Abstract
Activation of the spinal phospholipase A2 (PLA2) - cyclooxygenase (COX) – prostaglandin signaling pathway is widely implicated in nociceptive processing. Although the role of spinal COX isoforms in pain signal transmission has been extensively characterized, our knowledge of PLA2 enzymes in this cascade is limited. Among all PLA2 groups, cytosolic calcium-dependent PLA2 group IVA (cPLA2IVA) appears to be the predominant PLA2 enzyme in the spinal cord. In the present study we sought to (i) characterize anatomical and cellular distribution and localization of cPLA2IVA in dorsal horn of rat spinal cord, (ii) verify efficacy and selectivity of intrathecal (IT) delivery of an antisense oligonucleotide (AS) targeting rat cPLA2IVA mRNA on spinal expression of this enzyme, and (iii) examine the effect of down-regulation of spinal cPLA2IVA on peripheral tissue injury-induced pain behavior. Here we demonstrate that cPLA2IVA is constitutively expressed in rat spinal cord, predominantly in dorsal horn neurons and oligodendrocytes but not in astrocytes or microglia. Intrathecal injection of AS significantly down-regulated both protein and gene expression of cPLA2IVA in rat spinal cord, while control missense oligonucleotide (MS) had no effect. Immunocytochemistry confirmed that the reduction occurred in neurons and oligodendrocytes. cPLA2IVA.AS did not alter expression of several other PLA2 isoforms, such as secretory PLA2 (groups IIA and V) and calcium-independent PLA2 (group VI), indicating that the AS was specific for cPLA2IVA. This selective knockdown of spinal cPLA2IVA did not change acute nociception (i.e. paw withdrawal thresholds to acute thermal stimuli and intradermal formalin-induced first phase flinching), however, it significantly attenuated formalin-induced hyperalgesia (i.e. second phase flinching behavior), which reflects spinal sensitization. Thus the present findings suggest that cPLA2IVA may specifically participate in spinal nociceptive processing.
Keywords: Rat, Intrathecal, Neurons, Oligodendrocytes, Pain
Introduction
The PLA2-COX cascade, yielding free arachidonic acid and the subsequent generation of eicosanoid metabolites, plays an important role in many physiological and pathological events including pain signal transduction (Cummings et al., 2000, Balboa et al., 2002, Kudo and Murakami, 2002, Svensson and Yaksh, 2002). It has been long appreciated that eicosanoids, particularly prostaglandins, play a significant role in the development of an exaggerated pain state (hyperalgesia) after tissue injury and inflammation (Vane, 1971, Yaksh, 1982). The action of prostaglandins occurs not only at the site of injury where afferent terminals are sensitized (Ferreira, 1972), but also in the spinal cord where prostaglandins enhance noxious input-evoked excitability by acting either pre-synaptically on central terminals of primary afferents to facilitate release of excitatory neurotransmitters or post-synaptically to activate second order neurons (Yaksh, 1982, Malmberg and Yaksh, 1992, Malmberg and Yaksh, 1995, Southall et al., 1998, Ebersberger et al., 1999, Samad et al., 2001, Ghilardi et al., 2004).
Eicosanoids are derived from arachidonic acid, which is released from membrane phospholipids through the action of a variety of phospholipases (Kudo and Murakami, 2002). In rat spinal cord, several PLA2 isoforms including group IVA (cytosolic calcium-dependent PLA2, cPLA2IVA), IIA, V (secretory PLA2, sPLA2IIA and sPLA2V), and VI (calcium-independent PLA2, iPLA2VI) have been found (Kishimoto et al., 1999, Ong et al., 1999, Lucas et al., 2005, Svensson et al., 2005b, Liu et al., 2006), with cPLA2IVA and iPLA2VI being the most predominant (Lucas et al., 2005). cPLA2IVA hydrolyzes intracellular phospholipids at the sn-2 position (Six and Dennis, 2000), and due to its high substrate selectivity it specifically releases arachidonic acid (Kudo and Murakami, 2002). In contrast, iPLA2VI has no significant fatty acid specificity and it is believed that its primary function is associated with membrane remodeling (Balsinde et al., 1997).
It is well documented that blocking generation of prostaglandins in spinal cord through the inhibition of COX has antinociceptive effect (Yaksh, 1982, Malmberg and Yaksh, 1992, Malmberg and Yaksh, 1995, Southall et al., 1998, Ebersberger et al., 1999, Samad et al., 2001, Ghilardi et al., 2004). However, recent reports indicate that arachidonic acid itself, as well as some arachidonic acid derivatives which are generated independently of COX activity, are also involved in spinal nociception (Evans et al., 2001, Morita et al., 2004, Lucas et al., 2005, Svensson et al., 2005b, Yaksh et al., 2006). We therefore hypothesized that inhibiting PLA2 activity may be a more effective approach for pain relief. In this regard, previous work has indicated that spinal cPLA2IVA, but not iPLA2VI, participate in the regulation of spinal sensitization. Intrathecal injection of chemical inhibitors such as arachidonyl trifluoromethylketone and ethyl 4-[(2-oxohexadecanoyl)amino] butanoate (AX048), which block the enzymatic activity of both cPLA2IVA and iPLA2VI, attenuate tissue injury-induced hyperalgesia as well as the spinal PGE2 release in experimental models of pain (Lucas et al., 2005, Yaksh et al., 2006). In contrast, inhibitors such as bromoenol lactone, which selectively inhibits iPLA2 were without effect. All together, the role of cPLA2IVA in generation of arachidonic acid, its presence in spinal cord and the noted pharmacology support an important role of cPLA2IVA in spinal nociception. However, as the inhibitors listed above also act on several systems, for example, inhibition of fatty acid amide hydrolase and activation of cannabinoid receptor 1 (Deutsch et al., 1997, Fernando and Pertwee, 1997), it is difficult to rule out the possibility that the antinociceptive effect of these inhibitors could be achieved through cPLA2IVA independent mechanisms. Hence, the aim of the present work was to assess the role of cPLA2IVA in nociception by the use of cPLA2IVA targeted antisense oligonucleotides to down-regulate specifically the expression of this enzyme and evaluate the effect of the selective knockdown of spinal cPLA2IVA on tissue injury-induced hyperalgesia.
Experimental Procedures
Animals
All experiments were carried out according to protocols approved by the Institutional Animal Care and Use Committee of the University of California, San Diego (under the Guild for Care and Use of Laboratory Animals, National Institute of Health publication 85-23, Bethesda, MD, USA). Male Holzmann rats (300–350g, Harlan, Srague-Dawley, IN, USA) were used in the study, and were housed in individual standard cages at room temperature on an ad libitum diet, maintained on a 12 hours light/12 hours dark cycle (light on at 07:00 h). Testing was performed during the light cycle between 09:00 h – 16:00h.
Oligonucleotides
ISIS 122523 (GATCACACAGTGCCATGCTG) is an antisense oligonucleotide that targets rat cPLA2 IVA mRNA (Accession # U38376.1) at position 609–628. ISIS 141923 (CCTTCCCTGAAGGTTCCTCC) is a missense control, which we define as a randomized sequence that is not complementary with any other known gene in the mouse, rat or human genomes. Both the antisense and misense are 20-mer oligonucleotides, with a full phosphorothioate backbone and 2’methoxyethyl modifications at positions 1–5 and 16–20. This chemical modification is known to improve oligonucleotide stability and target affinity (Butler et al., 2005). We have previously achieved successful knockdown of several protein isoforms in rat spinal cord with IT delivery of 2’methoxyethyl AS (Hua et al., 2002, Butler et al., 2005, Svensson et al., 2005a). All oligonucleotides were dissolved in sterile saline.
Intrathecal Catheter Implantation/Drug Delivery and Tissue Harvesting
For chronic IT infusion of oligonucleotides, lumbar catheters were surgically implanted into rat intrathecal space following a modification of a previously described protocol (Yaksh and Rudy, 1976). Animals were anesthetized with 2% isofluorane in a 1:1 room air mixture and secured in a sterotaxic head holder. A skin incision was made at the dorsal midline of the skull. Muscle behind the neck was separated from the occipital crest exposing the cisternal membrane. A bent 22-G needle was used to make an incision in the cisternal membrane through which an 8.5 cm polyethylene catheter (PE-5) was inserted. The catheter was advanced caudally into the intrathecal space to L1–L3 spinal segments and flushed with 10 µl of saline. A mini osmotic pump (model 2001, 1 µl/hour; Alzet, Palo Alto, CA, USA) was filled with ISIS 122523 or ISIS 141923 at concentrations to deliver either a 100 µg or 200 µg constant total daily infusion for 5 days. The pump was connected to the catheter and placed subcutaneously behind the shoulders. The skin incision was closed using 3.0 USP black braided silk suture. Rats received a 5 mL subcutaneous injection of lactated Ringer’s solution and allowed to recover under a heat lamp. Rats showing motor weakness or signs of paralysis after recovery from anesthesia or during the 5-day infusion period were immediately euthanized. The spinal cord tissue samples for western blots or immunocytochemistry were collected at the 6th day of IT infusion. For the western blots, the animals were anesthetized in 4% isoflurane, decapitated by guillotine, then spinal cords were hydroextruded with cold saline and a 2 cm portion of spinal cord lumbar enlargement was collected. For immunocytochemistry, the details of the spinal cord dissection are discussed below.
Immunohistochemistry
Naïve and AS/MS-treated rats were anesthetized with 0.5 mL Nembutal solution (sodium pentobarbital 50 mg/ml, Abbott Laboratories, Chicago, IL) injected intraperitoneally. Rats were perfused intracardially with 250 mL saline followed by 250 mL 4% paraformaldehyde in 0.1M PBS. Spinal cords were removed by laminectomy and immersed in the same fixing solution overnight, followed by 20% sucrose in PBS for 24 hours and 30% sucrose for 48 hours. Using a cryostat, 10 µm transverse sections were cut from lumbar enlargement and mounted directly on glass slides. To reduce non-specific binding, slides were incubated in blocking buffer (5% normal goat serum, 1% BSA, 0.2% TritonX-100 in PBS) for 1 hour at room temperature and then incubated with primary cPLA2 antibody (1:250 diluted in blocking buffer; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 24 hours at 4°C. Slides were then washed 3 times with PBS followed by incubation in goat anti-rabbit Alexa-488 (1:250 diluted in blocking buffer; Invitrogen, Carlsbad, CA) for 1 hour at room temperature. The specificity of the cPLA2 staining was determined by substituting rabbit total IgG for the primary antibody, and this resulted in no immunostaining in these sections. To identify cells expressing cPLA2, double immunolabelling was performed using antibodies against Neuronal N (1:1000; Chemicon, Temecula, CA), glial fibrillary acidic protein (GFAP, 1:1000; Chemicon), OX-42/CD11b (1:1000; Biosource International, Dallas, TX)(Robinson et al., 1986, Ling et al., 1990), or adenomatous polyposis coli (APC, 1:500; EMD Chemicals, Gibbstown, NJ)(Bhat et al., 1996). After incubation with goat anti-mouse Alexa-594 (1:250; Invitrogen) slides were washed and mounted (prolong gold antifade medium; Invitrogen). Images were captured using a Leica LCS SP2 confocal microscope (Leica Microsystems, Bannockburn, IL) and processed with Adobe Photoshop CS3 (Adobe Systems, Mountain View, CA).
Western Blot
For protein analysis of PLA2 enzymes, tissue from spinal cord lumbar enlargement was immersed in 0.6 mL of protein extraction buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.5% TritonX-100, 0.5% SDS pH 7.4) and homogenized by sonication. Samples were centrifuged at 14,000 rpm in 4°C for 10 minutes, and the supernatants were subjected to polyacrylamide gel electrophoresis. To blot for cPLA2IVA / iPLA2VI, samples for each lane containing 0.1 M DTT and 40 µg protein in 1 × LDS loading buffer (Invitrogen), were heated in a water bath at 95°C for 5 minutes, and separated on a 3–8% Tris-Acetate gel (Invitrogen). To blot for sPLA2 IIA/sPLA2 V, samples for each lane containing 0.3 M DTT and 50 µg protein in 1 × LDS loading buffer, were heated in boiling water for 10 minutes, and separated on an 8–12% Bis-Tris gel using MES running buffer (Invitrogen). Proteins were transferred to a 0.45 µM nitrocellulose membrane (Invitrogen), and non-specific binding blocked with 5% non-fat milk dissolved in TBS-T (Tris-buffered saline solution with 0.1% Tween-20) for 1 hour at room temperature. Blots were incubated in one of the following rabbit primary antibodies: anti-cPLA2IVA (Cell Signaling Technology, Beverly, MA), anti-iPLA2 VI (Cayman Chemical, Ann Arbor, MI), anti-sPLA2 IIA (Cayman Chemical), or anti-sPLA2 V (Cayman Chemical) diluted in TBS-T 1:1000 at 4°C overnight. Secondary antibody was an anti-rabbit-HRP antibody (Cell Signaling). To normalize loading, membranes were stripped, blocked, and incubated with mouse primary antibodies: anti-β-actin (1:10000, Sigma, St Louis, MO) or anti- GAPDH (1:4000, Abcam, Cambridge, MA). Anti-mouse-HRP was used as a secondary antibody (1:5000, Cell Signaling). Blots were developed using Femto Sensitive enhanced chemiluminescent detection system (SuperSignal Piece, Rockford, IL) for all antibodies except for β-actin where a Pico Sensitive chemiluminescent detection system was used (Pierce). To verify the specificity of the cPLA2 antibody used in Western blots, we ran the spinal cord samples together with a positive control, a purified recombinant cPLA2IVA protein isolated from macrophages (a gift from Dr. Edward Dennis, Department of Biochemistry, University of California San Diego), which was recognized by the cPLA2 antibody as a single immunoreactive band with the same size as that in the spinal cord samples. Western blots were scanned and quantitated by densitometry using ImageQuant (Molecular Dynamics, Sunnyvale, CA, USA).
Real-Time RT-PCR
For mRNA analysis of PLA2 enzymes, spinal cord tissue was immediately placed in 2mL of Guanidinium Isothiocyanate buffer (Invitrogen) containing 8% β-mercaptoethanol and pH adjusted to 5.0 with glacial acetic acid. Tissue was homogenized using a mechanical tissue homogenizer. RNA was isolated and purified using RNeasy kit (Qiagen, Valencia, CA, USA). Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR) was used to quantitate mRNA levels of various PLA2 enzymes as previously described (Hua et al., 2002). Each 25 µL RT-PCR reaction contained 1.2× TaqMan Buffer, 5.5 mM MgCl2, 1.2 mM dNTP, 0.3mM forward and reverse primer, 0.1 mM probe, 0.16 U RNase inhibitor, 0.15 µL AmphiTAQ Platinum, 0.104 U MuLV, and 10 µL purified mRNA.
Primers for cPLA2IVA, sPLA2IIA, sPLA2V, iPLA2VI and cyclophilin A were synthesized by Eurogentec (San Diego, CA, USA; for structures see Table 1). RNA samples were run in triplicate with a standard curve for each amplicon, in addition to no-template and non-amplification controls for each primer-probe set. Data was collected using Applied Biosystems Prism 7700 thermal cycler and raw data analyzed using Sequence Detector Software 1.7.1 (Applied Biosystems). Standard curves for each amplicon were plotted from four different concentrations of control mRNA run in duplicate and considered acceptable if the correlation coefficient exceeded 0.99. The expression data for all samples were normalized to total RNA levels as determined by cyclophilin A (Hager et al., 1999).
Table 1.
Primers for four phospholipase A2 enzymes
| Gene | Primer/Probe | Sequence (5′-3′) |
|---|---|---|
| cPLA2IVA | F Primer | GCAAAGCACATTGTGAGTAACGA |
| R Primer | CGGTGCCTTTGGGTCCTT | |
| Probe | AGCTCTGACAGCGATGACGAGGCC | |
| sPLA2IIA | F Primer | CCAAGTTCCCCAGTGATCAAG |
| R Primer | GCTCCTTCTGGGTGAAGACAGA | |
| Probe | TCCAACCCTAGAAGCAGGCGGGC | |
| sPLA2V | F Primer | AGCACTCTCACGATCAGCATCA |
| R Primer | CCCAAGCATCAGGGTCTACAA | |
| Probe | CACGGAATCCATCCTTCCTGTGTTGC | |
| iPLA2VI | F Primer | TGTCCCACATAGGATGCAGTTC |
| R Primer | CCTTTACCCGGAATGGGTTT | |
| Probe | TTGGACGCCTCGTTAACACCCTCAGTAGT | |
| Cyclophillin | F Primer | CCCACCGTGTTCTTCGACA |
| R Primer | AAACAGCTCGAAGCAGACGC | |
| Probe | CACGGCTGATGGCGAGCCCX |
Behavioral Tests
After five days of AS or MS treatment, animals were subjected to two behavioral tests, thermal paw withdrawal test and formalin-induced flinching. To assess the thermally evoked paw withdrawal response, a Hargreaves-type testing device was used (Dirig et al., 1997). The device consists of a glass surface (maintained at 30°c) on which the rats are placed individually in Plexiglas cubicles. The thermal nociceptive stimulus originates from a focused projection bulb positioned below the glass surface. A timer is activated by the light source, and latency was defined as the time required for the paw to show a brisk withdrawal as detected by photodiode motion sensors that stopped the timer and terminated the stimulus.
Formalin-induced flinching was measured using an automated detection system (Yaksh et al., 2001). A metal band was placed on one hind paw of the rat and the animal was allowed to acclimate in a plexiglass chamber for 1 hour prior to testing. Immediately before testing, rats were restrained in a cloth towel and 50 µl of 2.5% formalin solution was injected into the dorsal side of the banded paw. Data collection began as soon as the animal was placed in testing chamber. Pain behavior was quantified by counting the number of flinches of the injected paw. Flinches were counted for 60 min, comprising a biphasic flinching response with Phase 1 (0–9 min) and Phase 2 (10–60 min).
Results
cPLA2IVA is expressed in neurons and oligodendrocytes in spinal dorsal horn
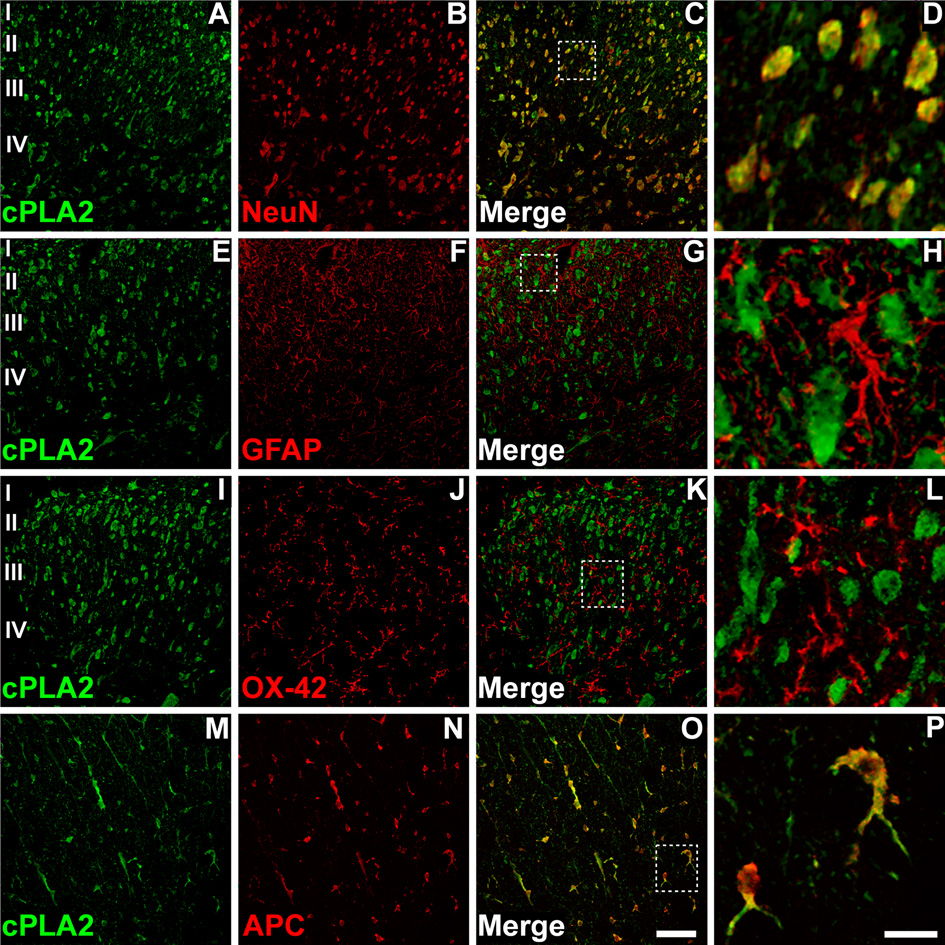
Cellular localization of cPLA2IVA in the spinal cord was characterized by immunohistochemistry. Spinal cord sections from naïve rats were stained with antibodies specifically for cPLA2IVA. cPLA2IVA immunoreactivity was seen in the cell soma primarily in dorsal horn grey matter (Figure 1A, E, I), while some staining was also observed in white matter, especially in the area of the dorsal column (Figure 1M). In order to define specific cell types that express cPLA2IVA, double immunostaining was performed. The spinal cord sections were incubated with the antibodies against cPLA2IVA and also with cellular markers for neurons (NeuN), astrocytes (GFAP), microglia (OX-42) or oligodendrocytes (APC). The majority of cPLA2IVA immuno-positive cells were co-stained with the neuronal marker NeuN through the grey matter, indicating that cPLA2IVA is expressed in dorsal horn neurons (Figure 1 A–D). cPLA2IVA did not appear to be expressed in astrocytes or microglia as no co-localization was observed between cPLA2IVA and GFAP (Figure 1 E–H) or OX-42 (Figure 1 I–L). Additionally, cPLA2IVA is exclusively expressed in oligodendrocytes in white matter, as shown (Figure 1 M–P) by co-localization between the cPLA2IVA immunoreactivity and cells colabeled with the APC antibody. cPLA2IVA immunoreactivity was observed in the cytoplasm of both neurons and oligodentrocytes (Figure 1D, P).
Figure 1. Distribution and cellular localization of cPLA2IVA in dorsal horn of naïve rat spinal cord.
cPLA2IVA immunoreactivity distributed throughout dorsal horn laminae I–IV (A, E, I). Double immunofluorescence confocal micrographs show that cPLA2IVA (A) and the neuronal marker NeuN (B) co-localize in the majority of neurons within the spinal cord (C, D). No co-localization was observed between cPLA2 and GFAP (E–H), or cPLA2 and OX-42 (I–L), suggesting that cPLA2IVA is not expressed in astrocytes or microglia. cPLA2IVA immunostaining also seen in white matter (M–P), where it co-localized with the oligodendrocyte marker APC (O, P). Scale bar in panel P is 20µm for figures D, H, L, P and 60µm for the remaining figures.
cPLA2 AS selectively inhibits protein expression of spinal cPLA2IVA
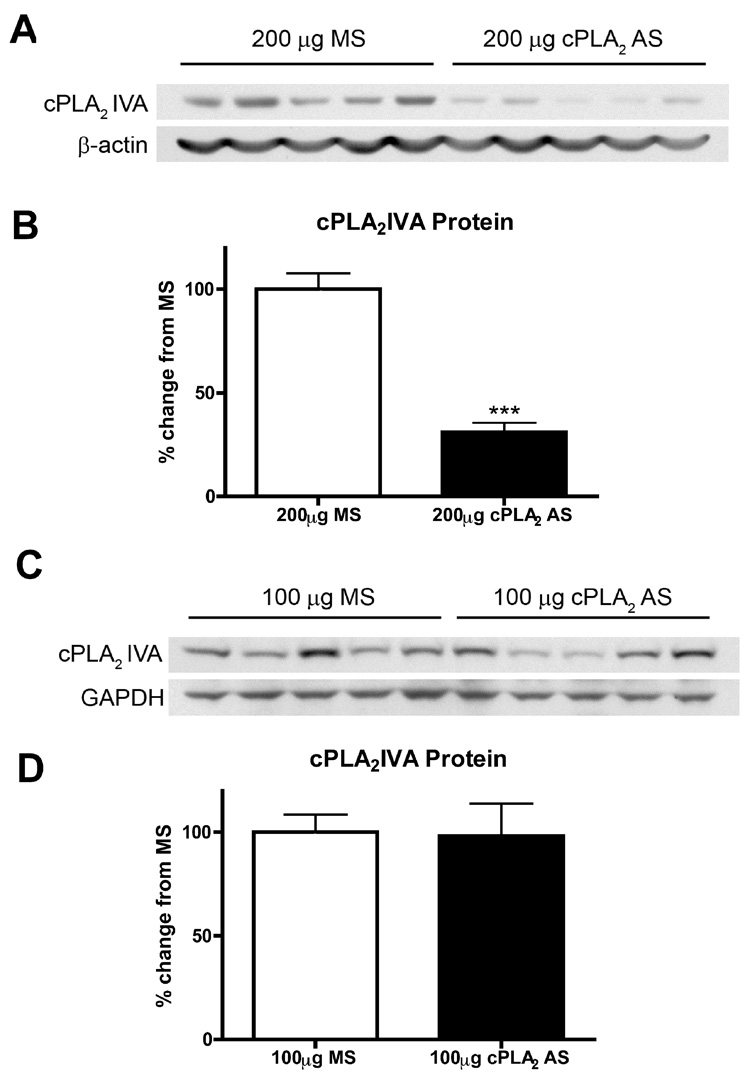
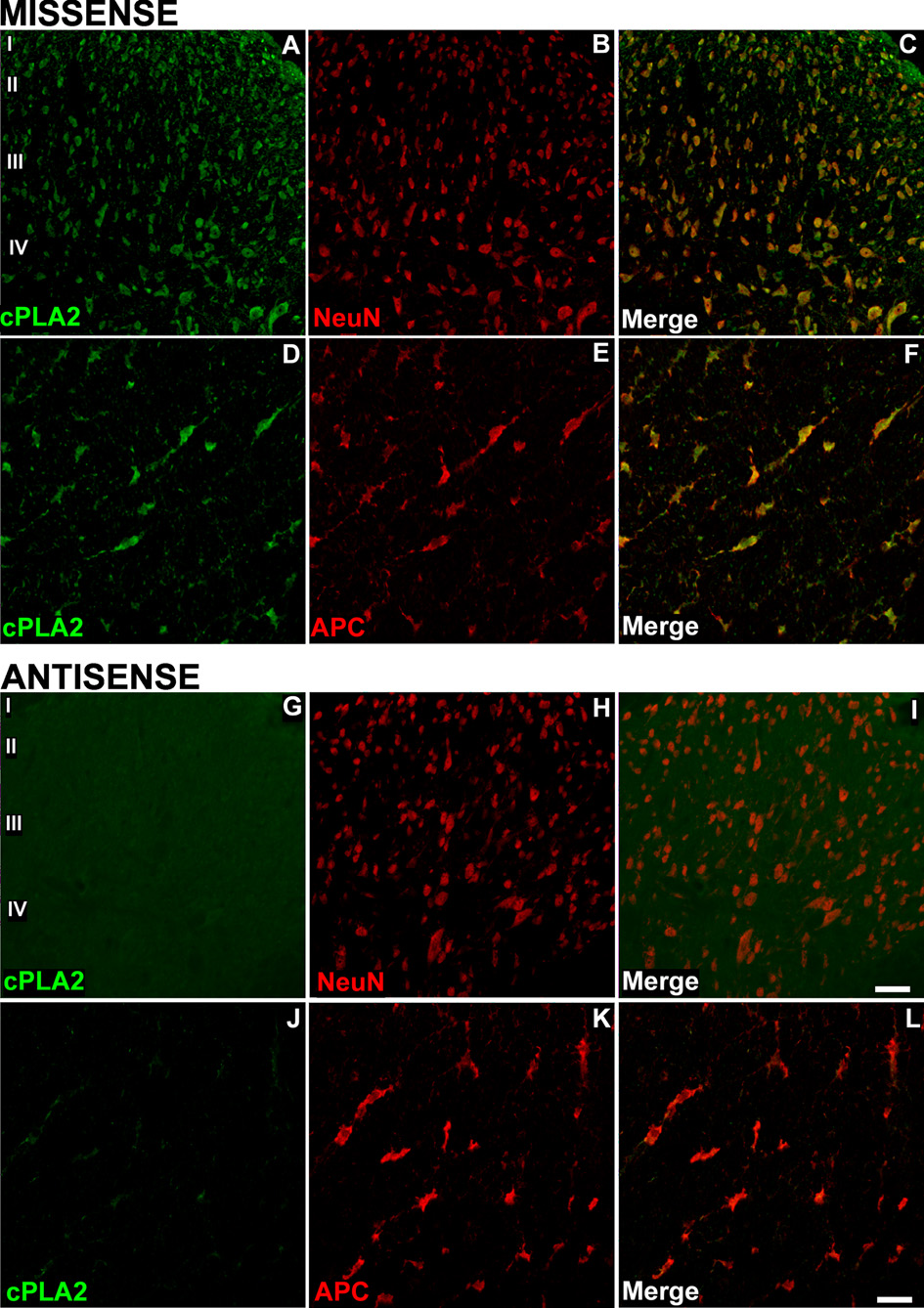
Rats were treated with a 5-day IT infusion of cPLA2 AS (ISIS 122523) or control MS (ISIS141923) at two doses, and the levels of spinal cPLA2 protein expression were analyzed by Western blotting. The cPLA2 AS at a dose of 200 µg per day significantly decreased protein expression of spinal cPLA2IVA compared to animals that received an equal dose of MS control (Figure 2A, B; p < 0.0001), while a lower dose of 100 µg per day did not produce any reduction compared to MS control (Figure 2C, D). Overall, there was approximately 70% knockdown of spinal cPLA2IVA protein after five days IT infusion of cPLA2 AS at a 200 µg daily dose in comparison to MS control. The protein level of cPLA2IVA in the rats treated with 5-day MS did not differ from those with 5-day saline infusion (data not shown). Inhibition of the cPLA2IVA expression was further confirmed with immunocytochemistry. The cPLA2IVA immunoreactivity was reduced in neurons as well as in oligodendrocytes of the AS-treated spinal cords (Figure 3).
Figure 2. Effect of intrathecal cPLA2 antisense oligonucleotides on expression of spinal cPLA2 IVA protein.
(A, C) Representative Western blots show levels of cPLA2IVA protein in rat spinal cord after a 5-day IT infusion of the cPLA2 AS or MS at two daily doses, i.e. (A) 200 µg and (C) 100 µg. (B, D) Densitometry of Western blots shows a significant decrease (70% reduction) in levels of spinal cPLA2IVA protein after (B) 200 µg cPLA2 AS (MS N=17; AS N=18. *** p <0.0001), but no such difference after (D) 100 µg cPLA2 AS (MS N=10; 100µg AS N=10, P >0.05). Bands were normalized to beta-actin and compared to cPLA2IVA protein levels in MS treated controls. Bars represent mean ± SEM.
Figure 3. cPLA2IVA expression is reduced in both neurons and oligodendrocytes after intrathecal cPLA2 antisense treatment.
The distribution and density of cPLA2IVA immunoreactivity in dorsal horn of rat spinal cord after 5-day IT MS is similar to that in the naïve rats (see Figure 1). The immunostaining was noted in both neurons (A–C) and oligodendrocytes (DF). After a 5-day IT AS, cPLA2IVA immunoreactivity was profoundly reduced both in neurons (G–I) and oligodendrocytes (J–L). Scale bar in panel L is 60µm for figures A–C and G–I and 30µm for the figures D–F and J–L.
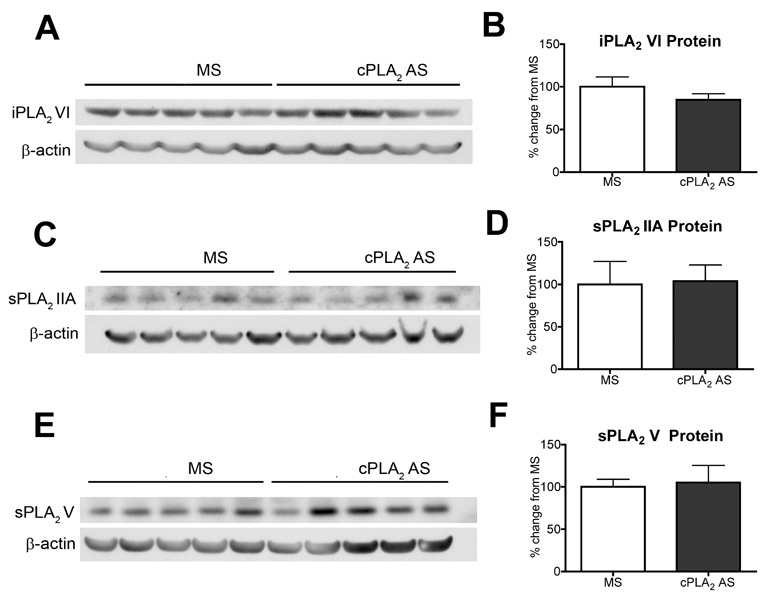
To verify target specificity, spinal cords from the animals treated with 200 µg of cPLA2 AS was also analyzed by Western blotting for the other three major PLA2 enzymes, i.e. iPLA2VI, sPLA2IIA, and sPLA2V. We did not detect any significant reduction or elevation in the protein levels of iPLA2VI (Figure 4A, B), sPLA2IIA (Figure 4C, D), and sPLA2V (Figure 4E, F) by the AS treatment in comparison to MS control group.
Figure 4. Protein expression of spinal iPLA2VI, sPLA2IIA, and sPLA2V after intrathecal cPLA2 antisense treatment.
Representative western blots show levels of spinal (A) iPLA2VI, (C) sPLA2IIA, (E) sPLA2V protein after IT infusion of 200 µg cPLA2 AS or control MS. Densitometry of Western blots (B, D, F, N=5 for each group) show no statistically significant difference in levels of spinal iPLA2VI, sPLA2IIA, and sPLA2V protein between AS and MS groups. Bars represent mean ± SEM.
cPLA2 AS specifically knocks down spinal cPLA2IVA mRNA
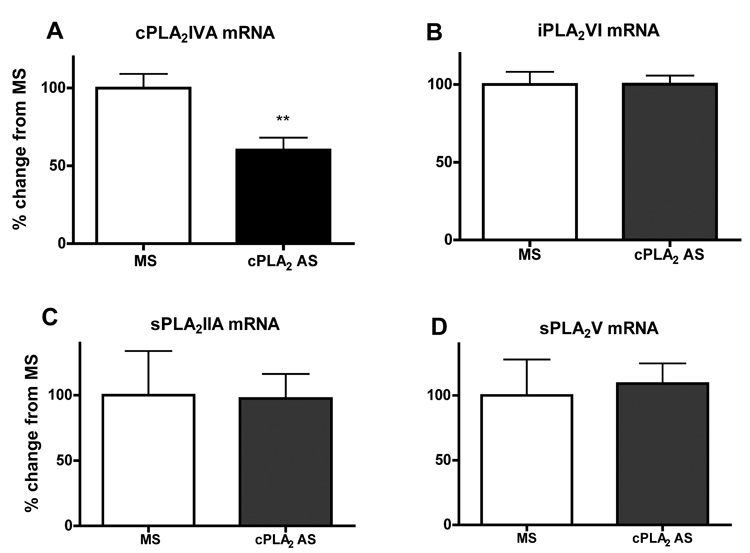
The effect of the AS treatment on spinal cPLA2IVA gene expression was also examined at the transcript level. Quantitative real-time RT-PCR revealed that intrathecal treatment with AS significantly reduced cPLA2IVA mRNA to 40% (p < 0.005) in comparison to those in the spinal cords treated with the MS control (Figure 5A). In agreement with the Western-blotting data, there was no difference in mRNA levels for iPLA2VI (Figure 5B), sPLA2IIA (Figure 5C), or sPLA2V (Figure 5D) between the AS-and the MS-treated group.
Figure 5. mRNA expression of spinal PLA2 isoforms after intrathecal cPLA2 antisense treatment.
Real Time RT-PCR shows the mRNA levels of (A) cPLA2IVA, (B) iPLA2VI, (C) sPLA2IIA, (D) sPLA2V after a 5-day IT cPLA2 AS (200 µg). There was a significant decrease in the levels of cPLA2 mRNA after the AS treatment compared to levels present in MS control (**p < 0.005). In contrast, the mRNA levels of iPLA2VI, sPLA2IIA, or sPLA2V in the same spinal cords was not affected by the AS treatment. MS group: N=7, AS group: N=9.
Knockdown of spinal cPLA2IVA does not affect acute nociception, but attenuates facilitated pain states
The withdrawal latencies for thermal stimulation in the AS-treated rats were not changed. The baseline thermal latencies in the rats treated with AS for 5 days were 10±0.9 seconds before and 12±0.3 seconds after at daily dose of 100µg; and 10±0.7 seconds before and 12±1.6 seconds after at 200 µg (p > 0.05). Unilateral injection of a formalin solution (2.5%, 50 µl) into the dorsum of one hind paw produced a biphasic flinching response: Phase I (0–10 min) and Phase II (11–60 min). Phase II flinching is thought to represent spinal facilitation initiated by afferent input (Dickenson and Sullivan, 1987, Yaksh, 1999). In the control MS (100–200 µg) group the total numbers of flinches in phase I was 163±11, and phase II was 971±11. Animals treated with 200 µg of cPLA2 AS, that exhibited a down-regulation of spinal cPLA2IVA protein (see Figure 2), displayed a 50% decrease in the number of phase II flinches compared to animals treated with MS control (p < 0.05, Figure 6), while the phase I flinching response was not affected (Figure 6). In contrast, cPLA2 AS at the dose of 100 µg neither reduced the protein expression (see Figure 2) nor suppressed the formalin-induced flinching behavior (Figure 6).
Figure 6. Effect of intrathecal cPLA2 antisense on formalin-induced hyperalgesia.
(A) Graph shows number of flinches per minute over time after unilateral injection of formalin (2.5%, 50uL) into dorsal hindpaw in rats that had received IT infusion of cPLA2 AS or MS at a daily dose of 200 µg for five days. (B) Bar graphs show sum of flinches after paw injection of formalin during phase I (0–9 min) and phase II (10–60 min) in rats treated with IT MS or cPLA2 AS (100µg–200µg). There was a significant decrease in the number of Phase II flinches in animals that received 200 µg cPLA2 AS compared to animals that received either 100µg of cPLA2 AS or MS control (*p < 0.05).
Discussion
Our previous studies, using non-selective PLA2 inhibitors, indicated that activity of spinal cPLA2 is involved in spinal nociceptive processing (Lucas et al., 2005, Yaksh et al., 2006). In the present study, we have demonstrated that intrathecal administration of cPLA2 antisense oligonucleotides, specifically targeting cPLA2IVA, down regulates gene and protein expression of this enzyme in the spinal cord, and that this selective suppression attenuates tissue injury-induced hyperalgesia. These findings provide further support for our hypothesis that spinal cPLA2IVA plays an important role in nociception by participating in the facilitation of spinal pain processing.
Spinal loalization
In agreement with earlier work, abundant cPLA2IVA expression was observed in naive rat spinal cord (Kishimoto et al., 1999, Ong et al., 1999, Lucas et al., 2005). The activity of cPLA2IVA is primarily dependent on cellular localization (translocation from cytosol to membrane in response to increased intracellular calcium) and phosphorylation (Channon and Leslie, 1990, Clark et al., 1995, Glover et al., 1995). However, as an increase in cPLA2 protein expression has been reported following spinal cord injury (Liu et al., 2006), it is plausible that transcriptional control provides an additional level of regulation (Clark et al., 1995). In support of this possibility, pathological conditions in the central nervous system such as global forebrain ischemia (Clemens et al., 1996) and kainate-induced neuronal injury (Ong et al., 1999) drive de novo synthesis of cPLA2IVA in brain glial cells including microglia and astrocytes. However, though not examined here, it is unlikely that an increase in cPLA2IVA protein expression is part of the mechanisms regulating spinal sensitization in our model. This assumption is based on the short duration of behavioral studies (1 hour) and the absence of increased spinal cPLA2IVA levels in other models of nociception (Samad et al., 2001, Lucas et al., 2005).
Our examination of cellular distribution of cPLA2IVA in the spinal cord of naïve rats and of rats with chronic intrathecal AS/MS revealed that the expression of this enzyme is restricted to neurons in the grey matter and oligodendrocytes in the white matter. No de novo expression of cPLA2IVA could be detected in other cell types in the spinal cord. A similar observation was made by another group studying the spinal cord in a rat trauma injury model (Liu et al., 2006). Although a small population of microglia in these injured cords express cPLA2IVA, neurons and oligodendrocytes are still the primary sources (Liu et al., 2006). Intrathecal administration of cPLA2 antisense down-regulated cPLA2IVA protein expression not only in neurons but also in oligodnedrocytes, and as this knockdown had antihyperalgesic effect, one cannot exclude a role for cPLA2IVA in oligodendrocytes in the regulation of spinal sensitization. Relevant to this, oligodendrocytes have also been shown to express COX2 and these cells are implicated the pain behavior associated with diabetic neuropathy (Ramos et al., 2007). Though increasing evidence suggest that other non-neuronal cells in the spinal cord, such as microglia and astrocytes, are involved in the regulation of nociception (Watkins et al., 2001, Svensson et al., 2003, McMahon et al., 2005, Svensson et al., 2005a), there are currently very few studies focusing on the role of oligodendrocytes in pain signal transduction.
Intrathecal antisense
The ability of the antisense oligonucleotide employed in this study to inhibit expression of cPLA2IVA was confirmed by assessing both transcript level and protein expression of cPLA2IVA. Intrathecal infusion of the antisense, at a rate that delivered a total daily dose of 200 µg, for 5 days, resulted in a substantial knockdown of cPLA2IVA mRNA (40% reduction) and protein (70% reduction) in the spinal cord tissue. The antisense oligonucleotide binds to the 5’ end of the protein coding region of rat cPLA2IVA mRNA, thus creating a substrate for RNase H-mediated degradation of the cPLA2 transcript (Crooke, 1992, Wahlestedt, 1994). We have previously shown that antisense oligonucleotides are effectively taken up by both neurons and non-neuronal cells after intrathecal delivery (Butler et al., 2005). This finding was confirmed in the present study as down-regulation of cPLA2 occurred not only in dorsal horn neurons but also in oligodendrocytes. The specificity of the knock down was defined in terms of lack of change in gene and protein expression of non-targeted but related proteins.
None of the other three major spinal PLA2 isoforms, i.e. iPLA2VI, sPLA2IIA and sPLA2V, were affected at the mRNA or the protein level by the dose and treatment regimen that significantly diminished the expression of spinal cPLA2IVA. This also indicates that there was no compensatory upregulation of these three PLA2 enzymes in response to decreased levels of cPLA2IVA within the timeframe of the treatment.
Spinal cPLA2 and hyperalgesia
The formalin model is a well-characterized rodent model of post-injury hyperalgesia (Dickenson and Sullivan, 1987, Puig and Sorkin, 1996, Yaksh, 1999, Yaksh et al., 2001). While the biphasic flinch response is evoked and maintained by persistent small afferent input (Puig and Sorkin, 1996), the second phase of flinching behavior reflects a facilitation of nociceptive processing at spinal and super-spinal level (Dickenson and Sullivan, 1987, Yaksh, 1999). It is important to note that while knockdown of cPLA2IVA attenuated the second phase flinching, the first phase flinching remained unchanged. In addition, thermal withdrawal thresholds were examined and AS treatment did not change the baseline of thermal latencies. These findings suggest that spinal cPLA2IVA is not involved in acute nociceptive processing, but participates in the complex biochemical events generated by persistent afferent input that lead to spinal sensitization (Svensson and Yaksh, 2002).
The hyperalgesic state observed in the formalin model, i.e. second phase flinching behavior, occurs as soon as 20 min following the injury. Thus, it is likely that an increase of enzymatic activity of cPLA2IVA rather than an increase in newly synthesized enzyme contributes to this augmented processing of nociception. We have demonstrated previously that activation of spinal neurokinin 1 (NK1) or N-methyl-D-Aspartate (NMDA) receptors results in hyperalgesia and an increase in PGE2 levels in the cerebrospinal fluid. Both endpoints are attenuated by inhibition of spinal cPLA2 (Lucas et al., 2005, Yaksh et al., 2006), suggesting that substance P (SP) and glutamate released from small afferents in response to noxious stimuli activate spinal cPLA2 and related cascade. Though other pain signaling systems are involved (Yaksh, 1999, Hunt and Mantyh, 2001), SP-NK1 and glutamate-NMDA are considered essential for development of hyperalgesia in several animal models of nociception including formalin-induced second phase pain behavior (Yamamoto and Yaksh, 1991, Chaplan et al., 1997, Suzuki et al., 2002). NK1- and NMDA-mediated spinal sensitization is closely associated with their ability to elevate intracellular level of calcium, see (Urban et al., 1994), which is also a critical factor regulating cPLA2IVA activity by either directly driving translocation of cPLA2IVA from the cytosol to the membrane (Channon and Leslie, 1990, Clark et al., 1995) or indirectly by activating various protein kinases such as ERK and p38 mitogenactivated protein kinases, which further enhance cPLA2IVA activity (Berenbaum et al., 2003, Svensson et al., 2003, Svensson et al., 2005a). Since both pharmacological inhibition (Lucas et al., 2005, Yaksh et al., 2006) as well as knockdown of cPLA2IVA by intrathecal AS attenuates hyperalgesia, these findings together point out the importance of cPLA2IVA in spinal pain processing.
We wish to emphasize that the anti-hyperalgesic effects associated with down-regulation of spinal cPLA2IVA could be due to effects on the production of lipid mediators in addition to eicosanoids. Thus, arachidonic acid, a product of cPLA2 activity, is regarded as a retrograde messenger in neurons and a neuromodulator for synaptic plasticity in CNS (Massicotte, 2000). Indeed, arachidonic acid itself has been shown to potentiate NMDA receptor activity resulting in modulation of neuronal excitability (Miller et al., 1992). Additionally, arachidonic acid is subject to non-enzymatic, free radical oxidation to bioactive isoprostanes, which produces nociception in animals and enhances sensitivity in sensory neurons (Evans et al., 2001). Concomitant with the release of arachidonic acid, lysophospholipid is formed and can be metabolized into platelet activating factor, which produces a prominent allodynia after spinal delivery (Morita et al., 2004). Since many of these lipid mediators derived from the linkages upstream of COX possess pro-nociceptive activity, we hypothesize that a more effective antinociception could be achieved by blocking PLA2, specifically cPLA2IVA.
In summary, the present work provides evidence that cPLA2IVA is constitutively expressed spinal dorsal horn neurons and oligodendrocytes. Selective knockdown of this enzyme in spinal cord by intrathecal AS does not affect acute pain sensation, but alleviates injury-induced exaggerated pain state. This finding provides additional support for the role of cPLA2IVA in facilitated processing of spinal nociception.
Acknowledgment
This work was supported by National Institute of Health Grants NS 16541 and DA 02110 (TLY).
List of Abbreviations
- APC
adenomatous polyposis coli
- AS
antisense oligonucleotide
- COX
cyclooxygenase
- cPLA2IVA
cytosolic calcium-dependent phospholipase A2 group IVA
- GFAP
glial fibrillary acidic protein
- iPLA2
calcium-independent phospholipase A2
- IT
intrathecal
- MS
missense oligonucleotide
- NK1
neurokinin 1 receptor
- NMDA
N-methyl-D-Aspartate receptor
- PLA2
phospholipase A2
- RT-PCR
Real-Time Reverse Transcription Polymerase Chain Reaction
- SP
substance P
- sPLA2
secretory phospholipase A2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balboa MA, Varela-Nieto I, Killermann Lucas K, Dennis EA. Expression and function of phospholipase A(2) in brain. FEBS Lett. 2002;531:12–17. doi: 10.1016/s0014-5793(02)03481-6. [DOI] [PubMed] [Google Scholar]
- Balsinde J, Balboa MA, Dennis EA. Antisense inhibition of group VI Ca2+independent phospholipase A2 blocks phospholipid fatty acid remodeling in murine P388D1 macrophages. J Biol Chem. 1997;272:29317–29321. doi: 10.1074/jbc.272.46.29317. [DOI] [PubMed] [Google Scholar]
- Berenbaum F, Humbert L, Bereziat G, Thirion S. Concomitant recruitment of ERK1/2 and p38 MAPK signalling pathway is required for activation of cytoplasmic phospholipase A2 via ATP in articular chondrocytes. J Biol Chem. 2003;278:13680–13687. doi: 10.1074/jbc.M211570200. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM. Expression of the APC tumor suppressor protein in oligodendroglia. Glia. 1996;17:169–174. doi: 10.1002/(SICI)1098-1136(199606)17:2<169::AID-GLIA8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Butler M, Hayes CS, Chappell A, Murray SF, Yaksh TL, Hua XY. Spinal distribution and metabolism of 2′-O-(2-methoxyethyl)-modified oligonucleotides after intrathecal administration in rats. Neuroscience. 2005;131:705–715. doi: 10.1016/j.neuroscience.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Channon JY, Leslie CC. A calcium-dependent mechanism for associating a soluble arachidonoyl-hydrolyzing phospholipase A2 with membrane in the macrophage cell line RAW 264.7. J Biol Chem. 1990;265:5409–5413. [PubMed] [Google Scholar]
- Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280:829–838. [PubMed] [Google Scholar]
- Clark JD, Schievella AR, Nalefski EA, Lin LL. Cytosolic phospholipase A2. J Lipid Mediat Cell Signal. 1995;12:83–117. doi: 10.1016/0929-7855(95)00012-f. [DOI] [PubMed] [Google Scholar]
- Clemens JA, Stephenson DT, Smalstig EB, Roberts EF, Johnstone EM, Sharp JD, Little SP, Kramer RM. Reactive glia express cytosolic phospholipase A2 after transient global forebrain ischemia in the rat. Stroke. 1996;27:527–535. doi: 10.1161/01.str.27.3.527. [DOI] [PubMed] [Google Scholar]
- Crooke ST. Therapeutic applications of oligonucleotides. Annual Review of Pharmacology and Toxicology. 1992;32:329–376. doi: 10.1146/annurev.pa.32.040192.001553. [DOI] [PubMed] [Google Scholar]
- Cummings BS, McHowat J, Schnellmann RG. Phospholipase A(2)s in cell injury and death. J Pharmacol Exp Ther. 2000;294:793–799. [PubMed] [Google Scholar]
- Deutsch DG, Omeir R, Arreaza G, Salehani D, Prestwich GD, Huang Z, Howlett A. Methyl arachidonyl fluorophosphonate: a potent irreversible inhibitor of anandamide amidase. Biochem Pharmacol. 1997;53:255–260. doi: 10.1016/s0006-2952(96)00830-1. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Subcutaneous formalin-induced activity of dorsal horn neurones in the rat: differential response to an intrathecal opiate administered pre or post formalin. Pain. 1987;30:349–360. doi: 10.1016/0304-3959(87)90023-6. [DOI] [PubMed] [Google Scholar]
- Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. J Neurosci Methods. 1997;76:183–191. doi: 10.1016/s0165-0270(97)00097-6. [DOI] [PubMed] [Google Scholar]
- Ebersberger A, Grubb BD, Willingale HL, Gardiner NJ, Nebe J, Schaible HG. The intraspinal release of prostaglandin E2 in a model of acute arthritis is accompanied by an up-regulation of cyclo-oxygenase-2 in the spinal cord. Neuroscience. 1999;93:775–781. doi: 10.1016/s0306-4522(99)00164-5. [DOI] [PubMed] [Google Scholar]
- Evans JH, Spencer DM, Zweifach A, Leslie CC. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes. J Biol Chem. 2001;276:30150–30160. doi: 10.1074/jbc.M100943200. [DOI] [PubMed] [Google Scholar]
- Fernando SR, Pertwee RG. Evidence that methyl arachidonyl fluorophosphonate is an irreversible cannabinoid receptor antagonist. Br J Pharmacol. 1997;121:1716–1720. doi: 10.1038/sj.bjp.0701303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SH. Prostaglandins, aspirin-like drugs and analgesia. Nat New Biol. 1972;240:200–203. doi: 10.1038/newbio240200a0. [DOI] [PubMed] [Google Scholar]
- Ghilardi JR, Svensson CI, Rogers SD, Yaksh TL, Mantyh PW. Constitutive spinal cyclooxygenase-2 participates in the initiation of tissue injury-induced hyperalgesia. J Neurosci. 2004;24:2727–2732. doi: 10.1523/JNEUROSCI.5054-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover S, de Carvalho MS, Bayburt T, Jonas M, Chi E, Leslie CC, Gelb MH. Translocation of the 85-kDa phospholipase A2 from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen. J Biol Chem. 1995;270:15359–15367. doi: 10.1074/jbc.270.25.15359. [DOI] [PubMed] [Google Scholar]
- Hager G, Eckert E, Schwaiger FW. Semiquantitative analysis of low levels of mRNA expression from small amounts of brain tissue by nonradioactive reverse transcriptase-polymerase chain reaction. J Neurosci Methods. 1999;89:141–149. doi: 10.1016/s0165-0270(99)00048-5. [DOI] [PubMed] [Google Scholar]
- Hua XY, Moore A, Malkmus S, Murray SF, Dean N, Yaksh TL, Butler M. Inhibition of spinal protein kinase Calpha expression by an antisense oligonucleotide attenuates morphine infusion-induced tolerance. Neuroscience. 2002;113:99–107. doi: 10.1016/s0306-4522(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- Kishimoto K, Matsumura K, Kataoka Y, Morii H, Watanabe Y. Localization of cytosolic phospholipase A2 messenger RNA mainly in neurons in the rat brain. Neuroscience. 1999;92:1061–1077. doi: 10.1016/s0306-4522(99)00051-2. [DOI] [PubMed] [Google Scholar]
- Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Ling EA, Kaur LC, Yick TY, Wong WC. Immunocytochemical localization of CR3 complement receptors with OX-42 in amoeboid microglia in postnatal rats. Anat Embryol (Berl) 1990;182:481–486. doi: 10.1007/BF00178913. [DOI] [PubMed] [Google Scholar]
- Liu NK, Zhang YP, Titsworth WL, Jiang X, Han S, Lu PH, Shields CB, Xu XM. A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann Neurol. 2006;59:606–619. doi: 10.1002/ana.20798. [DOI] [PubMed] [Google Scholar]
- Lucas KK, Svensson CI, Hua XY, Yaksh TL, Dennis EA. Spinal phospholipase A2 in inflammatory hyperalgesia: role of group IVA cPLA2. Br J Pharmacol. 2005;144:940–952. doi: 10.1038/sj.bjp.0706116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. Journal of Pharmacology and Experimental Therapeutics. 1992;263:136–146. [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Cyclooxygenase inhibition and the spinal release of prostaglandin E2 and amino acids evoked by paw formalin injection: a microdialysis study in unanesthetized rats. J Neurosci. 1995;15:2768–2776. doi: 10.1523/JNEUROSCI.15-04-02768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massicotte G. Modification of glutamate receptors by phospholipase A2: its role in adaptive neural plasticity. Cell Mol Life Sci. 2000;57:1542–1550. doi: 10.1007/PL00000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Miller B, Sarantis M, Traynelis SF, Attwell D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992;355:722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- Morita K, Morioka N, Abdin J, Kitayama S, Nakata Y, Dohi T. Development of tactile allodynia and thermal hyperalgesia by intrathecally administered platelet-activating factor in mice. Pain. 2004;111:351–359. doi: 10.1016/j.pain.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Ong WY, Horrocks LA, Farooqui AA. Immunocytochemical localization of cPLA2 in rat and monkey spinal cord. J Mol Neurosci. 1999;12:123–130. doi: 10.1007/BF02736926. [DOI] [PubMed] [Google Scholar]
- Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Ramos KM, Jiang Y, Svensson CI, Calcutt NA. Pathogenesis of spinally mediated hyperalgesia in diabetes. Diabetes. 2007;56:1569–1576. doi: 10.2337/db06-1269. [DOI] [PubMed] [Google Scholar]
- Robinson AP, White TM, Mason DW. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986;57:239–247. [PMC free article] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- Southall MD, Michael RL, Vasko MR. Intrathecal NSAIDS attenuate inflammation-induced neuropeptide release from rat spinal cord slices. Pain. 1998;78:39–48. doi: 10.1016/S0304-3959(98)00113-4. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Fitzsimmons B, Azizi S, Powell HC, Hua XY, Yaksh TL. Spinal p38beta isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. J Neurochem. 2005a;92:1508–1520. doi: 10.1111/j.1471-4159.2004.02996.x. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Lucas KK, Hua XY, Powell HC, Dennis EA, Yaksh TL. Spinal phospholipase A2 in inflammatory hyperalgesia: role of the small, secretory phospholipase A2. Neuroscience. 2005b;133:543–553. doi: 10.1016/j.neuroscience.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Yaksh TL. The spinal phospholipase-cyclooxygenase-prostanoid cascade in nociceptive processing. Annu Rev Pharmacol Toxicol. 2002;42:553–583. doi: 10.1146/annurev.pharmtox.42.092401.143905. [DOI] [PubMed] [Google Scholar]
- Urban L, Thompson SW, Dray A. Modulation of spinal excitability: cooperation between neurokinin and excitatory amino acid neurotransmitters. Trends Neurosci. 1994;17:432–438. doi: 10.1016/0166-2236(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C. Antisense oligonucleotide strategies in neuropharmacology. Trends Pharmacol Sci. 1994;15:42–46. doi: 10.1016/0165-6147(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Yaksh TL. Central and peripheral mechanisms for the analgesic action of acetylsalicylic acid. In: Barett HJM, et al., editors. Acetylsalicylic acid: new uses for an old drug. New York: Raven; 1982. pp. 137–151. [Google Scholar]
- Yaksh TL. Spinal systems and pain processing: development of novel analgesic drugs with mechanistically defined models. Trends Pharmacol Sci. 1999;20:329–337. doi: 10.1016/s0165-6147(99)01370-x. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Kokotos G, Svensson CI, Stephens D, Kokotos CG, Fitzsimmons B, Hadjipavlou-Litina D, Hua XY, Dennis EA. Systemic and intrathecal effects of a novel series of phospholipase A2 inhibitors on hyperalgesia and spinal prostaglandin E2 release. J Pharmacol Exp Ther. 2006;316:466–475. doi: 10.1124/jpet.105.091686. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, Yaksh MC. An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol. 2001;90:2386–2402. doi: 10.1152/jappl.2001.90.6.2386. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TS. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yaksh TL. Stereospecific effects of a nonpeptide NK1 selective antagonist, CP-96,345: Antinociception in the absence of motor dysfunction. Life Sciences. 1991;49:1955–1963. doi: 10.1016/0024-3205(91)90637-q. [DOI] [PubMed] [Google Scholar]