Abstract
Mutations in DJ-1 lead to a monogenic form of early onset recessive parkinsonism. DJ-1 can respond to oxidative stress, which has been proposed to be involved in the pathogenesis of sporadic Parkinson disease (PD). We have recently reported that DJ-1 interacts with mRNA in an oxidation dependent manner. Here, we confirm interaction of DJ-1 and RNA in human brain using immunoprecipitation followed by quantitative real time PCR. We confirmed previous reports that DJ-1 is more oxidized in cortex from cases of sporadic PD compared to controls. In the same samples, protein and RNA expression was measured for four DJ-1 target genes GPx4, MAPK8IP1, ND2 and ND5. While no alterations in mRNA expression were observed, an increase in protein expression was observed in PD cases for GPx4 and MAPK8IP1. In the same patients, we saw decreased mRNA and protein levels of two mitochondrial targets, ND2 and ND5. These results suggest that these proteins undergo regulation at the post-transcriptional level that may involve translational regulation by DJ-1.
Mutations in DJ-1 cause a rare form of recessive parkinsonism in humans [3]. Increased levels of DJ-1 protein are reported in both spinal fluid [14] and brain [5] of sporadic Parkinson disease (PD) patients, suggesting a role in the pathogenesis of sporadic PD.
DJ-1 protein has been shown to protect against oxidative stress in a number of models and organisms [4, 7, 8, 13]. This protection is dependent on the facile formation of a cysteine-sulfinic acid at cysteine-106 of the human protein under mildly oxidative conditions [4, 8]. The oxidation of cysteine 106 to a cysteine-sulfinic acid can be monitored using 2-D gel electrophoresis as a shift from in the pI of the protein [4, 9]. In sporadic PD cases, higher proportions of oxidized DJ-1 have been shown in cortex compared to controls [1, 5].
We recently reported that DJ-1 interacts with several RNA species in neuroblastoma cell lines and mouse brain, including transcripts coding for mitochondrial complex I components, selenoproteins, and PTEN/Akt cell signaling proteins [12]. Furthermore, we showed that this interaction was abolished under oxidative conditions and led to oxidation dependent increases in translation of anti-oxidant and pro-survival proteins [12].
In order to examine whether these RNA species were associated with DJ-1 human brain, we examined human tissue obtained from the archive at the Queen Square Brain Bank for Neurological Disorders, collected with the ethical approval of the London Multicentre Research Ethics Committee and with the informed consent of next-of-kin (Supplementary Table 1). Ethical approval for studies of gene expression in archival tissue was obtained from the National Hospital for Neurology and Neurosurgery and Institute of Neurology Joint Research Ethics Committee.
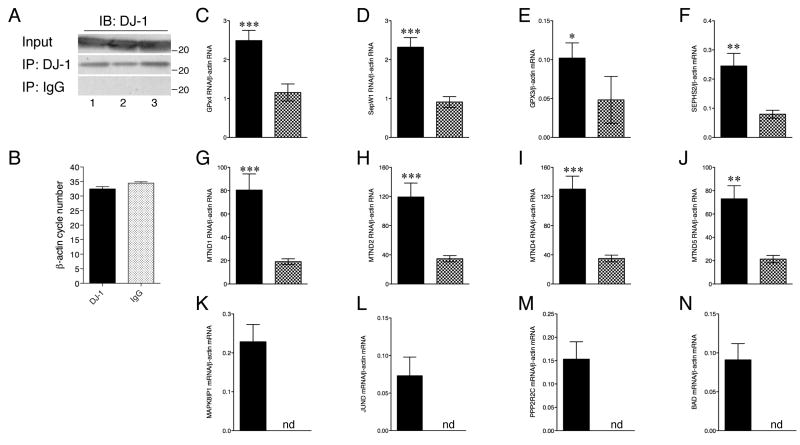
RNA immunoprecipitations (IP) were performed using either a DJ-1 specific antibody or a non-specific IgG antibody (Fig 1A) as described [12] on frozen brain tissue protein lysates homogenized in PLB buffer (0.5% Nonidet P-40, 10 mM Hepes, 100 mM KCl, 5 mM MgCl2, 1 mM DTT, RNase OUT and protease inhibitors). cDNA was produced by Oligo dT priming and Superscript III enzyme reaction (Invitrogen) as directed. Quantitative real time PCR (qRT-PCR) using specific probes (Supplementary Table 2) was performed as described [12]. As a control for non-specific pulldown in the IP, we measured amplification of β-actin, (Fig 1B). The abundance of β-actin mRNA in DJ-1 and IgG IPs differed by less than one PCR cycle value, consistent with previous observations [12]. Comparing DJ-1 and IgG IP samples after correction for β-actin amplification in the same IP, revealed significant (see figure 1 for P values) enrichment of previously identified transcripts in the DJ-1 IP sample, including selenoproteins (GPx4, SepW1, GPx3 and SEPHS2; Figure 1C-F) and mitochondrially encoded complex I components (ND1, ND2, ND4 and ND5; Figure 1G-J). While mRNA levels for PTEN/Akt pathway modifiers MAPK8IP1 (Fig 1J), JunD (Fig 1K) PPP2R2C (Fig 1L) and BAD (Fig 1M) were measurable in the DJ-1 IP samples, these same mRNAs were not detected in the IgG IP sample. Each target was repeated in at least three independent IPs from normal human brain with four replicate amplifications per IP. These consistent enrichments suggest the interaction between DJ-1 and RNA also occurs in human brain.
Figure 1. Immunoprecipitation of RNA bound to DJ-1 from human brain.
Brain tissue was homogenized and anti-DJ-1 and non-specific IgG antibodies (Santa Cruz Biotechnology) were used to immunoprecipitate RNA bound to DJ-1 or background, respectively. (A) Representative western blot using anti-DJ-1 antibody of input (upper panel), DJ-1 IP (mid panel) and IgG IP (lower panel). Each lane is a different brain sample. Molecular weights are on the right of all blots. (B-N) Quantitative real time PCR was used to measure abundance of transcripts associated with each sample. As a non-specific control, we measured levels of β-actin (B), which amplified at similar cycle numbers in qRT-PCR. Correcting for β-actin levels in each sample, we saw enrichments of RNA in DJ-1 IP samples (solid black) compared to IgG IP samples (checkered) for selenoproteins (C) GPx4, (D) SepW1, (E) GPx3 and (F) SEPHS2, mitochondrially encoded complex I components ND1 (G), ND2 (H), ND4 (I) and ND5 (J), and PTEN/Akt modulators (K) MAPK8IP1, (L) JunD (M) PPP2R2C and (N) BAD. While measurable in DJ-1 IP samples, no detection (nd) of PTEN/Akt modulators was observed after 40 cycles in IgG IP samples. Statistical significance was determined by Student's t-test comparing DJ-1 to IgG IP; * P<0.05, ** P<0.01, *** P<0.001.
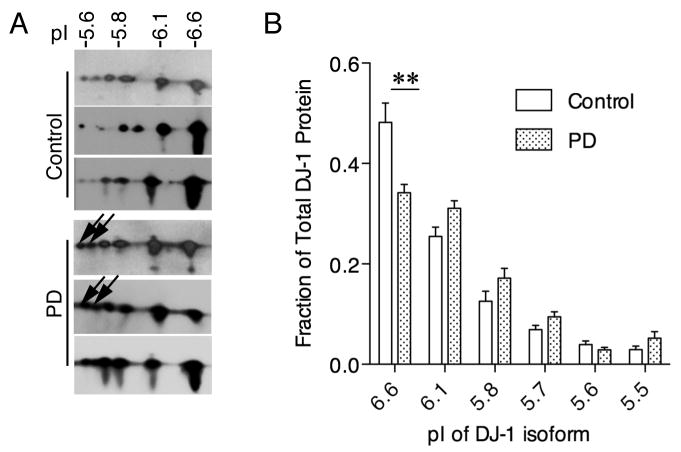
Our previous data using reporter constructs suggests that DJ-1 has effects on translation in an oxidation-dependent fashion, with decreased RNA interaction as DJ-1 is increasingly oxidized [12]. We therefore examined the oxidation state of DJ-1 in cortical tissue from nine idiopathic PD patients and nine controls. Cortical tissue was chosen due to availability and miminal cell loss compared to regions such as the substantia nigra. We separated protein lysates from PD and control brains based by isoelectric point by 2-D gel electrophoresis as described [4] prior to Western blotting with an antibody against DJ-1 (Fig 2A). The presence of low pI, highly oxidized isoforms was observed (arrows). The percentage of total DJ-1 in each isoform was quantified (Fig 2B) and significant decrease (P<0.01 by t-test, n=9) in reduced DJ-1 was observed. More total oxidized DJ-1 was present in PD patients compared to controls at several pI isoforms, although none of these reached statistical significance individually.
Figure 2. Increased oxidation of DJ-1 in sporadic PD.
(A) 2-D gel electrophoresis was performed on cleared lysate extracted from PD and control cortices, then Western blotted with an anti-DJ-1 antibody. Three representative blots from nine samples are shown. DJ-1 is shifted in PD brains to lower pI isoforms than in control brains as a result of increased oxidation as indicated by arrows. (B) Quantification of pI isoforms as a proportion of total DJ-1 protein reveals significantly less DJ-1 at the 6.6 pI isoform, indicating cumulatively more DJ-1 in more oxidized isoforms in PD cortex. Statistical significance was determined by Student's t-test, ** P<0.01 comparing control and PD (n=9).
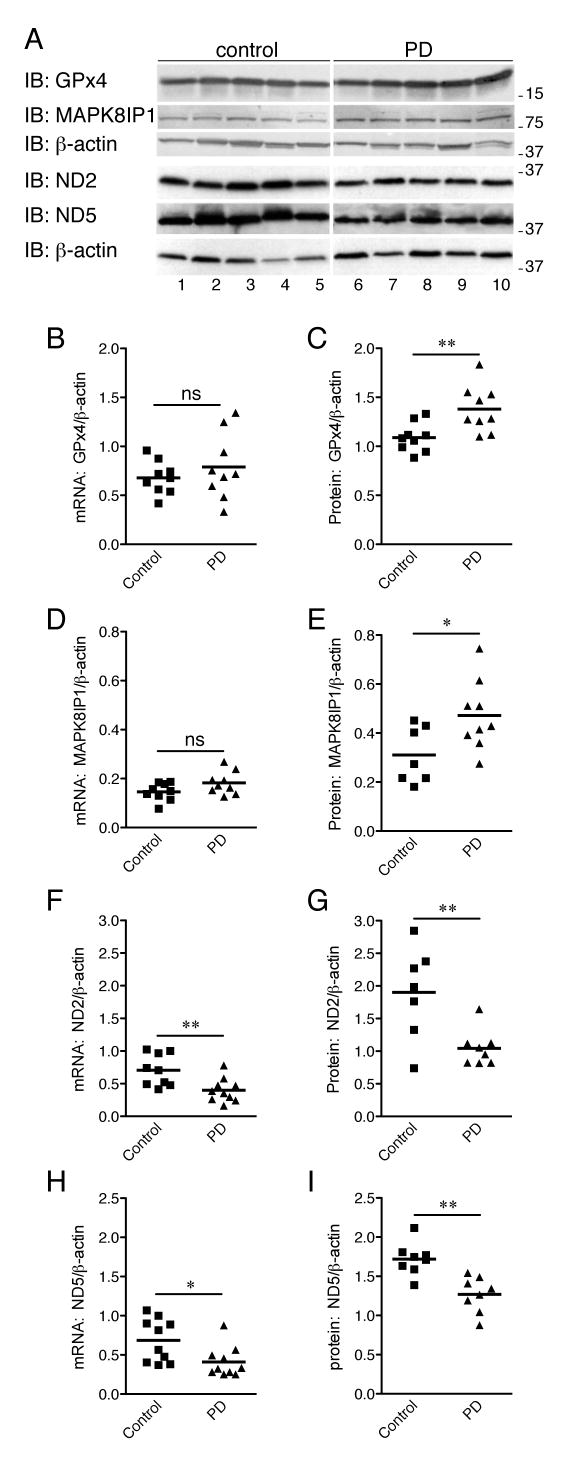
To examine the post-translational effects of oxidation on DJ-1 targets, RNA and protein were isolated from frontal cortex of the same nine PD patients and nine controls. Four target transcripts, GPx4, MAPK8IP1, ND2 and ND5, were investigated for changes in expression at both the protein and RNA levels. We observed no statistically significant difference in RNA expression levels for GPx4 or MAPK8IP1 using qRT-PCR (Fig 3B,D). Although expression levels overlapped, significantly higher expression of both GPx4 and MAPK8IP1 protein was observed in PD patients as a group compared to controls (Fig 3A,C,E) using Western blotting with antibodies specific to GPx4 (Abnova), MAPK8IP1 (Santa Cruz Biotechnology), after normalization to β-actin (Sigma). Significantly lower mRNA and protein expression of ND2 and ND5 (Santa Cruz Biotechnology) was observed in controls compared to PD patients (Fig 3F-I).
Figure 3. GPx4 and MAPK8IP1 protein expression, but not RNA expression, is higher in PD patients.
(A) Protein and RNA were extracted from cortex of nine human PD patients and nine controls and expression of GPx4, MAPK8IP1, ND2 and ND5 was examined as indicated. Protein levels of β-actin were used as a control; because the GPx4/MAPK8IP1 and ND2 blots were run on different occasions from replicate extracts, separate β-actin blots are shown. Each lane is a different brain sample. Molecular weights are on the right of all blots. (B-I) Using quantitative real time PCR, no differences were observed in abundance of mRNA for (B) GPx4, (D) MAPK8IP1 but mRNA levels of (F) ND2 or (H) ND5 were significantly lower. At the protein level, (C) GPx4 and (E) MAPK8IP1 showed increases in PD patients compared to controls while (G) ND2 and (I) ND5 showed decreases in PD patients compared to controls. Statistical significance determined by Student's t-test, * P<0.05, ** P<0.01, ns=not significant (P>0.05).
Here, we have confirmed the interaction of DJ-1 with specific mRNA species [12] in the human brain. We confirmed that oxidation of DJ-1 increases in the cortex of sporadic PD patients compared to controls, and showed that in these same patients, mRNA levels for known DJ-1 targets GPx4, MAPK8IP1 are not altered while protein levels are significantly different, suggesting a translational regulation mechanism that may be related to DJ-1 oxidation. This is consistent with previous observations of MAPK8IP1 protein levels associated with aging in mice, which is also associated with increased oxidation of DJ-1 [12]. We also saw decreased protein expression of ND2 and ND5, which is opposite in direction to the GPx4 and MAPK8IP1 data. However, this result is difficult to interpret in the context of DJ-1 oxidation as the mRNA levels for these two genes were lower in sporadic PD, presumably indicating damage to mitochondria in the disease state.
The observations linking oxidation of DJ-1 to altered protein vs mRNA levels in sporadic PD they are consistent with our previous observations that DJ-1 acts by modifying translation of pro-survival and anti-oxidant in response to the presence of oxidation. This would result in increased cell survival and improved anti-oxidant defense and in a rapid, localized and direct response to oxidative injury. Protein levels of cell survival and anti-oxidant pathway targets of DJ-1 are upregulated, while protein levels of mitochondrially-encoded oxidative phosphorylation targets appear downregulated. This may be related to the previously reported shift of localization of DJ-1 under stress, from cytoplasm to mitochondria [4]. The regulation of these transcripts simultaneously by DJ-1 would require DJ-1 to target different transcripts when oxidized versus unoxidized state, which will require further studies. It should also be noted that post-mortem studies are correlative in nature, limiting our ability to determine if DJ-1 regulates translation directly. Further studies would also benefit from having larger sample series. To date, we have not been able to amplify RNA from immunoprecipitated DJ-1 from PD brains (data not shown), although whether this relates to a specific change in DJ-1 in the disease state or is due to variability in post-mortem tissue quality is unclear.
In summary, these data from human brain provide evidence that DJ-1 participates in the cellular response to sporadic Parkinson disease through oxidation dependent translational regulation of oxidation responsive and mitochondrial mRNA transcripts.
Supplementary Material
Table 1.
Case/Control details for patient samples.
| Sample | Gender | Age | PMI |
|---|---|---|---|
| PD1 | M | 85.1 | 15 |
| PD2 | F | 74 | 5 |
| PD3 | M | 78 | 22 |
| PD4 | F | 82.4 | 15 |
| PD5 | M | 58 | 3 |
| PD6 | M | 79 | 20 |
| PD7 | M | 63 | 2 |
| PD8 | M | 80 | 6 |
| PD9 | M | 83 | - |
| CON1 | M | 75 | 19 |
| CON2 | F | 85.9 | 15 |
| CON3 | M | 86 | 18 |
| CON4* | M | 85.3 | 2 |
| CON5* | M | 80.8 | 4 |
| CON6 | M | 81.4 | 7 |
| CON7 | M | 91.3 | 7 |
| CON8* | F | 80.7 | 1 |
| CON9 | F | 97 | 16 |
Age = age at death (years); PMI = post mortem intervals (unknown for sample PD9, indicated by -); PD = Parkinson disease; CON = control; M = male; F = female.
samples were used for RNA immunoprecipitations.
Table 2.
Sequence of qRT-PCR primers used to quantify abundance of RNA in DJ-1 and IgG RNA immunoprecipitations
| Gene | Start (F) | Sequence | Start (R) | Sequence |
|---|---|---|---|---|
| BAD | 411F | TCCGGAGGATGAGTGACGAGTT | 546R | AAGTTCCGATCCCACCAGGACT |
| β-actin | 219F | AGAAGGATTCCTATGTGGGCG | 319R | CATGTCGTCCCAGTTGGTGAC |
| GPx3 | 12887F | GTGCTGGACAGTGACAACCCT | 1439R | GGAGGCAGTGGGAGATGCT |
| GPx4 | 450F | GAGATCAAAGAGTTCGCCGC | 605R | GGTGAAGTTCCACTTGATGGC |
| JUND | 1068F | GAAAGTGAAGACCCTCAAGAGTCA | 1167R | GTGGCTGAGGACTTTCTGCTT |
| MAPK8IP1 | 1517F | TGATGAACCCGACGTCCATT | 1627R | CCGTTGATGATGCAGGAGAAC |
| MTND1 | 50F | TGGCATTCCTAATGCTTACCG | 202R | TGGTAGATGTGGCGGGTTTTA |
| MTND2 | 359F | AAGGCACCCCTCTGACATCC | 509R | AATCCACCTCAACTGCCTGC |
| MTND4 | 1101F | CGCCTTACCCCCCACTATTAA | 1253R | GAGCCCCATTGTGTTGTGGT |
| MTND5 | 1245F | TACCTCCCTGACAAGCGCC | 1395R | TCCTGCGAATAGGCTTCCG |
| PPP2R2C | 1296F | GAACATCATTGCCATCGCC | 1440R | TTGCGGTCGTGAAGGTCAT |
| SEPHS2 | 2010F | CTGGCTCTTTCCTGCTCTGG | 2161R | GGGCTCTGAGGCTTGCTCT |
| SEPW1 | 550F | GGACACCTGGTCTTTCCCTGA | 701R | GATGAAACCACGGGACAGGA |
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. RB acknowledges funding from PD Society UK and PD SPRING UK. We would like to thank Karin Pernold, Karin Lundströmer and Eva Lindqvist for technical assistance.
Footnotes
Disclosure Statement: The authors declare no conflicts of interest. Human subject protocols were approved and followed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bandopadhyay R, Kingsbury AE, Cookson MR, Reid AR, Evans IM, Hope AD, Pittman AM, Lashley T, Canet-Aviles R, Miller DW, McLendon C, Strand C, Leonard AJ, Abou-Sleiman PM, Healy DG, Ariga H, Wood NW, de Silva R, Revesz T, Hardy JA, Lees AJ. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson's disease. Brain. 2004;127:420–30. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- 2.Bogaerts V, Theuns J, Van Broeckhoven C. Genetic findings in Parkinson's disease and translation into treatment: a leading role for mitochondria? Genes Brain Behav. 2008;7:129–151. doi: 10.1111/j.1601-183X.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 4.Canet-Avilés RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi J, Sullards MC, Olzmann JA, Rees HD, Weintraub ST, Bostwick DE, Gearing M, Levey AI, Chin LS, Li L. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem. 2006;281:10816–10824. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keeney PM, Xie J, Capaldi RA, Bennett JP. Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci USA. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meulener MC, Xu K, Thomson L, Thompson L, Ischiropoulos H, Bonini NM. Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging. Proc Natl Acad Sci USA. 2006;103:12517–12522. doi: 10.1073/pnas.0601891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsumoto A, Nakagawa Y, Takeuchi A, Okawa K, Iwamatsu A, Takanezawa Y. Oxidized forms of peroxiredoxins and DJ-1 on two-dimensional gels increased in response to sublethal levels of paraquat. Free Radic Res. 2001;35:301–310. doi: 10.1080/10715760100300831. [DOI] [PubMed] [Google Scholar]
- 10.Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet neurology. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 11.Stokes AH, Hastings TG, Vrana KE. Cytotoxic and genotoxic potential of dopamine. J Neurosci Res. 1999;55:659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.van der Brug MP, Blackinton J, Chandran J, Hao LY, Lal A, Mazan-Mamczarz K, Martindale J, Xie C, Ahmad R, Thomas KJ, Beilina A, Gibbs JR, Ding J, Myers AJ, Zhan M, Cai H, Bonini NM, Gorospe M, Cookson MR. RNA binding activity of the recessive parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proc Natl Acad Sci U S A. 2008;105:10244–10249. doi: 10.1073/pnas.0708518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ved R, Saha S, Westlund B, Perier C, Burnam L, Sluder A, Hoener M, Rodrigues CM, Alfonso A, Steer C, Liu L, Przedborski S, Wolozin B. Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J Biol Chem. 2005;280:42655–42668. doi: 10.1074/jbc.M505910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waragai M, Nakai M, Wei J, Fujita M, Mizuno H, Ho G, Masliah E, Akatsu H, Yokochi F, Hashimoto M. Plasma levels of DJ-1 as a possible marker for progression of sporadic Parkinson's disease. Neurosci Lett. 2007;425:18–22. doi: 10.1016/j.neulet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, Beal MF, Nishimura I, Wakamatsu K, Ito S, Takahashi R, Lu B. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci USA. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.