Abstract
Objective
Calcium pyrophosphate dihydrate crystals (CPPD) are commonly found in osteoarthritic joints and correlate with a poor prognosis. Intra-articular corticosteroids, such as dexamethasone (Dxm), are commonly used therapies for osteoarthritis with or without CPPD deposition. Dxm has variable effects in mineralization models. We investigated the effects of Dxm on CPPD crystal formation in a well established tissue culture model.
Methods
Porcine articular chondrocytes were incubated with ATP to generate CPPD crystals. Chondrocytes incubated with or without ATP were exposed to 1–100 nM Dxm in the presence of 45Ca. Mineralization was measured by 45Ca uptake in the cell layer. We also investigated the effect of Dxm on mineralization-regulating enzymes such as alkaline phosphatase, NTPPPH and transglutaminase.
Results
Dxm significantly increased ATP-induced mineralization by articular chondrocytes. While alkaline phosphatase and NTPPPH activities were unchanged by Dxm, transglutaminase activity increased in a clear dose responsive manner. Levels of factor XIIIA mRNA and protein were increased by Dxm, while type II Tgase protein was unchanged. Transglutaminase inhibitors suppressed Dxm-induced increases in CPPD crystal formation.
Conclusion
These findings suggest a potential for Dxm to contribute to pathologic mineralization in cartilage and reinforce a central role for the transglutaminase enzymes in CPPD crystal formation.
Keywords: Dexamethasone, chondrocytes, calcium pyrophosphate dihydrate, osteoarthritis, transglutaminases
Calcium pyrophosphate dihydrate crystals (CPPD) occur commonly in osteoarthritic joints. At the time of knee replacement for osteoarthritis, for example, 60% of synovial fluids possess either CPPD and/or hydroxyapatite-like basic calcium phosphate crystals. (1) (2) Although the role of these crystals is not completely understood, they likely contribute to joint damage. Clinically, their presence predicts increased joint damage and rapid destruction of cartilage. (3) (2) In vitro, calcium crystals induce the production of proteases and catabolic cytokines from synovial cells and chondrocytes. (4)
While no specific therapies for this form of pathologic mineralization currently exist, intra-articular corticosteroids are widely used clinically for both osteoarthritis and calcium-crystal deposition diseases. (5) Corticosteroids, including dexamethasone (Dxm), are steroid hormones with a broad range of physiologic and pharmacologic effects. Pharmacologic effects on mineralization vary depending on the route of administration. While systemic corticosteroid administration may reduce bone mineralization, (6) intra-articular corticosteroids have been associated clinically with increased tissue mineralization. (7) This has been reproduced in vitro, as repeated injections of methylprednisolone resulted in increased articular calcifications in rabbit joints. (8) Previous work in fetal chondrocytes and other cell types have suggested that Dxm may increase mineralization by altering the chondrocyte phenotype. (9, 10) However, little is known about the effects of corticosteroids on mammalian articular cartilage mineralization.
CPPD crystal formation is relatively unique to articular cartilage. While the actors involved in the formation of these crystals are not fully delineated, key participants in this process have been identified. CPPD crystal formation in chondrocyte pericellular matrix is likely facilitated by extracellular organelles, known as matrix vesicles. (11) Excess quantities of extracellular ATP (12) or its metabolite pyrophosphate (13) are elaborated by abnormal chondrocytes that resemble the hypertrophic chondrocytes responsible for matrix mineralization in bone. (14) In addition, abnormalities of the extracellular matrix, characterized by increased levels of osteopontin (15) and high activities of the protein cross-linking transglutaminase enzymes (16), also contribute to CPPD crystal formation. Two transglutaminase enzymes, type II transglutaminase and factor XIIIA, have been well characterized in articular chondrocytes, (17) and the latter may play a particularly important role in CPPD crystal formation.
The purpose of the current investigation was to determine the effect of Dxm on CPPD crystal formation by articular chondrocytes, and to investigate the mechanism of this effect.
METHODS AND MATERIALS
Materials
All reagents were from Sigma Aldrich Chemical Co. (St. Louis, MO) unless otherwise noted.
Chondrocyte Cultures
Normal articular cartilage from 3–5 year old pigs (Johnsonville Foods, Watertown, WI) was collected shortly after slaughter from the tibial, patellar, and femoral surfaces of knee joints. Chondrocytes were enzymatically isolated and plated at high density (4.5×105 cells/cm2) as previously described. (18) Cells were plated in Dulbecco’s Modified Eagle’s Medium (DMEM, Mediatech, Herndon, VA) with 10% fetal calf serum and 1% Penicillin-Streptomycin-Fungizone® (PSF, Invitrogen, Carlsbad, CA). These conditions have been shown to maintain the differentiated chondrocyte phenotype for up to 10 days. (19) Media were changed 24 hours prior to each experiment and replaced with fresh DMEM containing 0.35 mg/ml bovine serum albumin and 1% PSF.
ATP-induced CPPD crystal formation (20)
Chondrocytes were plated in 24 well culture plates and treated with 1–100nM of Dxm in 0.1% (v/v) methanol or vehicle (methanol) alone. Media included 1mM ATP to assess CPPD formation, 1 mM β-glycerophosphate as a control for added phosphate, or no other addition. Cultures were incubated with1μCi/ml 45Ca (Perkins-Elmer, Boston, MA) for 96 hours at 37°C. The cell layer was washed thoroughly with cold Hank’s Balanced Salt Solution (HBSS, Mediatech, Herndon, VA) and treated with 0.1N NaOH. In this assay, 45Ca uptake by the cell layers in the presence of ATP correlates with CPPD crystal formation, proven by biochemical, morphologic (20) and spectroscopic methods (21). Radioactivity in the cell layer was quantified by liquid scintigraphy in a Packard 1900TR scintillation counter. Levels of protein in the samples were determined by Lowry assay. (22)
RNA extraction
Total RNA from chondrocytes was isolated by the acidified guanidinium isothiocyanate method, using TRIzol (Invitrogen). The RNA concentration was quantified using optical density at 260 nm. A ratio of OD260/280 between 1.8 and 2.0 was used as the range of acceptable purity for the RNA samples.
Semi-quantitative RT-PCR
Total RNA (1 μg) was reverse transcribed using random hexamer primers and the Superscript First Strand Synthesis System for RT-PCR (Invitrogen,) in a 20 μl reaction volume. The generated cDNA was amplified by PCR in an iCycler thermal cycler (BioRad, Hercules, CA) using PLATINUM™ Blue PCR Supermix (Invitrogen) with 0.4 μM primers and at the optimal hot start temperature of 94°C. Factor XIIIA, OPN, and type II transglutaminase were individually multiplexed with 18S ribosomal RNA from the QuantumRNA 18S kit (Ambion, Austin, TX) which served as an internal standard and was used to correct for any differences in starting amounts of RNA. All gene specific primers were synthesized by Integrated DNA Technologies (Coralville, IA) from published primer pair sequences as follows for factor XIIIA: 5′-TCA ATG ACA TCA AGA CCA GAA G-3′ and 5′-GAC ACC AGC AAA AAC CCA G-3′ (17) ; OPN: 5′-CAC CTA AGA AGA CGA GTC AG-3′ and 5′-CAT TCA CCA ACT AAG CTG AG-3′ (23) and type II transglutaminase: 5′-CCG TTT TCC ACT AAG AGA TGC -3′ and 5′-CCC AAA ATT CCA AGG TAT GTT -3′ (24). The number of amplification cycles was empirically determined to ensure PCR products were within the exponential phase of the amplification curve. PCRs were performed as follows: 2 min at 94°C, followed by 26–30 cycles of 94°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec, followed by 5 min at 72°C. Ten ol of PCR product were separated on a 2% agarose gel in Tris-borate EDTA buffer containing 1 μg/ml ethidium bromide. The gel was photographed under UV light and bands were quantified using densitometry with Kodak 1D Image Analysis software. Relative expression was calculated as the ratio of band intensity of PCR product/18S control band.
Western Blotting
Proteins used for Western blotting were obtained from cultured chondrocytes after Trizol was used to isolate RNA. Identical quantities of protein were loaded onto 10% NuPage® Bis-Tris gels (Invitrogen). After electrophoresis, protein were blotted onto poly(vinylidene) difluoride membranes (Invitrogen). These membranes were blocked in a TBS-igepal-5% skim milk buffer for 1 hour at room temperature. They were then exposed to either rabbit anti-factor X111A Ab-2 (1:1000, NeoMarkers, Fremont, CA) or rabbit anti-transglutaminase II Ab-4 (1:1000, NeoMarkers) for 1.5 hours. After washing, the membranes were exposed to peroxidase-goat anti-rabbit IgG (H+L) secondary antibody for 1 hour (1:2500, Invitrogen). Both the primary and secondary antibody exposures were done in a TBS-igepal-0.5% skim milk buffer. SuperSignal® West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL) was used to visualize immunoreactive protein bands.
Enzyme Assays
Alkaline phosphatase was measured using p-nitrophenol as a chromogenic substrate (Sigma Kit). Nucleoside triphosphate pyrophosphohydrolase (NTPPPH) generates pyrophosphate from ATP and other nucleoside triphosphates. Activity was measured using 2mM p-nitrophenol thymidine monophosphate as the substrate, as previously described. (25)
Collagen content
Collagen content was determined by measuring levels of hydroxyproline. (26) All results were corrected for protein using the Lowry assay.
Elaboration of matrix vesicles
Articular cartilage vesicle elaboration by chondrocyte monolayers was assessed by isolating vesicles from the cell layer and measuring the protein content of the vesicle-containing fraction. This method uses differential centrifugation of scraped cell layers to isolate the articular cartilage vesicle fraction. (27) Protein levels were measured using the Lowry assay.
Transglutaminase activity: (28)
Transglutaminase activity was measured with a standard radiometric assay based on the ability of these enzymes to incorporate radiolabeled putrescine into dimethyl casein. Chondrocytes were treated with 1–100nM Dxm or vehicle and incubated at 37 °C for 96 hours. Media were removed and the chondrocyte cell layer was washed with HBSS. The cells were scraped in 0.1% EDTA, sonicated, and 150 μl of cell suspension were added to 150 ul of 2X reaction mixture (250 mM Tris-HCl, 30mM CaCl2 10 mg/ml N,N′dimethyl casein, 200 μM putrescine, pH 8.2). One μCi 3H-putrescine (GE Healthcare, Piscataway, NJ) was added to each sample and incubated for 30 min at 37 °C. Protein was precipitated in 50% trichloroacetic acid (TCA) and spotted on Whatman glass filters. Filters were washed with 10% TCA and counted in a Packard 1900TR scintillation counter. Results were expressed in Units (picomoles of (3H)putrescine incorporated into dimethyl casein/30 minutes) and corrected for protein using the Lowry assay.
Transglutaminase inhibitors
We inhibited transglutaminase activity by using two previously characterized transglutaminase inhibitors. Cystamine (125uM) and monodansylcadaverine (1 mM) inhibit chondrocyte transglutaminase activity by 50–90% without significant toxicity. (16)
Cytotoxicity
Cytotoxicity was assessed using a commercial assay based on leakage of lactate dehydrogenase from injured cells (Genotech, St. Louis, MO).
Statistics
All experiments were repeated at least three times. The Students′ t test was used to determine statistical significance between groups.
RESULTS
We evaluated the effect of Dxm on CPPD crystal formation in the well-characterized ATP-induced mineralization model. In this model, 45Ca precipitated by the cell layer in the presence of ATP reflects the generation of CPPD crystals (20, 21). Controls included cultures incubated with media alone or with β glycerophosphate to account for nonspecific incorporation and an alternative phosphate source respectively. ATP-induced mineralization increased by 37 ± 1.9% over vehicle control at 10 nM Dxm (p<0.01), while 1nM and 100nM Dxm concentration increased mineralization by 34 ± 4.5% and 36 ± 2.2% respectively (p<0.05) (Figure 1). β glycerophosphate produced no significant changes in 45Ca incorporation over control values. No differences were seen between the vehicle and no vehicle controls (Data not shown). Dxm had no effect on cell viability at any of the concentrations tested (Data not shown). Fourier Transform Infrared (FTIR) Spectroscopy of cell layers incubated with ATP and Dxm confirmed the presence of CPPD crystals.(21)
Figure 1. The effect of Dxm on CPPD crystal formation by chondrocytes.

Chondrocytes were incubated with 45Ca in the presence of 1mM ATP (black bars), 1 mM β-glycerophosphate (grey bars), or no additives (white bars), and with or without various concentrations of Dxm. 45Ca in the cell layer was measured after 96 hours using liquid scintigraphy and corrected for cell protein. Results represent means ± standard deviations (n= 6). Dxm increased ATP-dependent 45Ca uptake at all concentrations tested (p<.05).
We then explored the potential mechanism of this effect. Previous studies suggested that Dxm could affect the elaboration of matrix vesicles, (29) or the activity of mineralization- regulating enzymes such as alkaline phosphatase. (10) The elaboration of matrix vesicles was measured in chondrocyte cultures treated with vehicle alone or with Dxm. There was no change in the protein content of the vesicle fraction in the presence of Dxm, suggesting that vesicle formation was not increased by Dxm (Table 1). Similarly, Dxm had no effect on activities of the mineralization -regulating enzymes, alkaline phosphatase or NTPPPH (Table 1).
Table 1.
Levels of mineralization-regulating enzymes and collagen content with Dxm treatment.
| Table 1 | Vehicle | 10nM Dexamethasone |
|---|---|---|
| Alk Phos (U/mg protein) | 0.30 ± 0.06 | 0.24 ± 0.08 |
| NTPPPH (U/mg protein) | 231.9 ± 2.3 | 244.2 ± 3.0 |
| Hydroxyproline content (ug /mg protein) | 0.26 ± 0.02 | 0.24 ± 0.02 |
| Elaboration of matrix vesicles (mg Dxm/mg vehicle) | 100% | 100 ± 11% |
Specific activity of alkaline phosphatase (Alk Phos) and NTPPPH were assayed after a 96 hour incubation with either vehicle or 10 nM Dxm. Hydroxyproline and vesiculation were measured in the cell layer after a similar incubation. Results were corrected for protein in the sample. There were no significant differences in the specific activities of these enzymes, elaboration of vesicles, or hydroxyproline content after Dxm exposure.
We then examined the effect of Dxm on levels of extracellular matrix proteins implicated in CPPD crystal formation. As Dxm often increases fibrosis in tissues, and collagen may alter mineralization (30), we asked whether the hydroxyproline content of the cultures was affected by Dxm treatment. As shown in Table 1, there was no change in collagen content under conditions in which Dxm stimulated CPPD crystal formation (Table 1). Previous work also supports a role for extracellular osteopontin in CPPD deposition disease. (15) Levels of osteopontin mRNA and protein were unchanged in chondrocytes treated with Dxm (Figure 2).
Figure 2. The effect of Dxm on protein and mRNA levels of osteopontin.

Semi-quantitative RT-PCR was performed on chondrocytes treated with vehicle or 10 nM Dxm for 96 hour with primers for OPN and compared to 18S. Band intensities were quantified with densitometry. Bars represent means ± SEM (n = 5). Identical quantities of protein from chondrocytes incubated with vehicle and 10 nM Dxm were loaded onto 10 % NuPage Bis-Tris gels. After electrophoresis, proteins were transferred onto PVDF membranes and exposed to antibodies to OPN. Immunoreactive bands were detected with secondary antibody and chemiluminescence. In this representative figure (n=5), lane 1 is control, lane 2 is Dxm treatment.
Next, we examined the effect of Dxm on transglutaminase activity. These cross-linking enzymes are increased in aging and osteoarthritic cartilage and clearly contribute to CPPD crystal formation. (16) Transglutaminase activity was dramatically increased by Dxm (Figure 3). The peak effect was seen at 100 nM Dxm with a 76 ± 9 % increase over the vehicle control (p<0.001). The 10 nM concentration, which had been the peak of the mineralization effect, showed a 44 ± 10 % increase over vehicle control (p<0.01).
Figure 3. The effect of Dxm on transglutaminase activity.
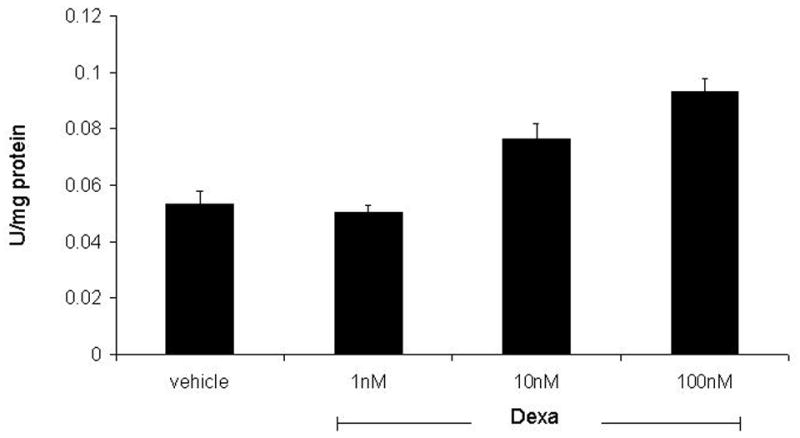
Chondrocytes were treated with vehicle or various concentrations of Dxm for 96 hours and assayed for transglutaminase activity using a radiometric assay. Bars represent means ± standard deviations (n=6). Dxm increased transglutaminase activity at concentrations greater than 1 nM (p<0.01).
We then investigated the effect of Dxm on levels of transglutaminase mRNA and protein. Ten nM Dxm was chosen because it produced a peak effect on mineralization and significantly increased transglutaminase activity. There are two transglutaminase enzymes in chondrocytes and the activity assay cannot distinguish between them. As shown in Figure 4, Dxm produced a significant increase in factor XIIIA mRNA (p<0.05) and a trend for slightly decreased levels of type II Tgase mRNA (p>0.05). Western blotting confirmed a slight increase in factor XIIIA protein levels with Dxm treated cells, while no consistent changes in protein levels of type II Tgase were noted (Figure 4).
Figure 4. The effect of Dxm on protein and mRNA levels of the transglutaminase enzymes factor XIIIA and type II transglutaminase.
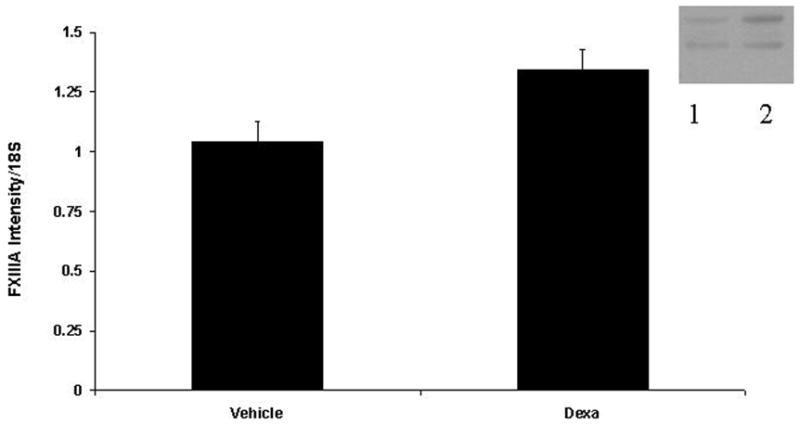
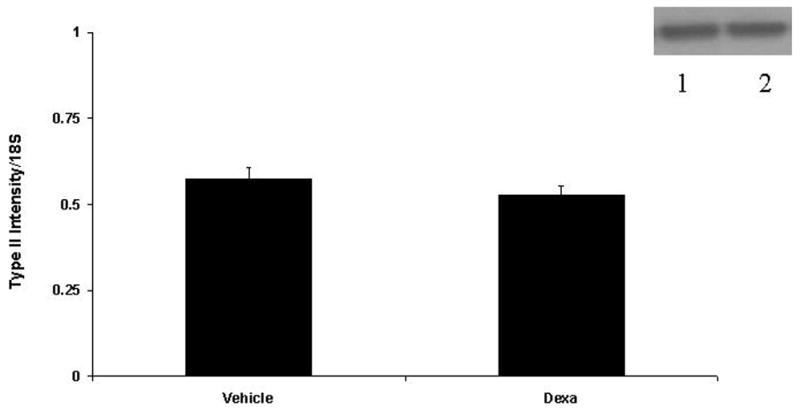
Semi-quantitative RT-PCR was performed on mRNA from chondrocytes treated with vehicle or 10 nM Dxm for 96 hour with primers for factor XIIIA (A) and type II transglutaminase (B) and compared to 18S. Band intensities were quantified with densitometry. Bars represent means ± SEM (n = 5). Identical quantities of protein from chondrocytes incubated with vehicle and 10 nM Dxm were loaded onto 10 % NuPage Bis-Tris gels. After electrophoresis, proteins were transferred onto PVDF membranes and exposed to antibodies to either Factor XIIIA (A) or type II transglutaminase (B). Immunoreactive bands were detected with secondary antibody and chemiluminescence. In this representative figure (n=5), lane 1 is control, lane 2 is Dxm treatment.
To determine whether the effect of Dxm on CPPD crystal formation was dependent on transglutaminase activity, we repeated the mineralization experiments with and without the transglutaminase inhibitors, cystamine and monodansylcadaverine. As shown in Figure 5, there was a marked decrease in Dxm’s ability to stimulate CPPD crystal formation in the presence of either transglutaminase inhibitor (p<0.05).
Figure 5. The effect of transglutaminase inhibitors on Dxm’s ability to stimulate CPPD crystal formation.

Chondrocytes were incubated with vehicle or Dxm in media containing 45Ca with ATP (black bars), β glycerophosphate (grey bars) or no additives (white bars). Some of the chondrocytes were exposed to the transglutaminase inhibitors cystamine (125 μM) or monodansylcadaverine (MDC, 1mM). After 96 hours cell layers were washed and 45Ca uptake in the cell layer was determined by liquid scintigraphy and corrected for cell protein. Results are expressed as means ± standard deviations (n=6). Both of the transglutaminase inhibitors significantly reduced the effects of Dxm on CPPD crystal formation (p< 0.001).
DISCUSSION
The present study was undertaken to investigate the effect of Dxm on CPPD crystal formation by articular chondrocytes. Because of the common therapeutic use of intra-articular corticosteroids for both osteoarthritis and CPPD deposition disease, chondrocytes could conceivably be exposed to significant concentrations of corticosteroids. Using a well-established model of CPPD crystal formation, we demonstrated a 34–40% increase in CPPD crystal formation in chondrocytes exposed to Dxm. The formation of CPPD crystals by ATP-exposed chondrocytes has been verified through multiple methods (20), and most recently has been proven by synchrotron FTIR spectroscopy (21). The observation that Dxm did not change 45Ca precipitation in the presence of an alternative phosphate source such as β-glycerophosphate supports the specificity of this effect, and these crystals were characterized as CPPD crystals by FTIR. We believe that these results clearly illustrate that Dxm significantly stimulates CPPD formation in primary articular chondrocytes.
Clinically, there is good support for the concept that pharmacologic doses of corticosteroids may increase pathologic mineralization. Soft tissue calcifications after intra-articular steroid injections are well described. (31) Additionally, instances of intra-articular calcification have been observed after corticosteroid injections in young patients with juvenile arthritis. (7) While the tissue location of these calcifications in many of these clinical studies is uncertain, rabbits given repeated intra-articular injections of methylprednisolone developed dramatic cartilage calcifications. (8) The calcifications in the rabbit model were basic calcium phosphate, as small animals such as rodents and rabbits have never been shown to make CPPD crystals.
With further investigation we were able to determine a possible mechanism for Dxm’s effect on chondrocytes. The transglutaminase enzymes, factor XIIIA and type II transglutaminase, participate in CPPD crystal formation by chondrocytes. (28) (16) We noted a significant increase in transglutaminase activity in chondrocytes treated with Dxm. Previous work showed a similar effect of Dxm on transglutaminase activity in hamster fibrosarcoma cells. (32).
Dxm increased both mRNA and protein levels of factor XIIIA in chondrocytes, while levels of mRNA and protein for type II transglutaminase remained unchanged. In contrast, both mRNA and protein levels of factor XIIIA increased with Dxm exposure. Furthermore, treatment with transglutaminase inhibitors significantly decreased Dxm’s effect on mineralization, reinforcing this probable mechanism. We did note differences in the “dose-response” curves for Dxm in the mineralization and transglutaminase assays. This is not unexpected as mineral formation is a complex process dependent on multiple factors, while enzyme activity is a much simpler cellular response. The lack of a complete reversal of pathologic mineralization by the inhibitors also leaves open the possibility of additional mechanisms. However, other factors associated with CPPD crystal formation, such as articular cartilage vesicle elaboration, osteopontin levels, collagen content, and alkaline phosphatase and NTPPPH enzyme activities showed little change with Dxm.
Prior studies have explored the effects of Dxm in other mineralization models. Corticosteroids have physiologic effects on bone mineralization and participate in longitudinal bone growth. In pharmacologic doses, however, they contribute to osteoporosis. In growth plate and fetal chondrocytes, Dxm increased mineralization by stimulating production of insulin-like growth factor. (33) (34) (10) In osteoblasts, corticosteroids were found to inhibit cell growth, but increase alkaline phosphatase activity. (35) Additionally, in vascular pericytes, Dxm (in concentrations similar to those used here) promoted mineralization by reducing levels of inhibitory factors such as osteopontin which interfere with bone mineral formation. (9)
Other factors have been implicated in CPPD crystal deposition, including increased activity of matrix metalloproteases, alterations in proteoglycan profiles, increased TGFβ levels, and stimulation of pyrophosphate production. Dxm may have a role in matrix metalloprotease formation and activity (36) (37). Proteoglycan production has also been shown to decrease with Dxm treatment in both articular chondrocytes and tenocytes. (38) (39) Finally, Dxm may play an inhibitory role in the TGFβ intracellular signaling cascade. (40) These actions could contribute to Dxm’s effect on CPPD crystal formation, and further evaluation of these potential mechanisms is currently underway in our laboratory.
These studies are not without limitations. The dosage and form of Dxm may not fully mimic the conditions to which chondrocytes are exposed in vivo to intra-articular corticosteroids. However, in horses, 100 mg of intra-articular Dxm produced intra-articular levels of 10−6 to 10−8 M (41) (42). In addition, Dxm may affect multiple cell types and have multiple actions within the joint. For example, the effect of Dxm on “inflammatory “cytokines or proteases released by synovial cells may outweigh its effects on CPPD crystal formation.
In summary, we demonstrated that Dxm stimulates CPPD crystal formation by articular chondrocytes. It appears that this effect is at least partially mediated by an increase in transglutaminase activity which occurs through stimulation of factor XIIIA at the transcriptional level. While we certainly would not recommend removing corticosteroids from the already limited therapeutic armamentarium for CPPD deposition disease, this work suggests an additional potential detrimental effect of intra-articular steroids on articular cartilage which warrants further investigation.
Acknowledgments
This work was supported by NIH grants AG-015337 (AKR) and a VA Merit Review grant.
References
- 1.Derfus B, Kurian J, Butler J, Daft L, Carrera G, Ryan L, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol. 2002;29:570–4. [PubMed] [Google Scholar]
- 2.Nalbant S, Martinez J, Kitumnuaypong T, Clayburne G, Sieck M, Schumacher H., Jr Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthritis Cart. 2003;11:50–4. doi: 10.1053/joca.2002.0861. [DOI] [PubMed] [Google Scholar]
- 3.Ledingham J, Regan M, Jones A, Doherty M. Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Ann Rheum Dis. 1993;52:520–6. doi: 10.1136/ard.52.7.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung H. Calcium crystal effects on the cells of the joint: implications for the pathogenesis of disease. Curr Opinion Rheumatology. 2001;12:223–7. doi: 10.1097/00002281-200005000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Masuda I, Ishikawa K. Clinical features of pseudogout attack: A survey of 50 cases. Clin Orth Rel Res. 1987;229:173–81. [PubMed] [Google Scholar]
- 6.Canalis E. Mechanisms of glucocorticoid action in bone: Implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab. 1996;81:3441–7. doi: 10.1210/jcem.81.10.8855781. [DOI] [PubMed] [Google Scholar]
- 7.Gilsanz V, Bernstein B. Joint calcification following intra-articular corticosteroid therapy. Radiology. 1984;151:647–9. doi: 10.1148/radiology.151.3.6718724. [DOI] [PubMed] [Google Scholar]
- 8.Ohira T, Ishikawa K. Hydroxyapatite deposition in articular cartilage by intra-articular injections of methylprednisolone. J Bone Jt Surg. 1986;68:509–19. [PubMed] [Google Scholar]
- 9.Kirton J, Wilkinson F, Canfield A, Alexander M. Dexamethasone downregulates calcification-inhibitor molecules and accelerates osteogenic differentiation of vascular pericytes. Circ Res. 2006;98:1264–72. doi: 10.1161/01.RES.0000223056.68892.8b. [DOI] [PubMed] [Google Scholar]
- 10.Nadra R, Menuelle P, Chevallier S, Berdal A. Regulation by glucocorticoids of cell differentiation and insulin-like growth factor binding protein production in cultured fetal rat nasal chondrocytes. J Cell Biochem. 2003;88:911–22. doi: 10.1002/jcb.10396. [DOI] [PubMed] [Google Scholar]
- 11.Derfus B, Rachow J, Mandel N, Boskey A, Buday M, Kushnaryov V, et al. Articular cartilage vesicles generate calcium pyrophosphate dihydrate-like crystals in vitro. Arthritis Rheum. 1992;35:231–240. doi: 10.1002/art.1780350218. [DOI] [PubMed] [Google Scholar]
- 12.Ryan L, Rosenthal A. Metabolism of extracellular pyrophosphate. Curr Opin Rheum. 2003;15:311–4. doi: 10.1097/00002281-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Ryan L, Cheung H, McCarty D. Release of pyrophosphate by normal mammalian articular hyaline and fibrocartilage in organ culture. Arthritis Rheum. 1981;124:1522–7. doi: 10.1002/art.1780241211. [DOI] [PubMed] [Google Scholar]
- 14.Masuda I, Ishikawa K, Usuku G. A histologic and immunohistochemical study of calcium pyrophosphate dihydrate crystal deposition disease. Clin Orth. 1991;263:272–287. [PubMed] [Google Scholar]
- 15.Rosenthal A, Gohr C, Uzuki M, Masuda I. Osteopontin promotes pathologic mineralization in articular cartilage. Matrix Biol. 2007;26:96–105. doi: 10.1016/j.matbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinkel D, Gohr C, Uzuki M, Rosenthal A. Transglutaminase contributes to CPPD crystal formation in osteoarthritis. Frontiers in Bioscience. 2004;9:3257–61. doi: 10.2741/1477. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal A, Masuda I, Gohr C, Derfus B, Le M. The transglutaminase, Factor XIIIA, is present in articular chondrocytes. Osteoarthritis and Cartilage. 2001;9:578–81. doi: 10.1053/joca.2000.0423. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal A, Heinkel D, Gohr C. Thyroxine stimulates transglutaminase activity in articular chondrocytes. Osteoarthritis Cart. 2003;11:463–70. doi: 10.1016/s1063-4584(03)00074-8. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell P, Struve J, McCarthy G, Cheung H. Basic calcium phosphate crystals stimulate cell proliferation and collagenase message accumulation in cultured adult articular chondrocytes. Arthritis Rheum. 1992;35:343–350. doi: 10.1002/art.1780350314. [DOI] [PubMed] [Google Scholar]
- 20.Ryan L, Kurup I, Derfus B, Kushnaryov V. ATP-induced chondrocalcinosis. Arthritis Rheum. 1992;35:1520–4. doi: 10.1002/art.1780351216. [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal A, Mattson E, Gohr C, Hirschmugl C. Characterization of articular calcium-containing crystals by synchrotron FTIR. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.03.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with Folin-phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 23.Sun Y, Kandel R. Deep zone articular chondrocytes in vitro express genes that show specific changes with mineralization. J Bone Min Res. 1999;14:1916–25. doi: 10.1359/jbmr.1999.14.11.1916. [DOI] [PubMed] [Google Scholar]
- 24.D′Argenio G, Calvani M, Della Valle N, Cosenza V, Di Matteo G, Giorgio P, et al. Differential expression of multiple transglutaminases in human colon: impaired keratinocyte transglutaminaes expression in ulcerative colitis. Gut. 2005;54:496–502. doi: 10.1136/gut.2004.049411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenthal A, Cheung H, Ryan L. Transforming growth factor beta 1 stimulates inorganic pyrophosphate elaboration by porcine cartilage. Arthritis Rheum. 1991;34:904–911. doi: 10.1002/art.1780340717. [DOI] [PubMed] [Google Scholar]
- 26.Pratta M, Yao W, Decicco C, Tortorella M, Lui R-Q, Copeland R, et al. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278:45539–45. doi: 10.1074/jbc.M303737200. [DOI] [PubMed] [Google Scholar]
- 27.Derfus B, Camacho N, Olmez U, Kushnaryov V, Westfall P, Ryan L, et al. Transforming growth factor beta-1 stimulates articular chondrocyte elaboration of matrix vesicles capable of greater calcium pyrophosphate precipitation. Osteoarthritis and Cartilage. 2001;9:189–94. doi: 10.1053/joca.2000.0375. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal AK, Derfus BA, Henry LA. Transglutaminase activity in aging articular chondrocytes and articular cartilage vesicles. Arthritis Rheum. 1997;40(5):966–970. doi: 10.1002/art.1780400526. [DOI] [PubMed] [Google Scholar]
- 29.Lewinson D, Silbermann M. In vitro precocious accumulation of calcium and matrix vesicles formation in young cartilage cells: specific effects of corticosteroids. Calcif Tissue Int. 1984;36:702–10. doi: 10.1007/BF02405393. [DOI] [PubMed] [Google Scholar]
- 30.Kirsch T, Wuthier R. Stimulation of calcification of growth plate cartilage matrix vesicles by binding to type II and X collagens. J Biol Chem. 1994;269:11462–9. [PubMed] [Google Scholar]
- 31.Toussirot E, Kremer P, Benmansour A, Wendling D. Giant calcification in soft tissue after shoulder corticosteroid injection. J Rheumatol. 1996;23:181–2. [PubMed] [Google Scholar]
- 32.Johnson T, Scholfield C, Parry J, Griffin M. Induction of tissue transglutaminase by dexamethasone: its correlation to receptor number and transglutaminase-mediated cell death in a series of malignant hamster fibrosarcoma. Biochem J. 1998;331:105–12. doi: 10.1042/bj3310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canalis E, Pereira R, Delany A. Effects of glucocorticoids on the skeleton. J Ped Endocrinol Metabol. 2002;15:1341–5. [PubMed] [Google Scholar]
- 34.Smink J, Koedam J, Koster J, vanBuul-Offers S. Dexamethasone-induced growth inhibition of porcine growth plate chondrocytes is accompanied by changes in levels of IGF axis components. J Endocrinol. 2002;174:343–52. doi: 10.1677/joe.0.1740343. [DOI] [PubMed] [Google Scholar]
- 35.Cheng S-L, Yang J, Rifas L, Zhang S-F, Avioli L. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinol. 1994;134:277–86. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 36.Richardson D, Dodge G. Dose-dependent effects of corticosteroids on the expression of matrix-related genes in normal and cytokine-treataed articular chondrocytes. Inflamm res. 2003;52:39–49. doi: 10.1007/s000110300012. [DOI] [PubMed] [Google Scholar]
- 37.Su S, Denhade F, Zafarullah M. Regulation of tissue inhibitor of metalloproteinases-3 gene expression by transforming growth factor-beta and dexamethasone in bovine and human articular chondrocytes. DNA Cell Biol. 1996;15:1039–48. doi: 10.1089/dna.1996.15.1039. [DOI] [PubMed] [Google Scholar]
- 38.Strove J, Schoniger R, Huch K, Brenner R, Gunther K-P, Pulh W, et al. Effects of dexamethasone on proteoglycan content and gene expression of IL-1beta stimulated osteoarthrotic chondrocytes in vitro. Acta Orthop Scand. 2002;73:562–7. doi: 10.1080/000164702321022857. [DOI] [PubMed] [Google Scholar]
- 39.Wong M, Tang Y, Lee S, Fu B. Glucocorticoids suppress proteoglycan production by human tenocytes. Acta Orthop. 2005;76:927–31. doi: 10.1080/17453670610046118. [DOI] [PubMed] [Google Scholar]
- 40.Miyazaki Y, Tsukazaki T, Hirota Y, Yonekura A, Osaki M, Shindo H, et al. Dexamethasone inhibitorion of TGFbeta-induced cell growth and type II collagen mRNA expression through ERK-integrated AP-1 activity in cultured rat articular chondrocytes. Osteoarthritis Cart. 2000;8:378–85. doi: 10.1053/joca.1999.0313. [DOI] [PubMed] [Google Scholar]
- 41.Autefage A, Alvinerie M, Toutain P. Synovial fluid and plasma kinetics of methylprednisolone acetate in horses following intra-articular administration of methylprednisolone acetate. Equine Vet J. 1986;18:193–8. doi: 10.1111/j.2042-3306.1986.tb03594.x. [DOI] [PubMed] [Google Scholar]
- 42.Lillich J, Bertone A, Schmall L, Ruggles A, Sams R. Plasma, urine, and synovial fluid disposition of methylprednisolone acetate and isoflupredone acetate after intra-articular administration in horses. Am J Vet Res. 1996;57:187–92. [PubMed] [Google Scholar]


