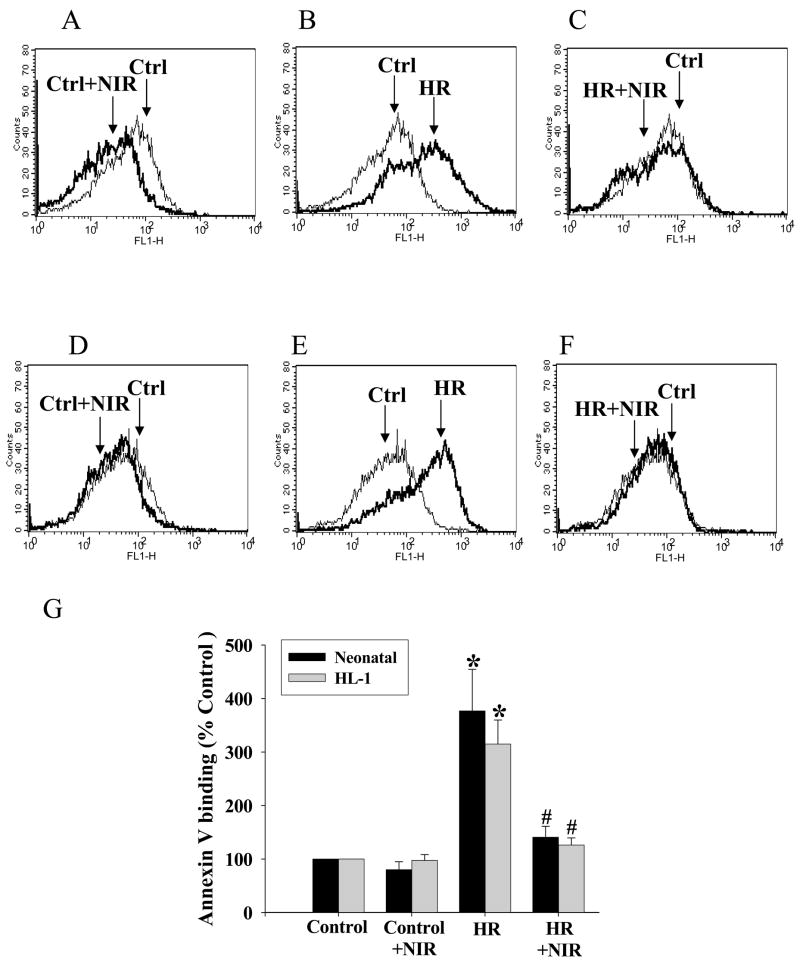
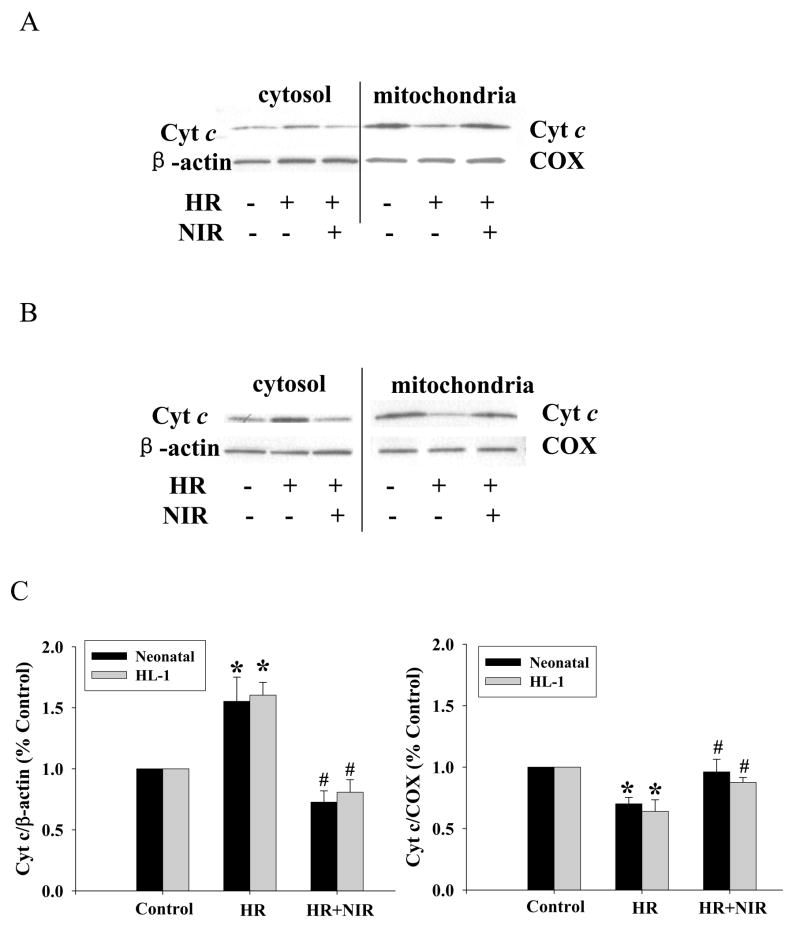
Caspase 3 activity plays a central role in the late stages of apoptosis. We tested the effects of three different energy densities of NIR administered during reoxygenation immediately after hypoxia in rat neonatal cardiomyocytes and HL-1 cells on apoptosis as determined by caspase 3 acitivty. Caspase 3 activity was increased after HR (144±7% and 343±43% for neonatal and HL-1 cells, respectively; relative to normoxic controls). There was a significant decrease in caspase 3 activity in both cell types exposed to NIR at an energy density of 7.5 J/cm2 for 5 min post-hypoxia (96±9% and 230±12% for neonatal and HL-1, respectively) but smaller decreases at lower or higher energy densities. This was similar to the trend observed with LDH release measurements (Figure 2). NIR itself had no effect on caspase 3 activity in either cell type treated under normoxic conditions. Binding of annexin V to phosphatidyl serine (PS) in the outer leaflet of the plasma membrane is an early apoptotic marker. NIR (7.5 J/cm2) did not change the number of annexin positive cells (neonatal cardiomyocytes and HL-1 cells) in normoxic control cells (Figure 3A, D). HR increased the number of annexin positive cells demonstrating apoptosis (377±78% for neonatal and 315±45% for HL-1 cells, relative to normoxic control; Figure 3B, E). NIR administered at the onset of reoxygenation reduced annexin positive cells in both cell types (141±20% and. 126±13% for neonatal and HL-1 cells, respectively; Figure 3C, F). Exposure to NIR protected cells from induction of apoptosis caused by HR, as indicated by the smaller number of annexin binding cells (Figure 3G). Mitochondrial cytochrome c release into the cytosol (another apoptotic index) was assayed by Western blotting of the mitochondrial and cytosolic fractions from control cells and cells after exposure to HR with or without NIR (7.5 J/cm2). In neonatal cardiomyocytes (Figure 4A) and HL-1 cells (Figure 4B), HR caused release of cytochrome c from the mitochondria into the cytosol, as observed by a more intense band in the cytosol and weaker signal in the mitochondria versus untreated cells. Exposure to NIR at the time of reoxygenation prevented cytochrome c release from mitochondria. Protein levels of β–actin in cytosolic and COX subunit 1 in mitochondrial fractions remained unaltered demonstrating equal protein loading. A summary of these data is presented in Figure 4C.
Figure 2. NIR reverses HR-induced increase in caspase-3 activity.
Neonatal myocytes and HL-1 cells were subjected to normoxia (Control) or HR with or without treatment with NIR (1.5, 7.5, and 15 J/cm2). Caspase-3 activity was measured using specific caspase substrate AcDEVD-pNA as described under Materials and Methods. Values of the absorption were expressed as % of control which was set as 100%. * p<0.01 vs. control, # p<0.01 vs. HR, n=6.
Figure 3. NIR decreases annexin V binding after HR.
Neonatal myocytes (A–C) and HL-1 cells (D–F) were subjected to normoxia or HR with or without treatment with NIR (7.5 J/cm2). Cells binding FITC-labeled annexin V were analyzed by flow cytometry as described in Materials and Methods. Graphs represent typical histograms comparing fluorescent intensity of control cells (traced in thin line) with control+NIR (A and D), HR (B and E) and HR+NIR (C and F) (traced in bold line). An increase in labeled cells after HR was noted, which was reversed by NIR. (G) Data summary illustrating % change of annexin V binding over control (100%). * p<0.01 vs. control, # p<0.01 vs. HR, n=4.
Figure 4. NIR mitigates HR-induced release of cytochrome c from mitochondria into cytosol.
Neonatal myocytes (A) and HL-1 cells (B) were maintained under normoxia or exposed to HR with or without NIR (7.5 J/cm2). The mitochondrial and cytosolic fractions were analyzed by Western blotting using an antibody against cytochrome c, and then, the membrane was reprobed using antibodies against β–actin or COX as loading controls for cytosol and mitochondria, respectively. The band intensities were determined by densitometry and the ratios of Cyt c/β–actin or Cyt c/COX in each sample calculated. These ratios were normalized to control. * p<0.01 vs. control, # p<0.01 vs. HR, n=5.
The protective effect of NIR is related to NO
The role of NO in the protective effect of NIR on neonatal cardiomyocytes was assayed by LDH release and caspase 3 activity in the presence of the NO scavengers carboxy-PTIO and oxyHb. Exposure to NIR (7.5 J/cm2) in the presence of carboxy-PTIO or oxyHb did not result in a significant reduction in LDH release (350±49% for carboxy-PTIO or 326±52% for oxyHb, relative to normoxic control; Figure 5A) and caspase 3 activity (144±10% or 156±10% for carboxy-PTIO and oxyHb, respectively; Figure 5B) after HR. This was in contrast to the findings obtained when experiments were conducted in the absence of NO scavengers (LDH release: 151±21%; caspase 3 activity: 104±2%). Experiments were also performed in the presence of the nonspecific inhibitor of all NOS isoforms, L-NMMA to test the involvement of NOS. Interestingly, L-NMMA attenuated the protective effect of NIR based on LDH release (245±41%) and caspase 3 activity (122±6%), but to a lesser degree than the NO scavengers carboxy-PTIO and oxyHb. Addition of 150 nM sodium nitrite together with L-NMMA restored the NIR induced protection reducing LDH release to control level (106±28%), suggesting a role of nitrite reductase activity of myoglobin. NO scavengers, L-NMMA and sodium nitrite alone did not alter LDH release and caspase 3 activity. Thus, NO appears to be necessary for the protective effect of NIR, and may only partially be derived from NOS.
Figure 5. NO scavengers blunt the protective effect of NIR.
Neonatal myocytes were subjected to HR with or without treatment with NIR (7.5 J/cm2) in the presence of NO scavengers carboxy-PTIO or oxyHb, or NOS inhibitor L-NMMA. (A) LDH release into the medium and (B) caspase 3 activity were measure as described under Material and Methods. NO scavengers abolished protective effect of NIR completely and L-NMMA only partially. Addition of sodium nitrite (NO2−) in the presence of L-NMMA restored the NIR induced protection to control LDH-release values. Values for LDH or Caspase 3 activity were expressed as % of control which was set as 100%. * p<0.01 vs. control, # p<0.05 vs. HR (no intervention), n=6.
NIR increases NO, partially independent of NOS
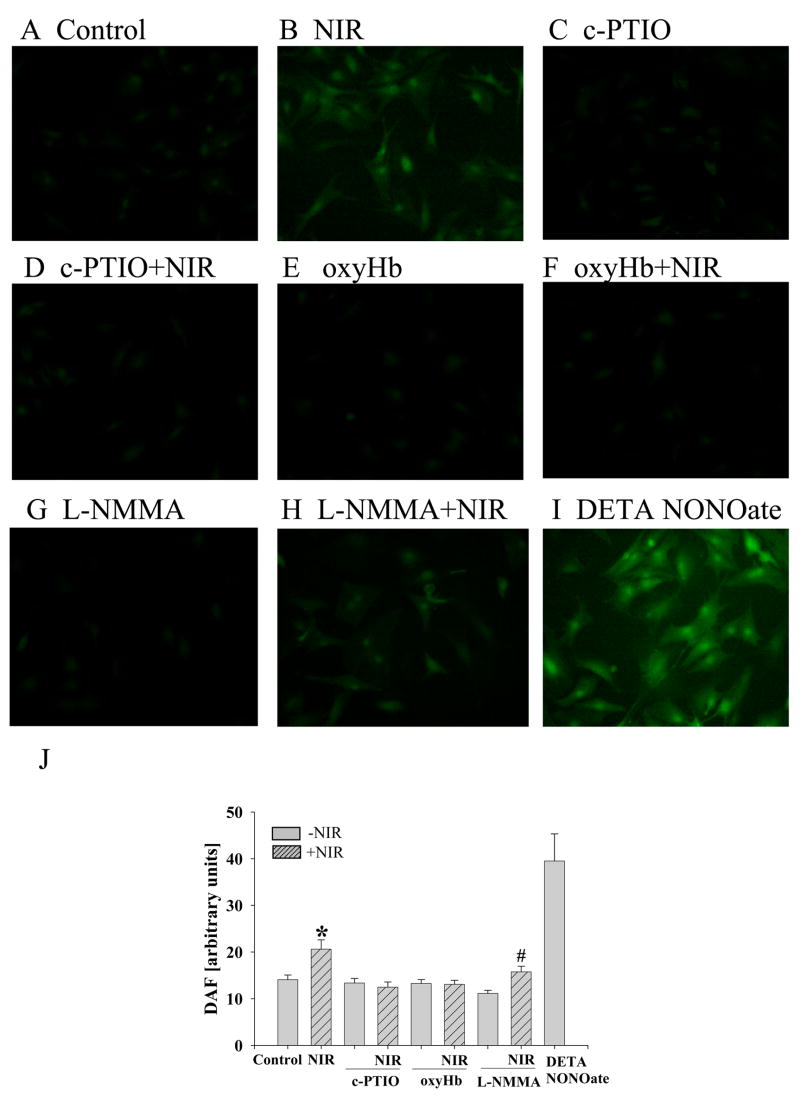
DAF fluorescence allows detection, bioimaging and quantification of NO with nanomolar sensitivity [36]. Compared to unexposed neonatal myocytes (14±1 arbitrary units A.U., Figure 6A), exposure to NIR (7.5 J/cm2) enhanced fluorescence intensity (21±2 A.U.), indicating increased NO release (figure 6B). Pretreatment with the NOS scavengers carboxy-PTIO and oxyHb completely abolished the increase in DAF fluorescence (12±1 A.U. and 13±1 A.U., Figure 6D and F) produced by NIR, while the NO scavengers had no effect in the absence of NIR (13±1 A.U. and 13±1 A.U., Figure 6C and E). L-NMMA attenuated the NIR-stimulated increase in DAF fluorescence intensity, but to a lesser extent than the NO scavengers (16±1 A.U., Figure 6H). L-NMMA had no effect on cells without NIR treatment (11±01 A.U., Figure 6G). These findings suggest that NIR released NO partially independent of NOS. Figure 6I illustrates the effect of the NO donor DETA NONOate on DAF fluorescence as a positive control. A summary of the data is presented in Figure 6J.
Figure 6. NIR increases NO level in cardiomyocytes.
The release of NO was measured after preloading cells with NO-indicator DAF-FM by fluorescence microscopy. Fluorescent images were examined 5 min after dye loading and washing-out (A) in untreated cells (control), or after exposure to (B) NIR (7.5 J/cm2), (C) c-PTIO, (D) c-PTIO+NIR, (E) oxyHb, (F) oxyHB+NIR, (G) L-NMMA, (H) L-NMMA+NIR and (I) DETA NONOate as positive control. (J) Data summary representing the average of DAF-FM fluorescence intensity (arbitrary units). * p<0.01 vs. control, # p<0.05 vs. NIR and L-NMMA without NIR, n=11–14 fields per treatment.
NIR reverses NO inhibition of oxygen consumption
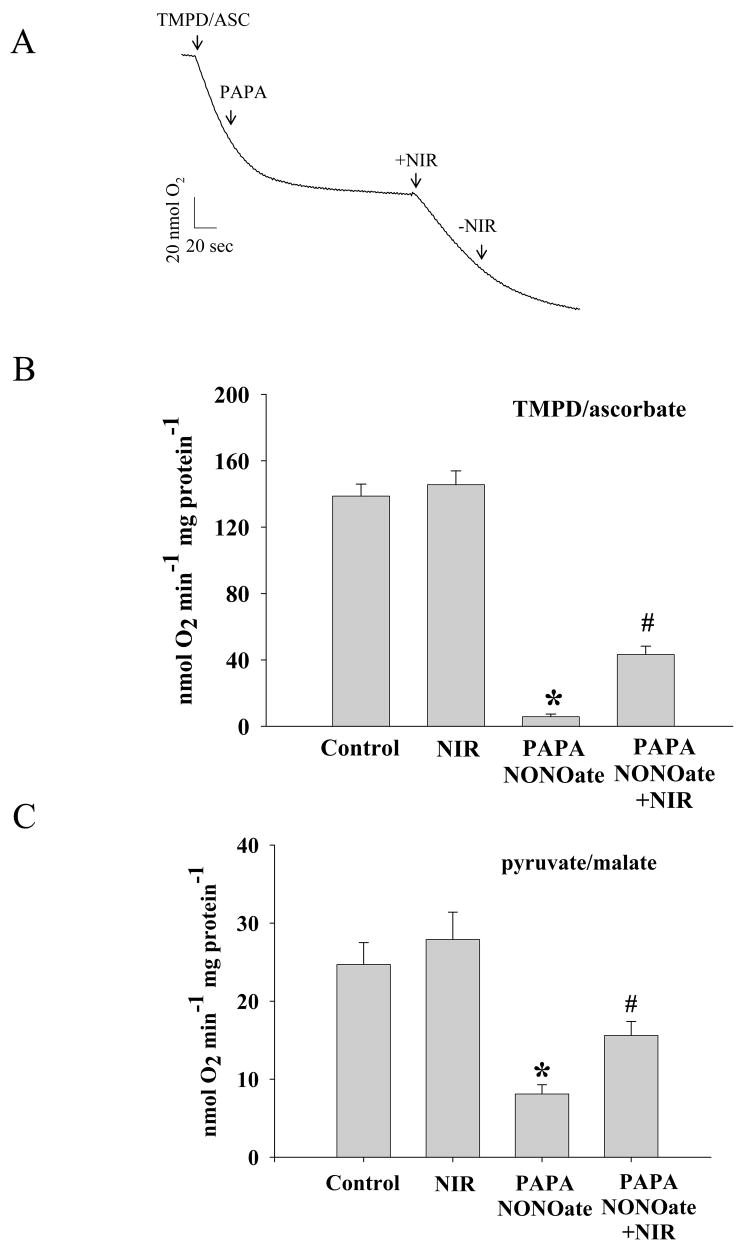
Electron transport chain complexes within mitochondria can absorb NIR, and cytrochrome c oxidase (complex IV) has been shown to be stimulated by NIR. Furthermore, NO binding to iron and copper sites within complex IV inhibits its activity [37]. Figure 7A shows the typical tracing of oxygen consumption by isolated mitochondria in the presence of TMPD and ascorbate as the substrates for complex IV. Isolated mitochondria were exposed to NIR in the presence and absence of NO donor PAPA NONOate, while their oxygen consumption was followed using TMPD and ascorbate (Figure 7B) or pyruvate and malate (Figure 7C) as substrates for complex IV and complex I, respectively. Addition of PAPA NONOate inhibited oxygen consumption significantly (5.8±1.6 vs. 138.7±7.2 nmol O2 min−1 mg protein−1 for TMPD and ascorbate, and 8.1±1.2 vs. 24.8±2.9 nmol O2 min−1 mg protein−1 for pyruvate and malate), but this inhibition was partially reversed when mitochondria were treated with NIR (43.4±5.0 nmol O2 min−1mg protein−1 for TMPD and ascorbate and 15.6±1.8 nmol O2 min−1mg protein−1 for pyruvate and malate). Thus, while NIR had no measurable effect on oxygen consumption in the absence of NO, it was able to partially reverse the inhibitory effect of NO on complex IV.
Figure 7. NO-induced inhibition of oxygen consumption is reversed by NIR.
(A) Recording of oxygen consumption of isolated mitochondria during state 4 respiration using TMPD/ascorbate (for complex IV) as substrate. As indicated with arrow, NO donor PAPANonoate was added and mitochondria exposed to NIR (3 J/cm2) in the respiratory chamber. (B) and (C) Data summary for oxygen consumption with TMPD/ascorbate (B) and pyruvate/malate (C) as substrates. * p<0.01 vs. control, # p<0.01 vs. PAPA NONOate, n=5.
ATP synthesis
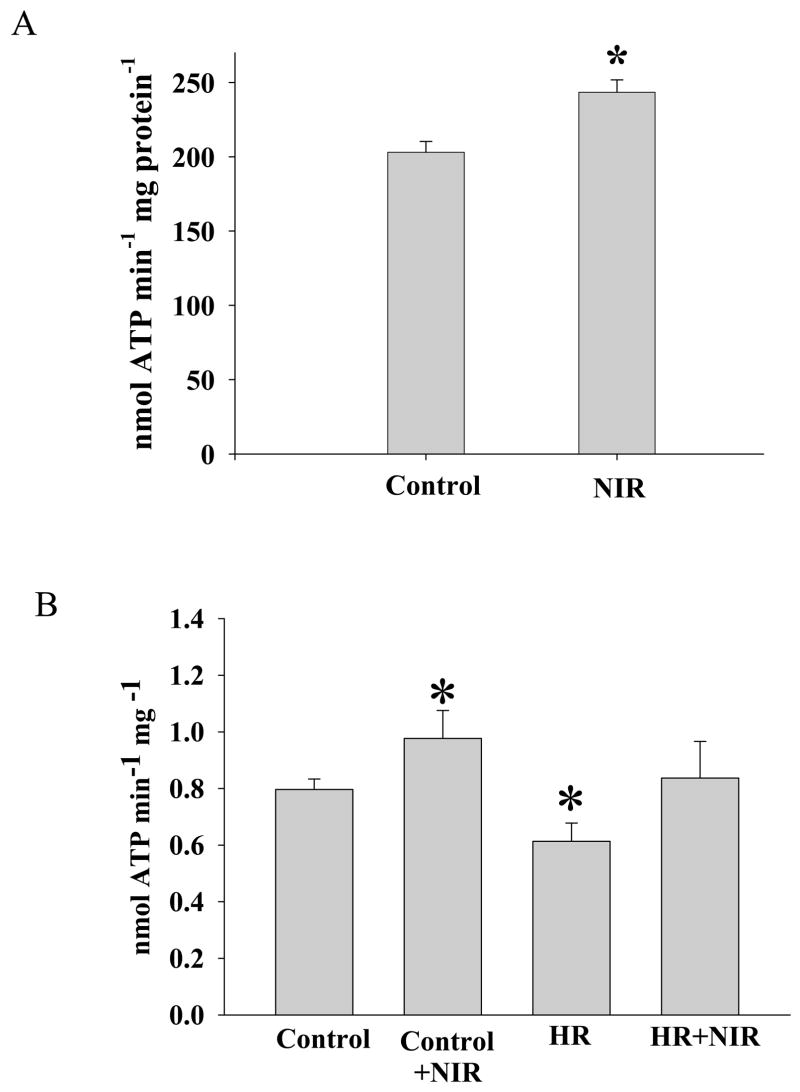
The effect of NIR on mitochondrial ATP synthesis was analyzed. When isolated cardiac mitochondria were exposed to NIR (7.5 J/cm2), the rate of ATP production increased significantly (243±8 nmol ATP min−1mg protein−1) as compared to control mitochondria not exposed to NIR (203±7 nmol ATP min−1mg protein−1) (Figure 8A). Similarly, cellular ATP synthesis of permeabilized HL-1 cells was increased after NIR exposure (1.06±0.17 vs. 0.80±0.06 nmol ATP/min/mg protein). After HR, mitochondrial ATP synthesis was reduced in HL-1 cells (0.57±0.06 nmol ATP/min/mg protein), and this effect was reversed by NIR treatment at the onset of reoxygenation (0.83±0.12 nmol ATP/min/mg protein).
Figure 8. NIR exposure increased mitochondrial ATP synthesis.
ATP production in (A) isolated mitochondria and (B) permeabilized HL-1 cells was quantified with a luciferase-based assay after exposure to NIR (7.5 J/cm2) or without light exposure (control). Cells were also exposed to HR with and without NIR treatment. * p<0.01 vs. control, n=4.
DISCUSSION
Recently, there has been increased interest in the diverse therapeutic effects of NIR [38], but the mechanisms for this photobiomodulation are not well understood. The present investigation characterizes mechanisms of NIR that may be of practical therapeutic benefit in the treatment of coronary artery desease. We demonstrated in cultured cardiomyocytes that exposure to NIR at the onset of reoxygenation following hypoxia enhances cell survival and attenuates apoptosis. Wavelength and energy doses of NIR selected for this study were comparable to those previously shown to be effective in in vivo experiments [13, 15, 25]. Further, we discovered that the protective effect of NIR was dependent on the presence of NO. NIR treatment produced increases in intracellular concentrations of NO, while NO scavengers abolished the protective effect of NIR. Interestingly, the increase in intracellular NO could only partially be blunted by pharmacological inhibition of NOS. Thus, NO seems to be released by at least two mechanisms, NOS and a second unidentified source. While the origin of NIR-induced NO was not examined in detail in this investigation, nitrite reductase activity of myoglobin seemed to participate in the production of NO. Exposure of mitochondria to NIR reversed the inhibitory effect of NO on mitochondrial oxygen consumption using substrates for COX. Several cardiac proteins, including myoglobin and COX [39] are known to bind NO, suggesting heme-carrying proteins as a potential NO donor in response to NIR. Finally, mitochondrial ATP synthesis was enhanced after NIR irradiation of mitochondria.
Ischemia and reperfusion of myocardium alters mitochondrial function directly or indirectly leading to necrosis or apoptosis [40, 41]. Disruption of the balance between pro-apoptotic factors and anti-apoptotic intracellular intermediates triggers mitochondria to produce apoptosis. Light in the NIR range has previously been shown in a variety of animal models to exhibit beneficial effects on the repair processes after ischemia and reperfusion in skeletal and cardiac muscle when applied repetitively after the injury over several days [13]. This observation in chronically treated animals was extrapolated to acute hypoxia and reoxygenation injury as an in vitro model for ischemia and reperfusion injury. Whether the coherence of laser radiation has benefits as compared to the monochromatic light from a LED with same wavelength and intensity is widely discussed. Coherent and noncoherent light produce the same biological effect on a cell monolayer or tissue surface, but additional effects of coherent light may manifest in deeper layers of tissue [42]. Light exposure in this study was accomplished with a LED array (670 nm) that has advantages over lasers including less risk of creating heat and the capacity to cover larger areas [43]. Only a single 5 min LED treatment at the onset of reoxygenation was chosen based on recent results that a 5 min application of NIR promoted cardioprotection from ischemia and reperfusion injury in vivo (P. Pratt, personal communication). Cardioprotection by NIR is analogous to the effect of ischemic or pharmacologic postconditioning, where brief episodes of ischemia or exposure to drugs or anesthetics such as isoflurane during early reperfusion after coronary occlusion reduce the extent of myocardial infarction [44, 45].
Mitochondria not only play an important role in the genesis, but also the prevention of cardiac tissue injury during IR [46, 47]. Mitochondria have been identified as trigger and effector organelles in cardioprotection by ischemic and pharmacologic preconditioning and postconditioning [46]. COX (complex IV) is the terminal component of the electron transport chain, oxidizing its electron donor cytochrome c and reducing oxygen to water. It has been recognized as a photoacceptor in the NIR range with absorption peaks at 680, 760 and 820 nm, and implicated to be directly involved in photobiomodulation [14, 15, 48, 49]. NIR at 670 and 830 nm partially reversed COX activity in primary neurons after inhibition with KCN, an inhibitor of complex IV. These actions resulted in preserved cellular ATP content and decrease in neuronal death [14].
NO has a wide range of physiological and pathological roles as an intracellular or intercellular messenger, including effect on mitochondria [50–52]. NO affects cellular decisions of life and death either by activating or inhibiting apoptotic pathways, depending on concentration, localization, cellular redox state and overall pro-versus anti-apoptotic state of the cell. [53, 54]. Pro-apoptotic pathways of NO are compatible with established signaling pathways associated with mitochondrial-mediated cell death. Anti-apoptotic actions of NO range from an immediate interference with pro-apoptotic signaling cascades, to long-lasting effects based on expression of cell protective proteins [55, 56]. In our experiments, NO scavengers carboxy-PTIO or oxyHb, or the nonspecific NOS inhibitor L-NMMA did not cause further increase in LDH release and caspase 3 activity after HR. This suggests that endogenous NO may not contribute to protection against HR in isolated cardiomyocytes in the absence of NIR. However, the protective effect of NIR exposure against cell injury (LDH release) and apoptosis (caspase 3 activity) was completely abolished by the NO scavengers, and partially by L-NMMA. In agreement with these data, pretreatment with L-NMMA reduced the NIR stimulated increase in endogenous fluorescence of intracellular DAF only partially while NO scavengers completely eliminated it. A low dose of sodium nitrite which was not protective itself completely restored the NIR-induced cellular protection in the presence of L-NMMA, indicating a possible role of nitrite reductase activity of myoglobin [57]. These findings demonstrate that the enhanced release of NO after exposure to NIR may be only partly attributable to activation of NOS, suggesting that NOS-independent sources of NO are important in protection against HR injury.
Both endogenous and exogenous sources of NO modulate mitochondrial function [58]. There is evidence that NO can be produced within mitochondria by a mitochondrial NOS in various tissue types, including the heart [59–61], although there is still considerable controversy regarding the existence and role of such an enzyme [62–64]. The reversible inhibition of the electron transport chain via binding of NO to COX is well characterized, although the physiological relevance of this effect is still under investigation [37, 65]. The generation of NO from nitrite through deoxymyoglobin may also inhibit mitochondrial oxygen consumption and has been suggested to increase myocardial viability through this regulation of mitochondrial metabolism [57, 66].
NO-induced inhibition of COX may regulate the formation of hydrogen peroxide from the respiratory chain for the purposes of signal transduction and controls O2 gradients in complex organs such as the liver or heart [67]. We did not observe a direct effect of NIR exposure on mitochondrial respiration (state 3) in the absence of NO donors, although baseline ATP synthesis of cardiac mitochondria was modestly increased after directly irradiating mitochondria with NIR. This is consistent with the well described benefit of NIR in increasing mitochondrial metabolism [1–4]. In agreement with better cellular survival after HR ATP production was also better preserved in NIR exposed cells, though it is not clear whether this is due to better cellular survival or due to a direct effect of NIR on mitochondrial metabolism. The reversal of NO-induced inhibition of complex IV-dependent respiration by NIR might be explained by light-induced conformational changes resulting in release of NO and thus increasing the respiratory function of mitochondria. However it should be noted that, primarily, the measurements of respiration in the presence of NO-donors served as proof of principle for potential effects NIR can have on heme-carrying proteins. In fact, under physiological conditions, NO released or produced after NIR induction from other sources may have a mild inhibitory and protective effect on the respiratory chain [66]. The released NO may participate in anti-apoptotic processes by activating prosurvival signaling pathways (Figure 9), in agreement with our data indicating improved cell survival and reduced apoptosis after exposure to NIR. Our data confirm the feasibility of NO binding to COX and NIR-induced NO release, but other proteins not examined in this study such as hemoglobin or myoglobin are well suited candidates that may have similar actions.
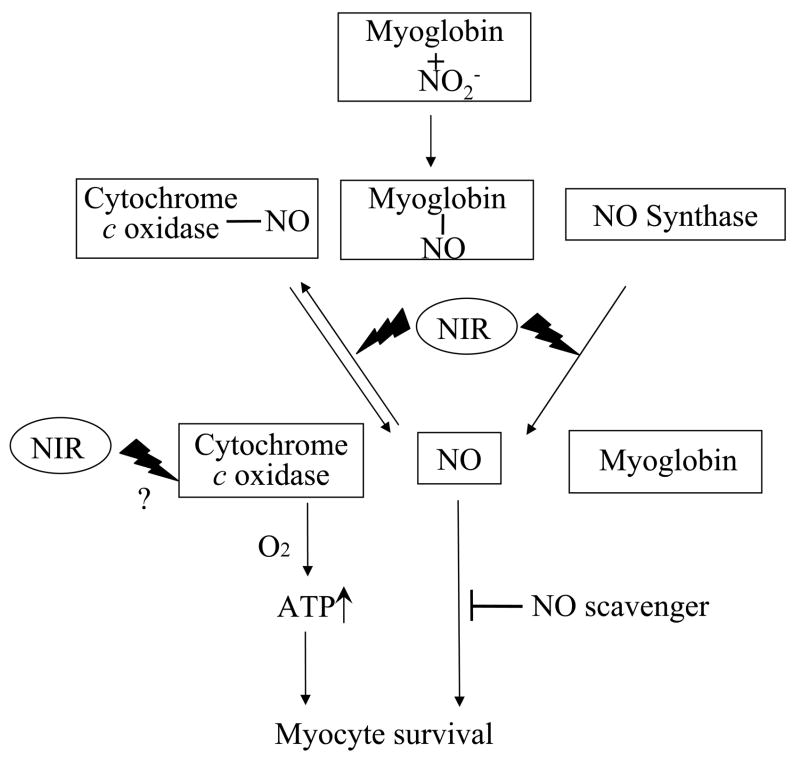
Figure 9. Diagram of suggested mechanism of NIR effect on cardiomyocytes.
NO, bound under hypoxic conditions to COX or myoglobin can be released after exposure to NIR, in addition to possibly enhanced synthesis of NO by NOS and myoglobin. COX activity and thus, mitochondrial metabolism are increased after NIR-exposure, and in addition, NO stimulates cell survival and inhibits apoptosis.
In summary, the present study demonstrates that treatment with NIR protects cardiomyocytes from HR injury. This protection is dependent on NO generated not only from NOS, but also from another source of NO, possibly COX. Future improvement in delivery of NIR could represent a noninvasive and non-pharmacological therapeutic means to protect against myocardial ischemia and reperfusion injury.
Acknowledgments
Financial Support: This study was supported in part by NIH (HL069996 (MM), HL49294 (ERJ), HL68627 (ERJ), HL054820 (DCW), GM066730 (DCW), and the Department of Anesthesiology, Medical College of Wisconsin.
The authors thank Ms. Chiaki Kwok, MS (Department of Anesthesiology, Medical College of Wisconsin, Milwaukee, WI) and Stephanie K. Gruenloh (Department of Pulmonary and Critical Care Medicine, Medical College of Wisconsin, Milwaukee, WI) for technical assistance.
Footnotes
Conflict of Interest: The authors have no conflicts of interest pursuant to the current work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Passarella S, Casamassima E, Molinari S, Pastore D, Quagliariello E, Catalano IM, Cingolani A. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett. 1984;175:95–9. doi: 10.1016/0014-5793(84)80577-3. [DOI] [PubMed] [Google Scholar]
- 2.Karu T, Pyatibrat L, Kalendo G. Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B Biol. 1995;27:219–23. doi: 10.1016/1011-1344(94)07078-3. [DOI] [PubMed] [Google Scholar]
- 3.Yu W, Naim JO, McGowan M, Ippolito K, Lanzafame RJ. Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem Photobiol. 1997;66:866–71. doi: 10.1111/j.1751-1097.1997.tb03239.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilden L, Karthein R. Import of radiation phenomena of electrons and therapeutic low-level laser in regard to the mitochondrial energy transfer. J Clin Laser Med Surg. 1998;16:159–65. doi: 10.1089/clm.1998.16.159. [DOI] [PubMed] [Google Scholar]
- 5.Conlan MJ, Rapley JW, Cobb CM. Biostimulation of wound healing by low-energy laser irradiation. J Clin Periodontol. 1996;23:492–6. doi: 10.1111/j.1600-051x.1996.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 6.Wong-Riley MTT, Bai XT, Buchmann E, Whelan HT. Lightemitting diode treatment reverses the effect of TTX on cytochrome oxidase in neurons. Neuroreport. 2001;12:3033–7. doi: 10.1097/00001756-200110080-00011. [DOI] [PubMed] [Google Scholar]
- 7.Toyokawa H, Matsui Y, Uhara J, Tsuchiya H, Teshima S, Nakanishi H, Kwon AH, Azuma Y, Nagaoka T, Ogawa T, Kamiyama Y. Promotive effects of far-infrared ray on full-thickness skin wound healing in rats. Exp Biol Med (Maywood) 2003;228:724–9. doi: 10.1177/153537020322800612. [DOI] [PubMed] [Google Scholar]
- 8.Yaakobi T, Maltz L, Oron U. Promotion of bone repair in the cortical bone of the tibia in rats by low energy laser (He-Ne) irradiation. Calcif Tissue Int. 1996;59:297–300. doi: 10.1007/s002239900126. [DOI] [PubMed] [Google Scholar]
- 9.Assia E, Rosner M, Belkin M, Solomon A, Schwartz M. Temporal parameters of low energy laser irradiation for optimal delay of post-traumatic degeneration of rat optic nerve. Brain Res. 1989;476:205–12. doi: 10.1016/0006-8993(89)91240-7. [DOI] [PubMed] [Google Scholar]
- 10.Weiss N, Oron U. Enhancement of muscle regeneration in the rat gastrocnemius muscle by low energy laser irradiation. Anat Embryol. 1992;186:497–503. doi: 10.1007/BF00185463. [DOI] [PubMed] [Google Scholar]
- 11.Bibikova A, Oron U. Promotion of muscle regeneration in the toad (Bufo virdis) gastrocnemius muscle by low-energy laser irradiation. Anal Rec. 1993;235:374–80. doi: 10.1002/ar.1092350306. [DOI] [PubMed] [Google Scholar]
- 12.Bibikova A, Oron U. Attenuation of the process of muscle regeneration in the toad gastrocnemius muscle by low energy laser irradiation. Lasers Surg Med. 1994;14:355–61. doi: 10.1002/lsm.1900140408. [DOI] [PubMed] [Google Scholar]
- 13.Oron U. Photoengineering of tissue repair in skeletal and cardiac muscles. Photomed Laser Surg. 2006;24:111–20. doi: 10.1089/pho.2006.24.111. [DOI] [PubMed] [Google Scholar]
- 14.Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation Directly Benefits Primary Neurons Functionally Inactivated by Toxins. J Biol Chem. 2005;280:4761–71. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 15.Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M, Whelan NT, Whelan HT. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci U S A. 2003;100:3439–44. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 17.Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996;79:949–56. doi: 10.1161/01.res.79.5.949. [DOI] [PubMed] [Google Scholar]
- 18.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, Gambert SR, Cigola E, Anversa P. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol. 1996;28:2005–16. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- 19.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–41. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 21.Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–7. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- 22.Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu Rev Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 23.Bienengraeber MW, Weihrauch D, Kersten JR, Pagel PS, Warltier DC. Cardioprotection by volatile anesthetics. Vascul Pharmacol. 2005;42:243–52. doi: 10.1016/j.vph.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Oron U, Yaakobi T, Oron A, Mordechovitz D, Shofti R, Hayam G, Dror U, Gepstein L, Wolf T, Haudenschild C, Haim SB. Low-Energy Laser Irradiation Reduces Formation of Scar Tissue After Myocardial Infarction in Rats and Dogs. Circulation. 2001;103:296–301. doi: 10.1161/01.cir.103.2.296. [DOI] [PubMed] [Google Scholar]
- 25.Tuby H, Maltz L, Oron U. Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Lasers Surg Med. 2006;38:682–8. doi: 10.1002/lsm.20377. [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association, American Physiologic Society. Guiding Principles for Research Involving Animals and Human Beings. Am J Physiol Regul Integr Comp Physiol. 2002;283:R281–3. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Laboratory Animals Resources, Commission of Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. 7. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 28.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–84. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi-Fanelli A, Antonini E, Caputo A. Studies on the relations between molecular and functional properties of hemoglobin. I. The effect of salts on the molecular weight of human hemoglobin. J Biol Chem. 1961;236:391–6. [PubMed] [Google Scholar]
- 30.Dhanasekaran A, Gruenloh SK, Buonaccorsi JN, Zhang R, Gross GJ, Falck JR, et al. Multiple anti-apoptotic targets of the PI3K-Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol. 2008;294:H724–35. doi: 10.1152/ajpheart.00979.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gebremedhin D, Harder DR, Pratt PF, Campbell WB. Bioassay of an endothelium-derived hyperpolarizing factor from bovine coronary arteries: role of a cytochrome P450 metabolite. J Vasc Res. 1998;35:274–84. doi: 10.1159/000025594. [DOI] [PubMed] [Google Scholar]
- 32.Wang GW, Klein JB, Kang YJ. Metallothionein inhibits doxorubicin-induced mitochondrial cytochrome c release and caspase-3 activation in cardiomyocytes. J Pharmacol Exp Ther. 2001;298:461–8. [PubMed] [Google Scholar]
- 33.Yu M, McAndrew RP, Al-Saghir R, Maier KG, Medhora M, Roman RJ, Jacobs ER. Nitric oxide contributes to 20-HETE-induced relaxation of pulmonary arteries. J Appl Physiol. 2002;93:1391–9. doi: 10.1152/japplphysiol.00247.2002. [DOI] [PubMed] [Google Scholar]
- 34.Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane preconditioning uncouples mitochondria and protects against hypoxia-reoxygenation. Am J Physiol Cell Physiol. 2007;292:C1583–90. doi: 10.1152/ajpcell.00221.2006. [DOI] [PubMed] [Google Scholar]
- 35.Wibom R, Hagenfeldt L, von Döbeln U. Measurement of ATP production and respiratory chain enzyme activities in mitochondria isolated from small muscle biopsy samples. Anal Biochem. 2002;311:139–51. doi: 10.1016/s0003-2697(02)00424-4. [DOI] [PubMed] [Google Scholar]
- 36.Lepiller S, Laurens V, Bouchot A, Herbomel P, Solary E, Chluba J. Imaging of nitric oxide in a living vertebrate using a diamino-fluorescein probe. Free Radic Biol Med. 2007;43:619–27. doi: 10.1016/j.freeradbiomed.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Mason MG, Nicholls P, Wilson MT, Cooper CE. Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proc Natl Acad Sci U S A. 2006;103:708–13. doi: 10.1073/pnas.0506562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lane N. Power games. Nature. 2006;443:901–3. doi: 10.1038/443901a. [DOI] [PubMed] [Google Scholar]
- 39.Cooper CE. Nitric oxide and iron proteins. Biochim Biophys Acta. 1999;1411:290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 40.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–8. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci. 2005;1047:248–58. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- 42.Karu TI. Low-power laser therapy. In: VoDinh T, editor. Biomedical Photonics Handbook. Vol. 48. Boca Raton; CRC Press: 2003. pp. 1–25. [Google Scholar]
- 43.Whelan HT, Smits RL, Jr, Buchman EV, Whelan NT, Turner SG, Margolis DA, et al. Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg. 2001;19:305–14. doi: 10.1089/104454701753342758. [DOI] [PubMed] [Google Scholar]
- 44.Zhao ZQ, Vinten-Johansen J. Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res. 2006;70:200–11. doi: 10.1016/j.cardiores.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 45.Chiari PC, Bienengraeber MW, Pagel PS, Krolikowski JG, Kersten JR, Warltier DC. Isoflurane protects against myocardial infarction during early reperfusion by activation of phosphatidylinositol-3-kinase signal transduction: evidence for anesthetic-induced postconditioning in rabbits. Anesthesiology. 2005;102:102–9. doi: 10.1097/00000542-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 46.Burwell LS, Brookes PS. Mitochondria as a Target for the Cardioprotective Effects of Nitric Oxide in Ischemia-Reperfusion Injury. Antioxid Redox Signal. 2008;10:579–99. doi: 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- 47.Sadek HA, Nulton-Persson AC, Szweda PA, Szweda LI. Cardiac ischemia/reperfusion, aging, and redox-dependent alterations in mitochondrial function. Arch Biochem Biophys. 2003;420:201–8. doi: 10.1016/j.abb.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 48.Chance B, Leigh JS, Miyake H, Smith DS, Nioka S, Greenfeld R, et al. Comparison of time-resolved and -unresolved measurements of deoxyhemoglobin in brain. Proc Natl Acad Sci U S A. 1988;85:4971–5. doi: 10.1073/pnas.85.14.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49:1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 50.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Kawahara K, Hachiro T, Yokokawa T, Nakajima T, Yamauchi Y, Nakayama Y. Ischemia/reperfusion-induced death of cardiac myocytes: possible involvement of nitric oxide in the coordination of ATP supply and demand during ischemia. J Mol Cell Cardiol. 2006;40:35–46. doi: 10.1016/j.yjmcc.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 52.Balligand JL. Regulation of cardiac beta-adrenergic response by nitric oxide. Cardiovasc Res. 1999;43:607–20. doi: 10.1016/s0008-6363(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 53.Kawahara K, Saitoh M, Nakajima T, Sato H, Tanaka M, Tojima T, Ito E. Increased resistance to nitric oxide cytotoxicity associated with differentiation of neuroblastoma-glioma hybrid (NG108-15) cells. Free Radic Res. 2002;36:545–54. doi: 10.1080/10715760290025924. [DOI] [PubMed] [Google Scholar]
- 54.Brüne B. Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ. 2003;10:864–9. doi: 10.1038/sj.cdd.4401261. [DOI] [PubMed] [Google Scholar]
- 55.Leist M, Single B, Naumann H, Fava E, Simon B, Kühnle S, Nicotera P. Inhibition of mitochondrial ATP generation by nitric oxide switches apoptosis to necrosis. Exp Cell Res. 1999;249:396–403. doi: 10.1006/excr.1999.4514. [DOI] [PubMed] [Google Scholar]
- 56.Brüne B. The Intimate Relation Between Nitric Oxide and Superoxide in Apoptosis and Cell Survival. Antioxid Redox Signal. 2005;7:497–507. doi: 10.1089/ars.2005.7.497. [DOI] [PubMed] [Google Scholar]
- 57.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–61. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 58.Davidson SM, Duchen MR. Effect of NO on mitochondrial function in cardiomyocytes: Pathophysiological relevance. Cardiovasc Res. 2006;71:10–21. doi: 10.1016/j.cardiores.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 59.Valdez LB, Zaobornyj T, Alvarez S, Bustamante J, Costa LE, Boveris A. Heart mitochondrial nitric oxide synthase. Effects of hypoxia and aging Mol Aspects Med. 2004;25:49–59. doi: 10.1016/j.mam.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Bates TE, Loesch A, Burnstock G, Clark JB. Immunocytochemical evidence for a mitochondrially located nitric oxide synthase in brain and liver. Biochem Biophys Res Commun. 1995;213:896–900. doi: 10.1006/bbrc.1995.2213. [DOI] [PubMed] [Google Scholar]
- 61.Ghafourifar P, Richter C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997;418:291–6. doi: 10.1016/s0014-5793(97)01397-5. [DOI] [PubMed] [Google Scholar]
- 62.Lacza Z, Snipes JA, Zhang J, Horváth EM, Figueroa JP, Szabó C, Busija DW. Mitochondrial nitric oxide synthase is not eNOS, nNOS or iNOS. Free Radic Biol Med. 2003;35:1217–28. doi: 10.1016/s0891-5849(03)00510-0. [DOI] [PubMed] [Google Scholar]
- 63.Zanella B, Giordano E, Muscari C, Zini M, Guarnieri C. Nitric oxide synthase activity in rat cardiac mitochondria. Basic Res Cardiol. 2004;99:159–64. doi: 10.1007/s00395-003-0454-3. [DOI] [PubMed] [Google Scholar]
- 64.French S, Giulivi C, Balaban RS. Nitric oxide synthase in porcine heart mitochondria: evidence for low physiological activity. Am J Physiol Heart Circ Physiol. 2001;280:H2863–7. doi: 10.1152/ajpheart.2001.280.6.H2863. [DOI] [PubMed] [Google Scholar]
- 65.Giulivi C, Kato K, Cooper CE. Nitric oxide regulation of mitochondrial oxygen consumption I: cellular physiology. Am J Physiol Cell Physiol. 2006;291:1225–31. doi: 10.1152/ajpcell.00307.2006. [DOI] [PubMed] [Google Scholar]
- 66.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2008;105:10256–61. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shiva S, Oh JY, Landar AL, Ulasova E, Venkatraman A, Bailey SM, Darley-Usmar VM. Nitroxia: The pathological consequence of dysfunction in the nitric oxide–cytochrome c oxidase signaling pathway. Free Radic Biol Med. 2005;38:297–306. doi: 10.1016/j.freeradbiomed.2004.10.037. [DOI] [PubMed] [Google Scholar]