Abstract
Salt-sensitive hypertension is common in the aged population. Increased fruit and vegetable intake reduces hypertension, but its effect on eventual diastolic dysfunction is unknown. This relationship is tested in the Dahl Salt-Sensitive (Dahl-SS) rat model of salt-sensitive hypertension and diastolic dysfunction. Table grape powder contains phytochemicals that are relevant to human diets. For 18 weeks, male Dahl-SS rats were fed one of five diets: low salt (LS), a low salt + grape powder (LSG), high salt (HS), a high salt grape powder (HSG), or high salt vasodilator hydralazine (HSH). Compared to the HS diet,the HSG diet lowered blood pressure and improved cardiac function; reduced systemic inflammation; reduced cardiac hypertrophy, fibrosis, and oxidative damage; and increased cardiac glutathione. The HSH diet similarly reduced blood pressure but did not reduce cardiac pathogenesis. The LSG diet reduced cardiac oxidative damage and increased cardiac glutathione. In conclusion, physiologically relevant phytochemical intake reduced salt-sensitive hypertension and diastolic dysfunction.
Keywords: Heart failure, Diet, Fruits, Vegetables
IN humans, the link between salt intake and blood pressure has been established in cross-sectional and longitudinal epidemiological studies. However, blood pressure response to changes in salt intake can vary from one individual to another, a phenomenon known as “salt sensitivity.” Salt sensitivity affects approximately 50% of hypertensive patients and 20% of normotensive patients (1), and its incidence increases linearly with age. Greater understanding of the pathology and sequelae of salt-sensitive hypertension is then critical to reducing the public health burden of hypertension and its associated pathologies.
Prolonged hypertension frequently contributes to the development of heart failure. Heart failure is defined by the inability of the heart to adequately meet oxygen demands of the body, characterized by inefficient systolic and/or diastolic actions of the heart chambers and valves. More than 90% of heart failure cases are preceded by prolonged hypertension (2). Heart failure is a significant and growing problem in our aging population. Heart failure is the #1 diagnosis in the Medicare system based on patient volume, the #1 discharge diagnosis in patients older than 62 years, and the #1 cause of hospital readmission (3). As such, preventive approaches that address risk factors for heart failure could impact public health burden.
Our group is focused on the effects of diet on hypertension-associated cardiac pathogenesis. The Dietary Approaches to Stop Hypertension (DASH) clinical trial revealed that diets rich in fruits and vegetables reduced blood pressure (4,5). One recent animal study carefully modeled the DASH diet nutrients to assess effects on hypertension in spontaneously hypertensive rats. However, the findings failed to reveal an antihypertensive effect (6). Importantly, this study did not include any non-nutritive phytochemicals contained in fruits and vegetables. The DASH diet phytochemical profile was distinctly different than the control diet, (7) and these compounds may be vital to the diet benefits.
The Dahl Salt-Sensitive (Dahl-SS) rat is a model that provides insight into the pathology and treatment of salt-sensitive hypertension. When fed a higher salt diet, the Dahl-SS rat predictably and gradually develops the clinically relevant sequelae of hypertension, renal hypertrophy, renal dysfunction, cardiac hypertrophy, and diastolic dysfunction. Many animal models of induced heart failure depend on infarct induction or surgical modification of the vasculature, which impose rapid morbidity and higher mortality. In contrast, the hypertension-induced model described here develops pathology over several months, allowing one to serially test for the more gradual effects of diet modification. As such, we propose that the Dahl-SS rat is a valuable model of diet effects on aging-related salt-sensitive hypertension and its associated cardiac pathologies.
The current study examines the cumulative cardiac effects of a diet supplemented with phytochemical-rich whole table grape powder. Although the use of one food source may be a simplified approach, table grapes are a relevant model to human diets because they are a widely available and affordable produce, and because they contain the major classes of commonly consumed, produce-derived flavonoids, including anthocyanins, flavanols (e.g., catechin, epicatechin, proanthocyanins), and flavonols (e.g., quercetin, kaempferol, isorhamnetin) (8). In addition, the table grape powder used in this study has already been shown to reduce other pathologies (9-13), and this evidence supports the in vivo efficacy and bioavailability of the grape powder constituents. Using the Dahl-SS rat model, we tested the hypothesis that table grape powder-enriched diets could lower hypertension-associated cardiac pathology and diastolic dysfunction. Furthermore, because grape product consumption is known to impart acute vasodilation (14-18), we compared grape treatment effects to those of hydralazine, a well-characterized vasodilator that has been shown at the selected dose to lower blood pressure in the Dahl-SS rat (19-21).
MATERIALS AND METHODS
Animal Care and Diets
Five-week-old Dahl-Rapp Salt-Sensitive rats (Harlan, Indianapolis, IN) were acclimated for 1 week on AIN-76a powdered diet (Research Diets, New Brunswick, NJ). Afterward, each rat was randomly assigned (n = 12 each) to one of five treatments: low salt diet (LS; AIN-76a with 2.8% added carbohydrate, glucose/fructose 1:1); low salt diet + grape powder (LSG; AIN-76a with 3.0% wt/wt added grape powder); high salt diet with 6% added NaCl (HS; AIN-76a with 2.8% wt/wt added carbohydrate); high salt diet + grape powder (HSG; AIN-76a with 3.0% wt/wt added grape powder); or high salt diet + hydralazine (20 mg/kg body weight/day, in drinking water). Hydralazine dose was selected based on previously published findings in the Dahl-SS rat (19-21), to obtain a similar percent reduction in systolic blood pressure as that observed with our grape powder. Diet nutrient content is described in Table 1, and grape powder phytochemical content is described in Table 2. The freeze-dried table grape powder was obtained from the California Table Grape Commission as a composite of green, red, and black California table grapes, processed and chemically characterized by the National Food Laboratory, Inc (Dublin, CA). Grape powder or added carbohydrate was mixed weekly into the AIN-76A base diet in-house using a commercial baking blender, and then stored in vacuum-sealed bags (Deni Magic Vac, Buffalo, NY) at 4°C. Hydralazine-fortified drinking water was made every 2 days, with concentration adjusted dynamically based on changing water intake and body weight over the course of the study. Animals were each fed 20 g of powdered diet/day. Ad libitum intake of AIN diet averages 19-21 g of AIN powder/day in the Dahl-SS rat (22), so provision of 20 g/day ensured complete daily consumption. For HS diets, NaCl was added directly to the food hopper and mixed carefully with the daily ration of powdered diet. Rats were housed three per cage on a 12-h light/dark cycle, and water was provided ad libitum. This project was approved by the Animal Care and Use Committee at the University of Michigan.
Table 1.
Diets and Estimated Nutrient Content
| Diet Components | LS | LSG | HS | HSG | HSH |
|---|---|---|---|---|---|
| Total protein | 20 | 21 | 20 | 21 | 20 |
| Total carbohydrates | 68 | 68 | 68 | 68 | 68 |
| Total fat | 5 | 5 | 5 | 5 | 5 |
| Total fiber | 5 | 5 | 5 | 5 | 5 |
| kcal/g of diet | 3.9 | 4 | 3.9 | 4 | 3.9 |
|
g/kg diet |
g/kg diet |
g/kg diet |
g/kg diet |
g/kg diet |
|
| Casein | 198 | 198 | 198 | 198 | 198 |
| Protein from grape | 0 | 1.1 | 0 | 1.1 | 0 |
| Corn starch | 150 | 150 | 150 | 150 | 150 |
| Sucrose | 500 | 500 | 500 | 500 | 500 |
| Sugar from grape | 0 | 27.7 | 0 | 27.7 | 0 |
| Dextrose | 14 | 0 | 14 | 0 | 14 |
| Fructose | 14 | 0 | 14 | 0 | 14 |
| Cellulose | 50 | 50 | 50 | 50 | 50 |
| Fiber from grape | 0 | 0.02 | 0 | 0.02 | 0 |
| Corn oil | 50 | 50 | 50 | 50 | 50 |
| AIN76a vitamin mix | 10 | 10 | 10 | 10 | 10 |
| AIN76a mineral mix | 35 | 35 | 35 | 35 | 35 |
| Vitamin C (grape) | 0 | 0.001 | 0 | 0.001 | 0 |
| Potassium (grape) | 0.3 | 0 | 0.3 | 0 | |
| Vitamin A (grape) | 0 | 99.6 IU | 0 | 99.6 IU | 0 |
Notes: Nutrient content of grape powder was analyzed by National Food Laboratory, Inc. (Dublin, CA). Nutrient content of the base AIN76a diet was provided by Research Diets, Inc.
LS = low-salt diet; LSG = low salt + grape powder diet; HS = high-salt diet; HSG = high salt + grape powder diet; HSH = high salt + vasodilator hydralazine diet.
Table 2.
Grape Powder Phytochemical Analysis
| Grape Powder Phytochemicals | Per kg Grape Powder |
|---|---|
| Anthocyanins | |
| Cyanidin | 380.0 mg |
| Malvidin | 170.3 mg |
| Peonidin | 33.5 mg |
| Monomeric flavanols | |
| Catechin | 19.1 mg |
| Epicatechin | 12.5 mg |
| Flavonols | |
| Quercetin | 49 mg |
| Kaempferol | 5.7 mg |
| Isorhamnetin | 4.4 mg |
| Stilbenes | |
| Resveratrol | 36.0 mg |
Note: Phytochemical analysis (per kg of grape powder) by National Food Laboratories, Inc.
Blood Pressure and Echocardiography Measures
During the 18-week study, blood pressure was measured bimonthly by with the IITC Mark 12 photoelectric/ oscillometric tail cuff system (IITC Life Sciences, Woodland Hills, CA) using the unit and method we described in detail previously and validated against telemetric approaches (22). Using preconditioned, conscious, restrained animals, the first two of ten measures were universally discarded because of acclimation to tail cuff pressure and operational noise. A run was accepted if at least six of the eight measures were adequate (having detectable pulses and free of gross artifacts). When the requisite determinations were obtained, the average was calculated and used as the mean heart rate and the mean systolic value for that session.
Echocardiography of all animals was performed at 0, 8, and 18 weeks of diet treatment, following the predicted Dahl-SS rat development of compensated cardiac hypertrophy and of diastolic dysfunction, respectively. All measurements were made by a trained research animal sonographer who was unaware of treatment assignment. Animals were anesthetized by 4% isoflurane and maintained with 1% isoflurane. Two-dimensionally guided M-mode recordings and Doppler tissue imaging were acquired as we described previously (22). Equations for each derived parameter are as described by Boluyt and colleagues (23), with the exception of midwall fractional shortening. In the Dahl-SS rat, endocardial fractional shortening overestimates left ventricle (LV) systolic function; midwall fractional shortening has been determined to be a more appropriate index of LV systolic function (24-26). Midwall fractional shortening was calculated according to the two-shell cylindrical model of Shimizu and colleagues (27).
Terminal Plasma Analysis
Conscious rats were decapitated, and trunk blood was collected. Whole blood was collected into an EDTA Vacutainer (BD Vacutainer Systems, Franklin Lakes, NJ) then spun at 4°C, 5000 × g for 20 minutes. The plasma was stored at -80°C until further analysis. Plasma tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) were measured by enzyme immunoassay kits (R&D Systems, Minneapolis, MN) according to manufacturers' instructions.
Organ Weights and Cardiac Hydroxyproline Content
The heart, kidneys, and lungs were harvested, blotted, and weighed. Organ weights were compared to tibial length rather than to body weight, because of the variable weight loss from cachexia. The LV was isolated and minced, then flash frozen and stored in aliquots in liquid nitrogen. Collagen component hydroxyproline was measured in LV homogenates as a quantitative index of fibrosis. Frozen LV tissue was homogenized in ice-cold phosphate-buffered saline containing a Complete Protease Inhibitor Mini-Tab cocktail (Roche, Indianapolis, IN). The tissue was homogenized with a 30-second pulse of a Polytron (Brinkmann, Westbury, NY) tissue homogenizer, and hydrolysis of the sample solution was carried out with 6 N HCl at 100°C for 24 hours. The hydrolyzed samples were dried under a stream of nitrogen. Hydroxyproline standard solutions were prepared in a range from 2.0 to 10.0 μg/mL, and 0.5 mL of each standard and cardiac homogenates were placed in glass tubes with 1.0 mL of isopropanol and vortexed. To this solution, 0.5 mL of oxidant (0.35 g of chloramine T in 5.0 mL of water and 20.0 mL of citrate buffer) was added, vortexed, and allowed to stand at room temperature for 4 minutes. Next, 3.25 mL of Ehrlich's reagent (3.0 mL of Ehrlich's reagent in 15.0 mL of isopropanol) was added, and the tubes were kept at 25°C for 18 hours. The intensity of red coloration was measured using a spectrophotometer at 560 nm. Total protein content was assessed using the bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). The amount of hydroxyproline was calculated using the standard curve and expressed as micrograms per milligram of total protein.
Cardiac Histology Area of Fibrosis
Four hearts from each group were used for histology determination of cardiac fibrosis. A transverse section of the LV was fixed in 10% neutral buffered formalin. Tissue sections were prepped and mounted, then stained with Masson's Trichrome Stain (MTS) for determination of fibrosis. Digital images were acquired with an Olympus BX40 digital microscope camera mounted on a Nikon DN100 light microscope. MTS-stained cross-sections of the heart were captured at ×200 magnification. The fibrotic areas stained blue with the MTS. True-color image analysis was performed using BIOQUANT Image Analysis software (BIOQUANT Life Science, Nashville, TN). Perivascular fibrosis was determined from 10 random measures around 10 distinct vessels. The area of fibrosis value was derived from the total area encompassing the vessel lumen plus the fibrotic ring divided by the area of the vessel lumen.
Cardiac Oxidative Damage and Cardiac Reduced/Oxidized Glutathione Ratio
Frozen LV tissue was homogenized in ice-cold phosphate-buffered saline containing a Complete Protease Inhibitor Mini-Tab cocktail (Roche) and a 0.01% volume of antioxidant 0.1 M butylated hydroxytoluene in acetonitrile to limit auto-oxidation during sample processing. The tissue was homogenized with a 30-second pulse of a tissue homogenizer (Polytron) then was centrifuged at 4°C for 10 minutes at 3000 × g. The supernatant was collected and stored at -80°C until further analysis. Total protein content was assessed using the BCA assay (Pierce). MDA detection was accomplished using the Biotech LPO-586 kit (Oxis Research, Portland, OR) according to the manufacturer's instructions and was expressed relative to total protein. Determination of the cardiac reduced/oxidized glutathione (GSH/GSSG) ratio was performed by using the Bioxytech GSH/GSSG-412 kit (Oxis Research) according to the manufacturer's instructions.
Statistical Methods
All results are expressed ± standard error of the mean. Groups were compared using analysis of variance (ANOVA). If the interaction was significant, between-group comparisons were conducted by using the Bonferroni post hoc test. Analysis was conducted using GraphPad PRISM 4(La Jolla, CA). A p value <.05 was considered statistically significant.
RESULTS
Body Weight and Blood Pressure
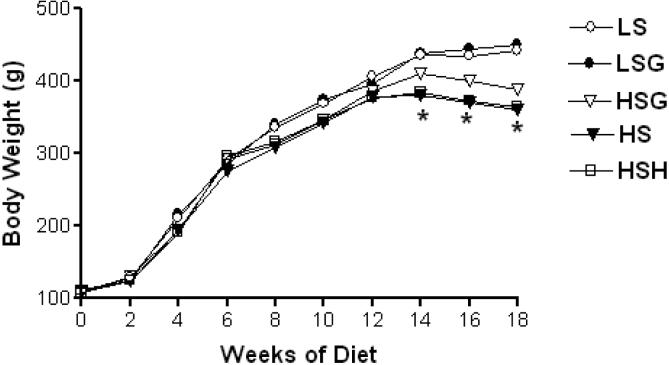
No significant differences were observed between LS and LSG groups in body weight gain during the course of the study. Cachexia is characteristic of human heart failure and is positively correlated with disease severity and mortality. Dahl-SS rats also develop cachexia as heart failure progresses, so it was expected that body weight would decrease in salt-fed groups at the later time points of the study. The results in Figure 1 show that, by 18 weeks of diet, body weight fell 22% in the HS group and 19% in the HSH group relative to the LS group, but it only fell 12% in the HSG group. However, this difference from HSG only approached significance (p < .08).
Figure 1.
Serial body weight. Each value given is the mean from 12 rats per group. Shown without error bars to preserve visualization. Both the high salt (HS) and high salt + vasodilator hydralazine (HSH) groups were significantly affected, *p < .05 vs low salt (LS) group. LSG = low salt + grape powder diet group; HSG = high salt + grape powder diet group.
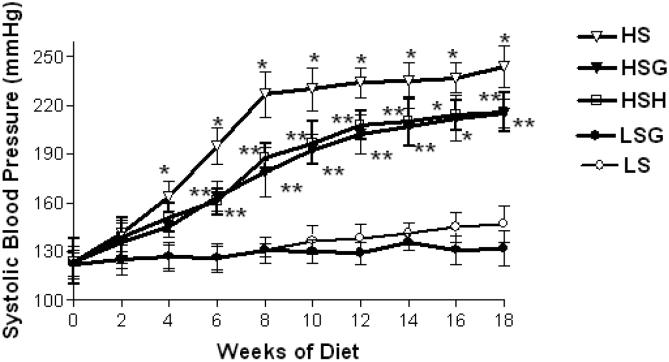
As shown in Figure 2, HSG did not prevent the development of hypertension, but the HSG diet significantly reduced systolic blood pressure relative to the HS diet. For both the HSG and HSH diets, the first statistically significant decrease versus the HS diet was detected at 6 weeks of treatment. The LSG group trended to have slightly lower systolic blood pressure versus the LS group, but the difference was not statistically significant at any time point.
Figure 2.
Systolic blood pressure. Each value given is an average from 12 rats per group ± standard error of the mean. *At least p < .05 vs low salt (LS) group; **p < .05 vs high salt (HS) group. HSG=high salt+grape powder diet group; HSH = high salt + vasodilator hydralazine diet group; LSG = low salt + grape powder diet group.
Echocardiography
Cardiac geometry and function were measured at study baseline (0 weeks), compensated hypertrophy (8 weeks), and diastolic dysfunction (18 weeks). For changes in cardiac geometry, Table 3 shows that HS rats showed greater LV end diastolic dimension (LVEDD) at 18 weeks. This remodeling was reduced with the HSG group but not in the HSH group. In the Dahl-SS rat, increasing relative wall thickness (RWT, or 2 × posterior wall thickness during diastole/LVEDD) is found to correlate strongly with contractile failure, more so than increasing LV mass (28). HS diets increased wall thickness, changes first evident at the 8-week compensated hypertrophy stage. When measured at 18 weeks, HSG reduced RWT and LV mass/body weight. This effect was not observed in the HSH group at any time point.
Table 3.
Serial Changes in Cardiac Geometry
| Cardiac Geometry | LS | LSG | HS | HSG | HSH |
|---|---|---|---|---|---|
| LVEDD | |||||
| 0 wk | 7.2 ± 0.4 | 7.0 ± 0.4 | 7.3 ± 0.4 | 7.1 ± 0.5 | 7.1 ± 0.3 |
| 8 wk | 7.8 ± 0.5 | 7.7 ±6 0.3 | 7.2 ± 0.3* | 7.6 ± 0.5 | 7.0 ± 0.4* |
| 18 wk | 8.1 ± 0.3 | 8.0 ± 0.5 | 8.9 ± 0.5* | 8.3 ± 0.3 | 8.8 ± 0.3* |
| LVESD | |||||
| 0 wk | 3.8 ± 0.2 | 3.7 ± 0.2 | 3.7 ± 0.1 | 3.5 ± 0.3 | 3.8 ± 0.2 |
| 8 wk | 4.0 ± 0.2 | 4.2 ± 0.3 | 3.8 ± 0.2 | 3.6 ± 0.2 | 3.7 ± 0.3 |
| 18 wk | 4.7 ± 0.3 | 4.6 ± 0.3 | 4.9 ± 0.3 | 4.9 ± 0.4 | 4.8 ± 0.3 |
| RW th | |||||
| 0 wk | 0.3 ± 0.01 | 0.3 ± 0.03 | 0.3 ± 0.02 | 0.3 ± 0.02 | 0.3 ± 0.03 |
| 8 wk | 0.3 ± 0.02 | 0.3 ± 0.03 | 0.6 ± 0.02* | 0.4 ± 0.02 | 0.6 ± 0.04* |
| 18 wk | 0.4 ± 0.02 | 0.4 ± 0.03 | 0.7 ± 0.02* | 0.5 ± 0.03 | 0.8 ± 0.03* |
| LV mass | |||||
| 0 wk | 2.5 ± 0.1 | 2.4 ± 0.1 | 2.5 ± 0.1 | 2.4 ± 0.2 | 2.6 ± 0.1 |
| 8 wk | 2.6 ± 0.1 | 2.5 ± 0.2 | 3.5 ± 0.2* | 2.7 ± 0.2 | 3.6 ± 0.3* |
| 18 wk | 2.8 ± 0.1 | 2.7 ± 0.2 | 4.3 ± 0.4* | 3.1 ± 0.2 | 4.4 ± 0.3* |
Notes: Echocardiography measures at 0 weeks of diet (baseline), 8 weeks of diet, and 18 weeks of diet. Data presented are mean ± standard error of the mean, n = 12 per group.
LVEDD = left ventricular end-diastolic dimension (mm); LVESD = left ventricular end systolic dimension (mm); RW th = relative wall thickness (mm); LV/BW = gram left ventricular mass/gram body weight; LS = low-salt diet; LSG = low salt + grape powder diet; HS = high-salt diet; HSG = high salt + grape powder diet; HSH = high salt + vasodilator hydralazine diet.
At least p < .05 vs LS, LSG, and HSG groups.
In addition to grape-associated changes in cardiac geometry, diastolic parameters were also positively affected by HSG diets (Table 4). Changes in diastolic parameters were assessed by using M-Mode echocardiography and Doppler tissue imaging. Mild or early diastolic dysfunction is commonly characterized by altered filling velocities, which are measured by the ratio of peak early filling velocity (E wave) to late filling velocity (A wave). The E/A ratio falls at compensated hypertrophy stage, indicating an abnormal relaxation pattern or early diastolic dysfunction. However, E/A sharply rises with cardiac decompensation, indicating increased LV end diastolic pressures and pseudonormalized mitral valve inflow. During compensated hypertrophy at 8 weeks, HS-fed rats displayed a lower E/A ratio indicating a relaxation abnormality. This effect was attenuated in the HSG group, but not in the HSH group. At 18 weeks, HS showed the expected E/A elevation, which was also significantly attenuated in the HSG group, but not in the HSH group. Isovolumetric relaxation time (IVRT) of the LV increased significantly between 8 and 18 weeks. Prolonged IVRT can be considered an indicator of increased myocardial stiffness due to fibrosis (29-32), but HSG significantly reduced IVRT. This effect was not observed in the HSH group. Collectively, these findings indicate that diastolic parameters are improved by the grape-containing diet, but not by the vasodilator hydralazine.
Table 4.
Serial Changes in Diastolic Parameters
| Diastolic Function | LS | LSG | HS | HSG | HSH |
|---|---|---|---|---|---|
| E/A | |||||
| 0 w | 2.7 ± 0.2 | 2.7 ± 0.3 | 2.6 ± 0.2 | 2.6 ± 0.3 | 2.4 ± 0.3 |
| 8 w | 2.9 ± 0.1 | 2.8 ± 0.3 | 2.0 ± 0.3* | 2.4 ± 0.2† | 2.2 ± 0.2* |
| 18 w | 2.6 ± 0.3 | 2.5 ± 0.1 | 6.2 ± 0.3* | 3.8 ± 0.1† | 5.8 ± 0.3* |
| E Dec time | |||||
| 0 w | 43.6 ± 3 | 44.0 ± 4 | 43.3 ± 6 | 43.2 ± 5 | 43.1 ± 4 |
| 8 w | 42.1 ± 3 | 41.7 ± 5 | 48.3 ± 3* | 45.7 ± 4 | 47.2 ± 4* |
| 18 w | 44.0 ± 2 | 43.0 ± 4 | 34.2 ± 3* | 39.9 ± 3 | 35.6 ± 3* |
| IVRT | |||||
| 0 w | 17.7 ± 3 | 17.3 ± 2 | 18.8 ± 2 | 16.7 ± 1 | 18.1 ± 3 |
| 8 w | 18.3 ± 2 | 18.1 ± 3 | 20.5 ± 2 | 20.1 ± 3 | 19.5 ± 2 |
| 18 w | 21.1 ± 2 | 20.4 ± 3 | 31.5 ± 3* | 24.3 ± 1† | 30.1 ± 3* |
Notes: Echocardiography measures at 0 weeks of diet (baseline), 8 weeks of diet, and 18 weeks of diet. Data presented are means ± standard error of the mean; n = 12 per group.
E/A = E wave to A wave; E Dec t = E wave deceleration time (in ms); IVRT = isovolumetric relaxation time (in ms); LS = low-salt diet; LSG, low salt + grape powder diet; HS = high-salt diet; HSG = high salt + grape powder diet; HSH = high salt + vasodilator hydralazine diet.
At least p < .05 vs LS, LSG.
p < .05 vs LS, LSG, HS, HSH.
Regarding systolic function (Table 5), the percent mid-wall fractional shortening and ejection fraction were not significantly altered by high-salt feeding, which is expected in this rat model; the Dahl-SS rat is a model of diastolic dysfunction rather than systolic dysfunction. However, cardiac index reflects cardiac contractile efficiency by measuring the volume of blood moved per minute (stroke volume × heart rate), per unit of body weight. As such, cardiac index can reflect both diastolic and systolic function. The 8-week measures in all groups did not show a significantly impaired cardiac index, which is expected during compensated hypertrophy. However, at 18 weeks, cardiac index was significantly lower in the HS-fed group but was significantly improved by HSG. This effect was not observed in the HSH group. Heart rate was not affected by treatment, so changes in cardiac index likely reflect changes in cardiac geometry and functionality independent of sympathetic outflow. As observed with diastolic function values, LSG did not confer benefits for systolic function over the LS group.
Table 5.
Serial Changes in Systolic Parameters
| Systolic Function | LS | LSG | HS | HSG | HSH |
|---|---|---|---|---|---|
| % Midwall FS | |||||
| 0 wk | 21.2 ± 2 | 21.2 ± 2 | 22.2 ± 2 | 20.3 ± 3 | 21.7 ± 3 |
| 8 wk | 20.4 ± 3 | 20.4 ± 3 | 18.3 ± 2 | 21.2 ± 2 | 17.9 ± 2 |
| 18 wk | 19.2 ± 2 | 19.2 ± 2 | 17.1 ± 2 | 18.3 ± 3 | 16.9 ± 2 |
| % Ejection fraction | |||||
| 0 wk | 73.0 ± 4 | 73.0 ± 4 | 71.3 ± 5 | 72.2 ± 6 | 72.1 ± 4 |
| 8 wk | 75.0 ± 8 | 75.0 ± 8 | 69.4 ± 5 | 70.3 ± 5 | 68.6 ± 6 |
| 18 wk | 72.1 ± 4 | 72.1 ± 4 | 70.1 ± 4 | 71.1 ± 5 | 70.4 ± 5 |
| Cardiac index | |||||
| 0 wk | 444 ± 32 | 440 ± 27 | 440 ± 23 | 438 ± 33 | 438 ± 17 |
| 8 wk | 435 ± 31 | 434 ± 25 | 431 ± 24 | 432 ± 31 | 426 ± 22 |
| 18 wk | 437 ± 22 | 436 ± 19 | 333 ± 26* | 375 ± 21† | 339 ± 23* |
| Heart rate | |||||
| 0 wk | 386 ± 22 | 394 ± 21 | 392 ± 20 | 382 ± 19 | 391 ± 18 |
| 8 wk | 397 ± 17 | 410 ± 19 | 412 ± 35 | 410 ± 21 | 417 ± 25 |
| 18 wk | 385 ± 23 | 404 ± 25 | 423 ± 14 | 418 ± 23 | 415 ± 24 |
Notes: Echocardiography measures at 0 weeks of diet (baseline), 8 weeks of diet, and 18 weeks of diet. Data presented are means ± standard error of the mean; n = 12 per group.
Cardiac index is mL of blood/minute/g body weight. Heart rate is beats/minute.
FS = fractional shortening; LS = low-salt diet; LSG = low salt + grape powder diet; HS = high-salt diet; HSG = high salt + grape powder diet; HSH = high salt + vasodilator hydralazine diet.
p < .05 vs LS and LSG.
p < .05 vs LS, LSG, HS, HSH.
Cardiac Hypertrophy, Histology, and Hydroxyproline Content
Cardiac hypertrophy correlates with increased blood pressure, increased fibrosis, and collagen deposition, and with reduced cardiac function. Importantly, cardiac hypertrophy precedes the development of more advanced, irreversible pathogenesis such as heart failure. Compared to the HS diet, the HSG diet was associated with significantly lower cardiac and renal hypertrophy (Table 6). Strikingly, the HSG cardiac weights were similar to those of the LS and LSG groups. The HSH diets reduced renal weight but did not reduce cardiac weight. The LSG diet did not impact cardiac weight relative to the LS group. The LSG diet trended to reduce kidney weight relative to the LS group, but the difference was not statistically significant. Hydroxyproline is a component of collagen and a quantitative index of fibrosis. Collagen accumulation occurs in the heart during heart failure and contributes to stiffening of the heart walls, impaired relaxation, impaired filling, and reduced cardiac output. As shown in Table 6, the HSG group had significantly reduced cardiac hydroxyproline content relative to the HS group. This effect was not observed in the HSH group. Also, the HSG diet was associated with a reduced perivascular area of fibrosis, which was not observed in the HSH group.
Table 6.
Changes in Organ Weight and Cardiac Fibrosis
| LS | LSG | HS | HSG | HSH | |
|---|---|---|---|---|---|
| Heart W/TL | 0.34 ± 0.01 | 0.33 ± 0.02 | 0.42 ± 0.1* | 0.32 ± 0.01† | 0.43 ± 0.3† |
| Kidney W/TL | 0.71 ± 0.04 | 0.65 ± 0.04 | 0.98 ± 0.05* | 0.82 ± 0.04† | 0.83 ± 0.06† |
| Lung W/TL | 0.42 ± 0.04 | 0.40 ± 0.04 | 0.54 ± 0.06 | 0.43 ± 0.05 | 0.52 ± 0.04 |
| Cardiac hydroxyproline | 5.2 ± 0.3 | 5.1 ± 0.2 | 9.2 ± 0.2* | 7.4 ± 0.3† | 9.1 ± 0.3* |
| Cardiac perivascular area fibrosis | 1.19 ± 0.04 | 1.17 ± 0.02 | 1.45 ± 0.1* | 1.3 ± 0.03† | 1.40 ± 0.* |
Notes: HW (g heart weight), KW (g kidney weight), and LW (g lung weight) are relative to tibial length (TL) in centimeters. Perivascular area fibrosis is expressed as microns squared. Hydroxyproline is expressed as milligrams per milligrams of total protein. Data are presented as means ± standard error of the mean; n = 12 per group.
LS = low-salt diet; LSG = low salt + grape powder diet; HS = high-salt diet; HSG = high salt + grape powder diet; HSH = high salt + vasodilator hydralazine diet.
At least p < .05 vs LS and LSG.
p < .05 vs LS, LSG, HS.
Cardiac GSH/GSSG, Oxidative Damage, and Plasma Inflammation
Malonyldialdehyde (MDA) is a by-product of the oxidation of lipids, and serves as a marker of oxidative stress. Data in Table 7 indicate that the HSG diet was associated with significantly lower MDA content relative to the HS diet, but the HSH diet did not provide this effect. Although MDA was relatively low in the healthy LS rat hearts, the LSG diet still conferred a significant treatment effect versus the LS diet. Cardiac GSH is decreased in the salt-fed Dahl-SS rat heart relative to GSSG (33,34). The HSG diet significantly improved the GHS/GSSG ratio over the HS group (Table 7), reflecting improved antioxidant defense. This effect was not observed in the HSH group. Interestingly, this effect was also observed in LSG rats as compared to LS rats, indicating that grape powder provision improved cardiac antioxidant defense even in the absence of concurrent disease. Also in Table 7, enzyme-linked immunosorbent assay (ELISA) for plasma markers of inflammation indicated that the HSG diet was associated with significantly reduced plasma IL-6 and TNF-α relative to the HS group. This effect was not observed in the HSH group. Although the LSG diet reduced IL-6 and TNF-α, the results were not statistically significant.
Table 7.
Cardiac GSH/GSSH, Cardiac MDA, and Plasma Inflammation
| LS | LSG | HS | HSG | HSH | |
|---|---|---|---|---|---|
| Cardiac GSH/GSSG | 148 ± 7 | 184 ± 9* | 45 ± 3* | 75 ± 5† | 53 ± 6* |
| Cardiac MDA | 0.4 ± 0.02 | 0.3 ± 0.02 | 1.4 ± 0.1* | 1.0 ± 0.06† | 1.3 ± 0.2* |
| Plasma TNF-α | 1.4 ± 0.02 | 1.1 ± 0.03 | 6.8 ± 0.3* | 4.3 ± 0.3† | 6.3 ± 0.4* |
| Plasma IL-6 | 0.9 ± 0.02 | 0.7 ± 0.02 | 5.4 ± 0.3* | 3.7 ± 0.2† | 5.1 ± 0.4* |
Notes: Cardiac reduced glutathione (GSH) in lM relative to oxidized glutathione (GSSG) in lM. Malonyldialdehyde (MDA) is expressed as mg/per mg total protein. Tumor necrosis factor-a (TNF-a) and interleukin 6 (IL-6) are in pg/mL. Data are presented as means 6 standard error of the mean; n = 12 per group.
At least p < .05 vs LS.
p < .05 vs LS, LSG, HS, HSH.
DISCUSSION
The current results demonstrate the broad effects of a phytochemical-enriched diet on the gradual development of hypertension-associated diastolic dysfunction. The focus on diastolic pathogenesis is of great significance in the aged population. Systolic dysfunction primarily concerns the heart's reduced ejection capacity, whereas diastolic dysfunction concerns the heart's reduced filling capacity. Whereas systolic failure has a higher mortality rate, diastolic heart failure has a strong association with normal aging and is more common than systolic heart failure in elderly persons (35,36). Importantly, numerous clinical trials have documented the benefits of pharmacologic treatment for systolic heart failure; however, the optimal treatment for diastolic heart failure has not yet been defined. Diastolic dysfunction develops over a prolonged period of time and is largely reversible, so the effects of diet patterns on disease course are of great interest for both preventive and interventional cardiology. The descriptive approach used here is intended to reveal the breadth of phytochemical-rich diet effects on many phenotypes relevant to hypertension and to diastolic heart failure pathogenesis.
The mechanisms behind the treatment effects are likely complex and involve interaction among a number of organ systems. For example, grape-related benefits may be derived in part from reduced blood pressure, and blood pressure is regulated dynamically by interactions among the kidney, brain, vasculature, and heart. In the HSG group, reduced systolic blood pressure was observed early and was sustained throughout the study. The lack of depressor effect in the LSG group as compared to the LS group suggests that grape-related depressor effects are largely observed in hypertensive as opposed to normotensive animals. The mechanisms of grape-associated vasodilation are not completely understood, but some studies have indicated that grape-product consumption may improve the availability of the vasodilator nitric oxide. However, hydralazine afforded a similar reduction in systolic blood pressure throughout the study, yet failed to impact eventual cardiac pathology, suggesting that reduced hypertension alone is not sufficient for cardioprotection. Hydralazine limits calcium release from smooth muscle sarcoplasmic reticulum, resulting in arterial and arteriolar relaxation. In the Dahl-SS rat and in other hypertensive rat models, hydralazine consistently reduces arterial pressure but does not impact cardiac hypertrophy or fibrosis (19-21), results which support our current findings. Hydralazine can elicit a reflex sympathetic stimulation at higher doses, but the dose provided here did not cause elevated heart rate or cardiac output. It is possible that differing routes of vasodilation lead to different protective phenotypes, but it is clear that reduced blood pressure alone does not protect against cardiac fibrosis or hypertrophy in this model.
The current results therefore imply that additional mechanisms beyond vasodilation are participating in grape-mediated cardioprotection. Hypertension contributes to cardiac oxidative stress, and grape enrichment may confer antioxidant effects. In the heart, unquenched reactive oxygen and reactive nitrogen species damage local lipids, proteins, and DNA, leading to cardiomyocyte death and suboptimal cardiac function. Although a specific relationship between oxidative stress and ventricular performance has not been clearly established, there is considerable association between oxidative stress and underlying components of cardiac pathogenesis including systemic inflammation, cardiomyocyte apoptosis, cardiac remodeling, mechanoenergetic uncoupling, and endothelial dysfunction. Furthermore, accumulated evidence suggests a significant correlation between oxidative stress and clinical indexes of cardiac functional capacity, such as New York Heart Association class and peak exercise oxygen consumption (37,38). By reducing oxidative stress, antioxidant-rich diets may thus impact the pathogenesis or severity of cardiac dysfunction.
Direct antioxidant effects in the heart tissue would require cardiac bioavailability of the grape phytochemicals. The predominant bioavailable components would likely include enterohepatic metabolites such as sulfate conjugates, glucuronides, and O-methylated forms, with very low levels of nonconjugated, parent compounds. However, these enterohepatic and intracellular metabolites have a reduced ability to donate hydrogen, and are less effective scavengers of radicals as compared to their parent compounds. Also, concentrations of these metabolites in the plasma or in tissues are lower (nanomolar, low micromolar) than those recorded for in vivo antioxidants such as ascorbate, uric acid, glutathione, and vitamin E (39). Consequently, bioavailable grape phytochemicals are unlikely to supersede these antioxidants for radical scavenging effects, and thus direct antioxidant action of grape metabolites in cardiac tissue may be relatively minor.
Instead, accumulating evidence suggests that the tissue antioxidant effects of phytochemicals may be mediated indirectly by their interactions with intracellular signaling cascades and with altered gene expression. For example, bioavailable phytochemicals may stimulate the transcription and translation of endogenous antioxidants in the heart. Polyphenols like those found in grapes can activate response elements in the genome, which regulate the transcription of glutathione-regulating enzymes. In the current study, both LSG and HSG diets were associated with elevated GSH/GSSG relative to their controls (LS and HS diets, respectively). Bioavailable grape phenolic phytochemicals may activate cardiac genes, which modify glutathione dynamics, like glutathione peroxidase and glutathione-S-transferase (40); this possibility is currently under investigation by our group.
Finally, grape-related benefits may derive in part from indirect effects on reduced cachexia and systemic inflammation. Cachexia is a catabolic state characterized by weight loss and muscle wasting, occurs frequently in patients with heart failure, and is a strong independent risk factor for heart failure-related mortality (41). Cachexia is also characteristic of Dahl-SS pathogenesis, and appeared in our rats after 14 weeks of diet. The onset of cachexia is associated with elevations in proinflammatory mediators, including IL-6 and TNF-α, both of which correlate with advancing heart failure (22,42). Grape diet effect on body weight loss approached significance, and significantly reduced plasma TNF-α and IL-6 (Table 7). Thus, limited cachexia may contribute to grape-associated benefits.
The phytochemical model presented here is limited by the use of only one fruit. We expect that treatment effect could be amplified were we to use a more complex mix of phytochemical-containing whole foods. Rationale for grape selection is supported from several studies showing a depressor effect of grape juice and wine consumption (14-18). Results found here may not extend to the dietary supplement grape seed extract, which contains higher levels of high-molecular-weight tannins of questionable bioavailability, and which lacks anthocyanins. Instead, table grape powder derived from grape skin, flesh, and seed contains a broader phytochemical profile that is more relevant to that observed in fruit/vegetable-rich human diets. Our intent was to use a model food that has modest antioxidant potential and has demonstrated efficacy. This standardized, whole grape powder used here has been shown by other investigators to reduce both plasma and tissue markers of oxidative stress in vivo and ex vivo (9-13). These studies indicate that beneficial components of the whole table grape powder are bioavailable to tissues and that they could confer health benefits against diseases that involve oxidative stress such as hypertension-associated heart failure. The simplified model presented here may thus serve as a precursor to studies that model more complex dietary patterns and their effects on cardiac pathogenesis.
With regard to grape “dose” justification, allometric scaling or the bioequivalence between rodents and humans is unknown. As such, the dose of grape powder given per day was made relative to body weight. One human serving of fresh grapes is 3/4 cup, or approximately 126 g. With loss on drying, one human serving of freeze-dried whole grape powder equals 23 g. The rat body weight equivalent of 9 servings of grape/day then averaged 600 mg/day, or 3% of the daily diet. In this manner, the dose used here attempted to model the 9 servings/day of fruit/vegetables in the DASH diet trials (4). Other methods of estimating bioequivalence could lead to different dose justifications, including adjustments made relative to metabolic rate, food intake, food intake relative to body weight, differences in body surface area, or target organ weight relative to body weight. Regardless of the approach to estimate bioequivalence, the level of whole fruit powder used here is likely to be physiologically relevant to human diets.
Although we controlled for macronutrient and calorie intake in the current design, we cannot conclusively exclude any benefit from the additional micronutrients from grape (as described in Table 1). However, the 3% wt/wt grape powder enrichment supplied a modest 6 mg increase in potassium and a 0.02 mg increase in vitamin C intake per day. Previous studies in the Dahl-SS rat suggest that more potassium (43) (5-10 times higher than provided here) and more vitamin C (44) (5000 times higher than provided here) are required to reduce blood pressure. Further studies may be warranted to ascertain the specific contribution of micronutrients in the absence and presence of phytochemicals. Clinical trials in patients with heart failure have failed to detect benefits from antioxidant micronutrient supplementation, and indeed some have observed adverse effects (45,46). In contrast to dietary supplements, whole food models allow synergistic interaction between micronutrients and phytochemicals that may improve their bioavailability or potency. Therefore, whole foods approaches may confer both increased efficacy and safety versus dietary supplements for the prevention or treatment of heart failure.
Summary
The diet incorporation of grape-derived phytochemicals improved cardiac glutathione reserve and reduced experimental hypertension-induced cardiac fibrosis and diastolic dysfunction in the Dahl-SS rat. This benefit correlated with reduced cardiac oxidative damage and improved cardiac antioxidant reserve. The findings support the efficacy of phytochemical-enriched diets against hypertension-associated cardiac pathology. This association may have particular importance to our aging population, which has reduced intake of both fruit and vegetables. The 2000 edition of the Dietary Guidelines for Americans (47) revealed that, in individuals older than 60 years, only 35% of women and 39% of men met the two-servings-per-day objective for fruits, and only 6% of both women and men met the three-servings-per-day objective for vegetables. Because grape supplementation occurred at the onset of the study, this model examines preventive effects versus interventional effects. Ongoing studies in our laboratory are assessing the effect of diet change after the development of hypertension and compensated hypertrophy, respectively. In this manner, we may reveal the further value of phytochemical-enriched diets to interventional cardiology.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH)-National Center for Complementary and Alternative Medicine grant AT001639 (to S. F. Bolling), by NIH-National Heart Lung and Blood Institute grant F019691 (to E.M. Seymour), and by core funding assistance to S. F. Bolling from NIH-National Institute on Aging grant AG013283 (University of Michigan Nathan Shock Center of Excellence in the Basic Biology of Aging). Funding and grape powder were also provided by an unrestricted grant from the California Table Grape Commission.
We thank the University of Michigan Center for Integrative Genomics for their assistance with the serial in vivo measures. We also thank veterinary pathologist Dr. John E. Wilkinson for his assistance with histology and data interpretation.
Footnotes
CONFLICT OF INTEREST This project was supported in part by the California Table Grape Commission (CTGC). The CTGC did not participate in data analysis or manuscript preparation.
REFERENCES
- 1.Council on High Blood Pressure Research, AHA . Hypertension Primer—The Essentials of High Blood Pressure. 3rd Ed Lippincott Williams & Wilkins; Philadelphia: 2003. [Google Scholar]
- 2.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 3.Knebel F, Baumann G. Heart failure: state-of-the-art treatment and new therapeutic options. Clin Nephrol. 2003;60(Suppl 1):S59–S66. [PubMed] [Google Scholar]
- 4.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 5.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 6.Doyle L, Cashman KD. The effect of nutrient profiles of the Dietary Approaches to Stop Hypertension (DASH) diets on blood pressure and bone metabolism and composition in normotensive and hypertensive rats. Br J Nutr. 2003;89:713–724. doi: 10.1079/BJN2003833. [DOI] [PubMed] [Google Scholar]
- 7.Most MM. Estimated phytochemical content of the dietary approaches to stop hypertension (DASH) diet is higher than in the Control Study Diet. J Am Diet Assoc. 2004;104:1725–1727. doi: 10.1016/j.jada.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Wu Q, Wang M, Simon JE. Determination of proanthocyanidins in fresh grapes and grape products using liquid chromatography with mass spectrometric detection. Rapid Commun Mass Spectrom. 2005;19:2062–2068. doi: 10.1002/rcm.2029. [DOI] [PubMed] [Google Scholar]
- 9.Cui J, Cordis GA, Tosaki A, Maulik N, Das DK. Reduction of myocardial ischemia reperfusion injury with regular consumption of grapes. Ann N Y Acad Sci. 2002;957:302–307. doi: 10.1111/j.1749-6632.2002.tb02930.x. [DOI] [PubMed] [Google Scholar]
- 10.Cui J, Juhasz B, Tosaki A, Maulik N, Das DK. Cardioprotection with grapes. J Cardiovasc Pharmacol. 2002;40:762–769. doi: 10.1097/00005344-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Fuhrman B, Volkova N, Coleman R, Aviram M. Grape powder polyphenols attenuate atherosclerosis development in apolipoprotein E deficient (E0) mice and reduce macrophage atherogenicity. J Nutr. 2005;135:722–728. doi: 10.1093/jn/135.4.722. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Simonyi A, Li W, et al. Dietary grape supplement ameliorates cerebral ischemia-induced neuronal death in gerbils. Mol Nutr Food Res. 2005;49:443–451. doi: 10.1002/mnfr.200500019. [DOI] [PubMed] [Google Scholar]
- 13.Zern TL, Wood RJ, Greene C, et al. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J Nutr. 2005;135:1911–1917. doi: 10.1093/jn/135.8.1911. [DOI] [PubMed] [Google Scholar]
- 14.Takahara A, Sugiyama A, Honsho S, et al. The endothelium-dependent vasodilator action of a new beverage made of red wine vinegar and grape juice. Biol Pharm Bull. 2005;28:754–756. doi: 10.1248/bpb.28.754. [DOI] [PubMed] [Google Scholar]
- 15.Coimbra SR, Lage SH, Brandizzi L, Yoshida V, da Luz PL. The action of red wine and purple grape juice on vascular reactivity is independent of plasma lipids in hypercholesterolemic patients. Braz J Med Biol Res. 2005;38:1339–1347. doi: 10.1590/s0100-879x2005000900008. [DOI] [PubMed] [Google Scholar]
- 16.Soares De Moura R, Costa Viana FS, Souza MA, et al. Antihypertensive, vasodilator and antioxidant effects of a vinifera grape skin extract. J Pharm Pharmacol. 2002;54:1515–1520. doi: 10.1211/002235702153. [DOI] [PubMed] [Google Scholar]
- 17.Chou EJ, Keevil JG, Aeschlimann S, et al. Effect of ingestion of purple grape juice on endothelial function in patients with coronary heart disease. Am J Cardiol. 2001;88:553–555. doi: 10.1016/s0002-9149(01)01738-6. [DOI] [PubMed] [Google Scholar]
- 18.Flesch M, Schwarz A, Bohm M. Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am J Physiol. 1998;275(4 Pt 2):H1183–H1190. doi: 10.1152/ajpheart.1998.275.4.H1183. [DOI] [PubMed] [Google Scholar]
- 19.Ohno T, Kobayashi N, Yoshida K, Fukushima H, Matsuoka H. Cardioprotective effect of benidipine on cardiac performance and remodeling in failing rat hearts. Am J Hypertens. 2008;21:224–230. doi: 10.1038/ajh.2007.51. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto E, Kataoka K, Shintaku H, et al. Novel mechanism and role of angiotensin II induced vascular endothelial injury in hypertensive diastolic heart failure. Arterioscler Thromb Vasc Biol. 2007;27:2569–2575. doi: 10.1161/ATVBAHA.107.153692. [DOI] [PubMed] [Google Scholar]
- 21.Matsui H, Shimosawa T, Uetake Y, et al. Protective effect of potassium against the hypertensive cardiac dysfunction: association with reactive oxygen species reduction. Hypertension. 2006;48:225–231. doi: 10.1161/01.HYP.0000232617.48372.cb. [DOI] [PubMed] [Google Scholar]
- 22.Seymour EM, Parikh RV, Singer AA, Bolling SF. Moderate calorie restriction improves cardiac remodeling and diastolic dysfunction in the Dahl-SS rat. J Mol Cell Cardiol. 2006;41:661–668. doi: 10.1016/j.yjmcc.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Boluyt MO, Converso K, Hwang HS, Mikkor A, Russell MW. Echocardiographic assessment of age-associated changes in systolic and diastolic function of the female F344 rat heart. J Appl Physiol. 2004;96:822–828. doi: 10.1152/japplphysiol.01026.2003. [DOI] [PubMed] [Google Scholar]
- 24.Doi R, Masuyama T, Yamamoto K, et al. Development of different phenotypes of hypertensive heart failure: systolic versus diastolic failure in Dahl salt-sensitive rats. J Hypertens. 2000;18:111–120. doi: 10.1097/00004872-200018010-00016. [DOI] [PubMed] [Google Scholar]
- 25.de Simone G, Devereux RB, Camargo MJ, et al. Midwall left ventricular performance in salt-loaded Dahl rats: effect of AT1 angiotensin II inhibition. J Hypertens. 1995;13(12 Pt 2):1808–1812. [PubMed] [Google Scholar]
- 26.Ono K, Masuyama T, Yamamoto K, et al. Echo doppler assessment of left ventricular function in rats with hypertensive hypertrophy. J Am Soc Echocardiogr. 2002;15:109–117. doi: 10.1067/mje.2002.115034. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu N, Yoshiyama M, Takeuchi K, et al. Doppler echocardiographic assessment and cardiac gene expression analysis of the left ventricle in myocardial infarcted rats. Jpn Circ J. 1998;62:436–442. doi: 10.1253/jcj.62.436. [DOI] [PubMed] [Google Scholar]
- 28.Qu P, Hamada M, Ikeda S, et al. Time-course changes in left ventricular geometry and function during the development of hypertension in Dahl salt-sensitive rats. Hypertens Res. 2000;23:613–623. doi: 10.1291/hypres.23.613. [DOI] [PubMed] [Google Scholar]
- 29.Arques S, Roux E, Sbragia P, et al. Accuracy of the isovolumic relaxation time in the emergency diagnosis of new-onset congestive heart failure with preserved left ventricular systolic function in the setting of B-type natriuretic peptide levels in the mid-range. Int J Cardiol. 2008;124:400–403. doi: 10.1016/j.ijcard.2006.12.084. [DOI] [PubMed] [Google Scholar]
- 30.Kosmala W, Spring A, Witkowska M. Relationship between systolic and diastolic function of the left ventricle in patients with impaired relaxation of the left ventricle without symptoms of heart failure. Attempt at quantitative estimation of diastolic function in the impaired relaxation stage. Pol Arch Med Wewn. 1997;98:414–423. [PubMed] [Google Scholar]
- 31.Tarmonova L, Shutov AM, Chernysheva EV. Factors influencing left ventricular diastolic function in elderly patients with chronic heart failure. Klin Med (Mosk) 2007;85:26–29. [PubMed] [Google Scholar]
- 32.Yu WC, Chiou KR, Lin YP, et al. Non-invasive determination of left ventricular relaxation time constant by Transthoracic Doppler echocardiography. J Chin Med Assoc. 2004;67:317–322. [PubMed] [Google Scholar]
- 33.Bayorh MA, Ganafa AA, Socci RR, Silvestrov N, Abukhalaf IK. The role of oxidative stress in salt-induced hypertension. Am J Hypertens. 2004;17:31–36. doi: 10.1016/j.amjhyper.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Somova LI, Nadar A, Gregory M, Khan N. Antioxidant status of the hypertrophic heart of Dahl hypertensive rat as a model for evaluation of antioxidants. Methods Find Exp Clin Pharmacol. 2001;23:5–12. doi: 10.1358/mf.2001.23.1.619173. [DOI] [PubMed] [Google Scholar]
- 35.De Keulenaer GW, Brutsaert DL. Diastolic heart failure: a separate disease or selection bias? Prog Cardiovasc Dis. 2007;49:275–283. doi: 10.1016/j.pcad.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Persson H, Lonn E, Edner M, et al. Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence: results from the CHARM Echocardiographic Substudy-CHARMES. J Am Coll Cardiol. 2007;49:687–694. doi: 10.1016/j.jacc.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 37.Keith M, Geranmayegan A, Sole MJ, et al. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 38.Mallat Z, Philip I, Lebret M, et al. Elevated levels of 8-isoprostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97:1536–1539. doi: 10.1161/01.cir.97.16.1536. [DOI] [PubMed] [Google Scholar]
- 39.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Puiggros F, Llopiz N, Ardevol A, et al. Grape seed procyanidins prevent oxidative injury by modulating the expression of antioxidant enzyme systems. J Agric Food Chem. 2005;53:6080–6086. doi: 10.1021/jf050343m. [DOI] [PubMed] [Google Scholar]
- 41.Akashi YJ, Springer J, Anker SD. Cachexia in chronic heart failure: prognostic implications and novel therapeutic approaches. Curr Heart Fail Rep. 2005;2:198–203. doi: 10.1007/BF02696650. [DOI] [PubMed] [Google Scholar]
- 42.Paulus WJ. Cytokines and heart failure. Heart Fail Monit. 2000;1:50–56. [PubMed] [Google Scholar]
- 43.Manger WM, Simchon S, Stier CT, Jr, et al. Protective effects of dietary potassium chloride on hemodynamics of Dahl salt-sensitive rats in response to chronic administration of sodium chloride. J Hypertens. 2003;21:2305–2313. doi: 10.1097/00004872-200312000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Tian N, Moore RS, Braddy SJ, et al. Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007;293:H3388–H3395. doi: 10.1152/ajpheart.00981.2007. [DOI] [PubMed] [Google Scholar]
- 45.Nightingale AK, Schmitt M, Frenneaux MP. Vitamin C in heart failure: hype or hope? Hypertension. 2004;43:e5–e6. doi: 10.1161/01.hyp.0000112025.25724.8a. author reply e5-e6. [DOI] [PubMed] [Google Scholar]
- 46.Marchioli R, Levantesi G, Macchia A, et al. Vitamin E increases the risk of developing heart failure after myocardial infarction: Results from the GISSI-Prevenzione trial. J Cardiovasc Med (Hagerstown) 2006;7:347–350. doi: 10.2459/01.JCM.0000223257.09062.17. [DOI] [PubMed] [Google Scholar]
- 47.Johnson RK, Kennedy E. The 2000 Dietary Guidelines for Americans: what are the changes and why were they made? The Dietary Guidelines Advisory Committee. J Am Diet Assoc. 2000;100:769–774. doi: 10.1016/s0002-8223(00)00225-x. [DOI] [PubMed] [Google Scholar]