What clinician has not dreamed of enlisting the immune system to battle cancer? Exquisitely specific, self-regulating, and equipped to search anywhere metastases could hide – immuno-therapy appears to have it all. Yet clinicians are also only too aware of its limited current role in cancer management (1). For many of the commonest malignancies, effective immunotherapy remains tantalizingly just out of sight. Of course, pessimism towards this entire diverse field is neither fair nor accurate (2). Immunotherapies comprise three broad categories: (a) adoptive approaches, by which immune effectors are expanded and returned to the patient; monoclonal antibodies are a special case; (b) immunization approaches, using tumor antigens and dendritic cells (DC), often genetically modified to augment their potency; and (c) biological response modifiers (BRMs) including recombinant cytokines, manipulation of immunoregulatory receptors, and, most germane to this Perspective, microbial products. BRMs date from the treatment of sarcomas with bacterial extracts (3) and includes the ongoing use of Bacillus Calmette-Guérin (BCG) (4), but has received variable attention in recent years.
The March issue of The Journal contains an intriguing study that heralds a resurgence in BRM research (5). Shingu and colleagues examined topical delivery of the mycoplasmal lipoprotein Macrophage-activating lipopeptide 2 (MALP-2) in a rat model of lung metastases of the MADB106 mammary adenocarcinoma line. Results show a 44% reduction in lung metastasis colonies at three weeks after treatment when MALP-2 was given just before, but not 1 or 3 days after, tumor inoculation. In a previous study in this model (6), both natural killer (NK) cells and macrophages (Mø) co-localized with tumor cells within 15–30 minutes. NK cell depletion resulted in an acute decrease in Mø co-localization, and a >5-fold increase in lung metastases. In the current study (5), MALP-2 treatment increased both Mø recruitment to tumor implants and expression of mRNA for the innate receptor TLR2, which recognizes MALP-2 (7); because NK cells lack TLR2, both effects were likely directly on Mø. The study is notable for the meticulous technique characteristic of Dr. Pabst and his colleagues. But what distinguishes it from previous empiric approaches is its appreciation of three factors likely to drive BRM research in the 21st century: timing of the intervention, cooperation between individual cell types, and specificity of innate immune receptors.
Spread of micro-metastases in the peri-operative period: timing is everything
Every clinician has encountered patients who refuse indicated cancer surgery because they know a someone whose cancer progressed rapidly “after they let air on the tumor”. Physicians, believing that dissemination begins as soon as tumors establish a blood supply (8), generally try to refute such folklore. Still, a number of lines of evidence suggest that our patients may be correct that the post-operative period is perilous. The first comes from use of RT-PCR which identified circulating tumor-specific mRNA during operations for a variety of tumors, but also without surgery (9, 10). Enhanced dissemination during surgery has been shown in small numbers of patients with breast or gastro-intestinal primaries (11–13). Whether metastases usually disseminate to a greater degree intra-operatively than at other times, whether “no-touch isolation” can improve survival (14, 15), and even whether circulating tumor RNA is of prognostic importance (16) remain controversial. Equally important is evidence that survival of metastases may be enhanced by the immunosuppressive effect of surgery. Pain, sympathetic outflow, and opiates all contribute to post-operative immunosuppression, acting indirectly as well as directly on leukocyte adrenergic and opiate receptors (17, 18). Most severely impacted is the NK cell (19).
NK cells: the body’s rapid response team
Rapidly recruited to sites of tissue damage (20), NK cells serve functions that cannot be replaced by other immune effectors (21). As their name implies, NK cells require neither advice nor consent from other cell types before executing their targets (22). Their lytic function is controlled both by activatory receptors that recognize motifs unique to transformed cells, and by inhibitory receptors that recognize MHC class I antigens, allowing NK cells “multiple-choice” of targets (23). Because each NK clone randomly bears several classes of inhibitory receptors, the NK repertoire can sense the loss of even a single HLA-A, -B or –C allotype, and thus eliminate transformed cells that would escape cytotoxic T cells (24). NK cells are also important both because they promote development of type 1 T cells through INF-γ production and intimate interactions with DC, and for antigen-independent activation of phagocytes early in inflammation (as in the MADB106 model) (20, 21, 25).
Cancer patients often have impaired NK cell function and post-therapy NK activity correlates with metastasis-free survival time (25). Nevertheless, it has been difficult to prove unequivocally that NK cells combat tumors in humans. The definitive results from NK cell-deficient mice (26) are difficult to extrapolate because primates have evolved a unique type of killer inhibitory receptors (KIR) entirely absent in rodents (23). Probably the most compelling data for NK cells comes from the anti-leukemic effect of mismatched bone marrow transplants (27). On balance, continued focus on NK cells in cancer immunotherapy seems advisable.
Mø activation: awakening the sleeping giant
Mø can kill cancer cells either by antibody-directed cellular cytotoxicity (ADCC) or by direct lysis. ADCC is rapid but depends on anti-tumor antibodies, which are unlikely with established primary tumors. Direct lysis is slower (1–3 days) and appears to involve a host of mechanisms, including toxic oxygen radicals, nitric oxide, and TNF-α. Unlike other functions of resident tissue Mø such as ingestion of microbes or apoptotic cells, both types of tumoricidal activity require Mø activation (28). One way Mø can be activated to kill tumor cells is by cytokines such as IFN-γ, IL-12 and GM-CSF. Ongoing human trials of these cytokines (either as BRMs or by adoptive transfer of genetically-engineered cells) have been largely phase I or I/II and show limited clinical benefit to date {reviewed in (29)}. Pending the results of ongoing phase II trials, the way remains open for renewed study of microbial-derived BRMs.
PAMPs & PRRs: activating innate immunity
Innate immune responses are triggered when invariant microbial elements called pathogen-associated molecular patterns (PAMPs) stimulate pathogen recognition receptors (PRRs) on Mø and DC (30). Cloning of one family of PRRs, the Toll-like receptors (TLRs), has accelerated understanding of pathogen recognition (31). Five of the ten human TLRs have been linked to recognition of individual PAMPs: TLR4 to protein-free LPS, TLR3 to double-stranded RNA, TLR5 to bacterial flagellin, TLR7 to viruses, and TLR9 to unmethylated CpG dinucleotides. TLR2 is more versatile, recognizing bacterial peptidoglycan and lipoteichoic acid by itself (32), as well as tri-acylated lipopeptides (with TLR1) (33) and di-acylated lipopeptides (with TLR6) (34). The realization that the ability of complex microbial mixtures to induce Mø tumoricidal activity depends on the interaction of PAMPs with PRRs (e.g., BCG works because muramyl peptides stimulate TLR2) paved the way for use of purified PAMPs as BMRs. Compounds that activate TLR4 or TRL9 are currently in phase I and II trials with promising outcomes (35–37).
MALP-2: a tiny factor with a hefty punch
PAMPs are generally components of the microbial cell wall. The story of MALP-2 began with the insightful deduction that Mycoplasma can also efficiently induce Mø tumoricidal activity (38) and cytokine production (39, 40), and yet because these tiny prokaryotes lack cell walls, they must possess unique PAMPs. Early attempts to identify the responsible molecules were hampered by variation in the numbers and sizes of Mycoplasmal lipoproteins. It is now clear that such variation is due to post-translational modifications of a protein designated MALP-404, M161Ag, or P48 (41, 42). MALP-2 is the 2-kDa free amino-terminal segment of these precursors; it consists of 14 amino acids, the first of which is modified with dual ester-linked lipids. MALP-2 is recognized by TLR2 plus TLR6 (7, 34, 43), signaling though MyD88 and IRAK-1 to activate NF-κB (7). TLR2 stimulation may be preferable in BRMs, as it yields a more narrow spectrum of cytokines (compared to stimulation via TLR4), in part because it does not induce IFN-β-dependent Stat1 α/β activation (44). MALP-2 can be synthesized economically, and is several orders of magnitude more potent on a molar basis than PAMPs of conventional bacteria, even those that also stimulate TLR2. Thus, MALP-2 is a superb candidate to activate Mø tumoricidal activity.
Tumor-associated mononuclear cells: good guys or unwitting co-conspirators?
Failure to activate tumor-associated mononuclear cells (TAM) is more than a missed opportunity (45). TAM potently produce angiogenic ELR-containing CXC chemokines that accelerate neo-vascularization (46), matrix metalloproteinases and cathepsins that facilitate tumor invasiveness, and tissue factor that activates coagulation and envelops metastatic cells in a protective cloak. Reactive oxygen species from TAM can inactivate tumor-infiltrating NK cells and T cells (47), and induce NK cell apoptosis (48). Extensive TAM infiltration correlates with poor prognosis in carcinomas of the breast, cervix, and bladder, and there is conflicting evidence for its role in prostate, lung and brain tumors (49). In their willingness to defend the tumor, TAM may seem to aficionados of Star Trek to be like crew-members assimilated into The Borg.
Paradoxically, tumors may even use TAM and their own ongoing apoptotic death to subvert the immune system. The Mø and immature DC that comprise TAM are likely to ingest apoptotic cells avidly (50, 51), and thereby impair their capacity for inflammatory cytokine production via secretion of TGF-β and PGE2 (52). This mechanism can impede host defense against pathogens (53) and probably limits development of tumoricidal activity by newly recruited monocytes. TGF-β1 can also upregulate TAM production of urokinase plasminogen activator (uPA) (54), increasing tumor neo-vascularization, matrix destruction, and invasion. DCs that pick up tumor antigens in this environment would be anticipated to induce T cell tolerance (55). The decreased capacity of alveolar Mø (relative to other resident tissue Mø studied so far) to ingest apoptotic cells (56, 57) could mitigate these anti-inflammatory effects in the lungs, one of the most common sites of metastasis. Although this adaptation probably evolved to preserve host defenses against pathogens, it may also preserve the capacity of alveolar Mø to fight metastases.
Wrapping it all up
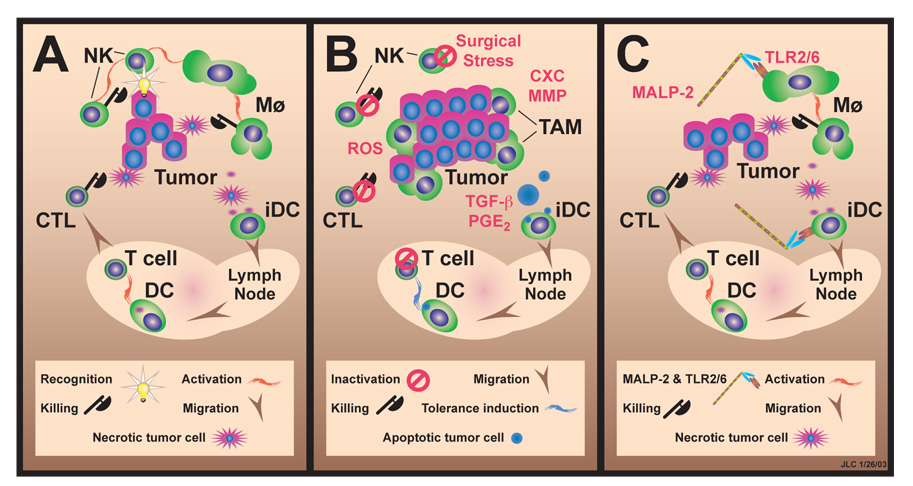
The study by Shingu et al (5) is one step in a journey to improved survival after curative intent resection of solid malignancies. The logic is as follows (Fig. 1). Operative stress and especially pain selectively depress NK cell function. In the absence of NK-derived signals, Mø are not activated to become tumoricidal, allowing metastasis to escape a vulnerable time-period immediately after lodging in the microvasculature. The metastases can then enslave Mø to promote their own survival and growth. By contrast, MALP-2 directly activates Mø via TLR2, bypassing depressed NK cell function, and allowing Mø to rid the lungs of tumor.
Figure 1. Proposed schema for modification of TAM by MALP-2.
A. Baseline tumor-immune cell interactions. NK cells activate Mø to become tumoricidal, and also kill some tumors; immature DC (iDC) pick up antigens from necrotic tumor cells, mature under influence of inflammatory cytokines, and migrate to lymph nodes where they activate specific anti-tumor responses, producing cytotoxic T cells (CTL). B. Suppressive effects of surgical stress. By suppressing NK cell function, pain, sympathetic hormones, and opiate analgesics prevent Mø activation; tumor cells induce TAM to produce angiogenic chemokines (CXC) and matrix metallo-proteinases (MMP) (which increase tumor survival and invasiveness), and reactive oxygen species (ROS) (which inactivate specific T cells and NK cells); iDC exposed to TGF-β and PGE2 carry antigens derived from apoptotic tumor cell to lymph nodes and tolerize specific T cells. C. Beneficial effect of BRMs. MALP-2 directly activates Mø (and probably iDC which also express TLR2/6) leading to tumor killing and development of effective adaptive immunity.
As we stated at the onset, an intriguing study – but can it be extrapolated to clinical practice? The experimental model system clearly has inherent limitations. First, and most obvious, it involved healthy rats with low tumor burden, not infirm humans with multiple co-morbidities. Second, only a single tumor cell line was studied; it is uncertain that common human tumors will be as dependent on NK cells for clearance. Third, only a single time-point was examined, and even then, considerable tumor burden remained. Fourth, no survival data are given. Hopefully, future studies will define these important end-points in this and other tumor model systems. As with all good science, this article raises additional questions. Will MALP-2 or similar adjuvants increase the risk of the systemic inflammatory response syndrome (SIRS) during concurrent stresses? Should other BRMs be considered for the inhalational route? What is the relative importance in the MALP-2 response of resident AMø, as opposed to monocytes? This issue is unclear because the ED-1 antibody used by Shingu et al to identify TAM stains both cell types (58). The most important outcome this article should be to stimulate further mechanistic human and animal studies grounded in the burgeoning understanding of innate immunity.
The seemingly esoteric data we have reviewed are relevant to the daily care of cancer patients. Careful control of post-operative pain {perhaps using the immunostimulatory analgesic tramadol (59, 60)} and of sympathetic outflow by avoiding hypotension (and perhaps by beta blockade) may prove not only good practice but of survival benefit. The putative and still controversial adverse effect of transfusion on recurrence after lung cancer resection {reviewed (61)} could reflect immunosuppression due to such stress rather than to the transfusion itself. Another potential source of stress can be overly aggressive attempts to keep dyspneic patients off mechanical ventilation post-operatively, which may ultimately be more deleterious in this setting than the small incremental daily risk of ventilator-associated pneumonia from continued mechanical ventilation. These data also suggest a new means of improving survival after resection of solid malignancies throughout the body. Timing appears to be crucial to the success of BRMs as immuno-adjuvants; administration at the time of elective surgery of low-stage patients may be more effective than in advanced disease. The narrow range of cytokines produced by TLR-2 stimulation (44) suggests that MALP-2 and similar BRMs may have less potential than more complex BRMs derived from intact organisms to induce lung injury if administered by inhalation. Perhaps some day soon, inhalation of BRMs the night before cancer surgery and infusion of a cocktail of anti-adhesive molecules on call to the OR will be as accepted as prophylactic antibiotics are now.
Acknowledgements
We thank Dr. John Osterholzer for reviewing the manuscript and Joyce O’Brien for secretarial support.
Supported by Merit Review funding and a Research Enhancement Award Program (REAP) grant from the Department of Veterans Affairs; and by RO1 HL56309, RO-1 HL6157, and P30 CA46592 from the USPHS.
Abbreviations used
- ADCC
antibody-dependent cellular cytotoxicity
- BRMs
biological response modifiers
- CTL
cytotoxic T cells
- DC
dendritic cell(s)
- KIR
killer inhibitory receptors
- NK cells
Natural Killer cells
- MMP
matrix metalloproteinase
- Mø
macrophage(s)
- PAMP
pathogen-associated molecule pattern
- PRR
pathogen recognition receptor
- ROS
reactive oxygen species
- SIRS
systemic inflammatory response syndrome
- TAM cell
tumor-associated mononuclear cell
- TLR
Toll-like receptor
- uPA
urokinase plasminogen activator
References
- 1.Pardoll DM. Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol. 2002;2:227–238. doi: 10.1038/nri774. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA. Progress in the development of immunotherapy for the treatment of patients with cancer. J Intern Med. 2001;250:462–475. doi: 10.1046/j.1365-2796.2001.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coley WB. The treatment of malignant tumors by repeated inoculation of erysipelas. With a report of ten original cases. Am J Med Sci. 1893;105:487–511. [PubMed] [Google Scholar]
- 4.Azuma I, Seya T. Development of immunoadjuvants for immunotherapy of cancer. Int Immunopharmacol. 2001;1:1249–1259. doi: 10.1016/s1567-5769(01)00055-8. [DOI] [PubMed] [Google Scholar]
- 5.Shingu K, Krushcinski C, Lühmann A, Tschernig T, von Hörsten S, Pabst R. Intratracheal macrophage-activating lipopeptide-2 (MALP-2) reduces metastasis in the rat lung. Am J Respir Cell Mol Biol. 2003 doi: 10.1165/rcmb.2002-0106OC. in press. [DOI] [PubMed] [Google Scholar]
- 6.Shingu K, Helfritz A, Kuhlmann S, Zielinska-Skowronek M, Jacobs R, Schmidt RE, Pabst R, von Horsten S. Kinetics of the early recruitment of leukocyte subsets at the sites of tumor cells in the lungs: natural killer (NK) cells rapidly attract monocytes but not lymphocytes in the surveillance of micrometastasis. Int J Cancer. 2002;99:74–81. doi: 10.1002/ijc.10279. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, Mühlradt PF, Akira S. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000;164:554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 8.Glaves D. Correlation between circulating cancer cells and incidence of metastases. Br J Cancer. 1983;48:665–673. doi: 10.1038/bjc.1983.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel H, Le Marer N, Wharton RQ, Khan ZA, Araia R, Glover C, Henry MM, Allen-Mersh TG. Clearance of circulating tumor cells after excision of primary colorectal cancer. Ann Surg. 2002;235:226–231. doi: 10.1097/00000658-200202000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamashita J, Matsuo A, Kurusu Y, Saishoji T, Hayashi N, Ogawa M. Preoperative evidence of circulating tumor cells by means of reverse transcriptase-polymerase chain reaction for carcinoembryonic antigen messenger RNA is an independent predictor of survival in non-small cell lung cancer: a prospective study. J Thorac Cardiovasc Surg. 2002;124:299–305. doi: 10.1067/mtc.2002.124370. [DOI] [PubMed] [Google Scholar]
- 11.Mori M, Mimori K, Ueo H, Karimine N, Barnard GF, Sugimachi K, Akiyoshi T. Molecular detection of circulating solid carcinoma cells in the peripheral blood: the concept of early systemic disease. Int J Cancer. 1996;68:739–743. doi: 10.1002/(SICI)1097-0215(19961211)68:6<739::AID-IJC8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg. 2000;232:58–65. doi: 10.1097/00000658-200007000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyazono F, Natsugoe S, Takao S, Tokuda K, Kijima F, Aridome K, Hokita S, Baba M, Eizuru Y, Aikou T. Surgical maneuvers enhance molecular detection of circulating tumor cells during gastric cancer surgery. Ann Surg. 2001;233:189–194. doi: 10.1097/00000658-200102000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Olmo D, Ontanon J, Garcia-Olmo DC, Vallejo M, Cifuentes J. Experimental evidence does not support use of the "no-touch" isolation technique in colorectal cancer. Dis Colon Rectum. 1999;42:1449–1456. doi: 10.1007/BF02235045. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi N, Egami H, Kai M, Kurusu Y, Takano S, Ogawa M. No-touch isolation technique reduces intraoperative shedding of tumor cells into the portal vein during resection of colorectal cancer. Surgery. 1999;125:369–374. [PubMed] [Google Scholar]
- 16.Bessa X, Elizalde JI, Boix L, Pinol V, Lacy AM, Salo J, Pique JM, Castells A. Lack of prognostic influence of circulating tumor cells in peripheral blood of patients with colorectal cancer. Gastroenterology. 2001;120:1084–1092. doi: 10.1053/gast.2001.23245. [DOI] [PubMed] [Google Scholar]
- 17.Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160:3251–3258. [PubMed] [Google Scholar]
- 18.Page GG, Blakely WP, Ben-Eliyahu S. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain. 2001;90:191–199. doi: 10.1016/s0304-3959(00)00403-6. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80:880–888. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–965. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 21.Trinchieri G. Natural killer cells wear different hats: effector cells of innate resistance and regulatory cells of adaptive immunity and of hematopoiesis. Semin Immunol. 1995;7:83–88. doi: 10.1006/smim.1995.0012. [DOI] [PubMed] [Google Scholar]
- 22.Herberman RB. Cancer immunotherapy with natural killer cells. Semin Oncol. 2002;29:27–30. doi: 10.1053/sonc.2002.33079. [DOI] [PubMed] [Google Scholar]
- 23.Colucci F, Di Santo JP, Leibson PJ. Natural killer cell activation in mice and men: different triggers for similar weapons? Nat Immunol. 2002;3:807–813. doi: 10.1038/ni0902-807. [DOI] [PubMed] [Google Scholar]
- 24.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 25.Long EO. Tumor cell recognition by natural killer cells. Semin Cancer Biol. 2002;12:57–61. doi: 10.1006/scbi.2001.0398. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 28.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 29.Klimp AH, de Vries EG, Scherphof GL, Daemen T. A potential role of macrophage activation in the treatment of cancer. Crit Rev Oncol Hematol. 2002;44:143–161. doi: 10.1016/s1040-8428(01)00203-7. [DOI] [PubMed] [Google Scholar]
- 30.Medzhitov R, Janeway CA., Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 31.Barton GM, Medzhitov R. Toll-like receptors and their ligands. Curr Top Microbiol Immunol. 2002;270:81–92. doi: 10.1007/978-3-642-59430-4_5. [DOI] [PubMed] [Google Scholar]
- 32.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi O, Kawai T, Mühlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 35.Davis ID. An overview of cancer immunotherapy. Immunol Cell Biol. 2000;78:179–195. doi: 10.1046/j.1440-1711.2000.00906.x. [DOI] [PubMed] [Google Scholar]
- 36.Persing DH, Coler RN, Lacy MJ, Johnson DA, Baldridge JR, Hershberg RM, Reed SG. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 2002;10:S32–S37. doi: 10.1016/s0966-842x(02)02426-5. [DOI] [PubMed] [Google Scholar]
- 37.Yu Z, Restifo NP. Cancer vaccines: progress reveals new complexities. J Clin Invest. 2002;110:289–294. doi: 10.1172/JCI16216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loewenstein J, Rottem S, Gallily R. Induction of macrophage-mediated cytolysis of neoplastic cells by mycoplasmas. Cell Immunol. 1983;77:290–297. doi: 10.1016/0008-8749(83)90029-1. [DOI] [PubMed] [Google Scholar]
- 39.Kostyal DA, Butler GH, Beezhold DH. A 48-kilodalton Mycoplasma fermentans membrane protein induces cytokine secretion by human monocytes. Infect Immun. 1994;62:3793–3800. doi: 10.1128/iai.62.9.3793-3800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rawadi G, Roman-Roman S. Mycoplasma membrane lipoproteins induced proinflammatory cytokines by a mechanism distinct from that of lipopolysaccharide. Infect Immun. 1996;64:637–643. doi: 10.1128/iai.64.2.637-643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calcutt MJ, Kim MF, Karpas AB, Mühlradt PF, Wise KS. Differential posttranslational processing confers intraspecies variation of a major surface lipoprotein and a macrophage-activating lipopeptide of Mycoplasma fermentans. Infect Immun. 1999;67:760–771. doi: 10.1128/iai.67.2.760-771.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seya T, Matsumoto M. A lipoprotein family from Mycoplasma fermentans confers host immune activation through Toll-like receptor 2. Int J Biochem Cell Biol. 2002;34:901–906. doi: 10.1016/s1357-2725(01)00164-9. [DOI] [PubMed] [Google Scholar]
- 43.Lien E, Sellati TJ, Yoshimura A, Flo TH, Rawadi G, Finberg RW, Carroll JD, Espevik T, Ingalls RR, Radolf JD, Golenbock DT. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 44.Toshchakov V, Jones BW, Perera PY, Thomas K, Cody MJ, Zhang S, Williams BR, Major J, Hamilton TA, Fenton MJ, Vogel SN. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat Immunol. 2002;3:392–398. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- 45.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 46.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 47.Kono K, Salazar-Onfray F, Petersson M, Hansson J, Masucci G, Wasserman K, Nakazawa T, Anderson P, Kiessling R. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell-and natural killer cell-mediated cytotoxicity. Eur J Immunol. 1996;26:1308–1313. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]
- 48.Hansson M, Asea A, Ersson U, Hermodsson S, Hellstrand K. Induction of apoptosis in NK cells by monocyte-derived reactive oxygen metabolites. J Immunol. 1996;156:42–47. [PubMed] [Google Scholar]
- 49.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 50.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 51.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freire-de-Lima CG, Nacimento DO, Soares MBP, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopes MF. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- 54.Hildenbrand R, Jansen C, Wolf G, Bohme B, Berger S, von Minckwitz G, Horlin A, Kaufmann M, Stutte HJ. Transforming growth factor-beta stimulates urokinase expression in tumor-associated macrophages of the breast. Lab Invest. 1998;78:59–71. [PubMed] [Google Scholar]
- 55.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Newman SL, Henson JE, Henson PM. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982;156:430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu B, Sonstein J, Christensen PJ, Punturieri A, Curtis JL. Deficient in vitro and in vivo phagocytosis of apoptotic T cells by resident murine alveolar macrophages. J Immunol. 2000;165:2124–2133. doi: 10.4049/jimmunol.165.4.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Brugge-Gamelkorn GJ, Dijkstra CD, Sminia T. Characterization of pulmonary macrophages and bronchus associated lymphoid tissue macrophages of the rat. An enzyme-cytochemical and immuno-cytochemical study. Immunobiol. 1985;169:553–562. doi: 10.1016/S0171-2985(85)80009-7. [DOI] [PubMed] [Google Scholar]
- 59.Scott LJ, Perry CM. Tramadol: a review of its use in perioperative pain. Drugs. 2000;60:139–176. doi: 10.2165/00003495-200060010-00008. [DOI] [PubMed] [Google Scholar]
- 60.Gaspani L, Bianchi M, Limiroli E, Panerai AE, Sacerdote P. The analgesic drug tramadol prevents the effect of surgery on natural killer cell activity and metastatic colonization in rats. J Neuroimmunol. 2002;129:18–24. doi: 10.1016/s0165-5728(02)00165-0. [DOI] [PubMed] [Google Scholar]
- 61.Dougenis D, Patrinou V, Filos KS, Theodori E, Vagianos K, Maniati A. Blood use in lung resection for carcinoma: perioperative elective anaemia does not compromise the early outcome. Eur J Cardiothorac Surg. 2001;20:372–377. doi: 10.1016/s1010-7940(01)00792-8. [DOI] [PubMed] [Google Scholar]