Abstract
Studies have demonstrated that long-term opioid treatment leads to an increased sensitivity to painful (hyperalgesia) or normally innocuous (allodynia) stimuli. The molecular mechanisms that lead to paradoxical pain sensitization upon chronic opioid treatment are not completely understood. Enhanced excitatory pain neurotransmitter (such as calcitonin gene-related peptide (CGRP)) release in the dorsal horn of the spinal cord may play a role in sustained morphine-mediated paradoxical pain. Recently we have demonstrated that inhibition of Raf-1 attenuates sustained morphine treatment-mediated augmentation of CGRP release in vitro, in cultured primary sensory neurons. In the present study, we show that knockdown of spinal Raf-1 levels in vivo by intrathecal administration of Raf-1-specific siRNA attenuates sustained morphine-mediated thermal hyperalgesia in rats.
Keywords: Opioids, Morphine, Raf-1, Hyperalgesia
Enhanced release of excitatory pain neurotransmitters, such as calcitonin gene related peptide (CGRP) from the central termini of the primary sensory neurons leads to a paradoxical pain sensitization upon sustained opioid treatment (Gardell et al., 2002). The mechanism of sustained morphine treatment-mediated augmentation of pain neurotransmitter release is currently not entirely clear.
We have found earlier that Raf-1-mediated augmentation of cAMP formation (Adenylyl cyclase (AC) superactivation) augments basal CGRP release upon sustained morphine treatment of cultured primary sensory neurons in vitro (Yue et al., 2008). The aim of the present study was to determine the effect of selective in vivo knockdown of spinal Raf-1 protein levels in the development of thermal hyperalgesia upon sustained morphine treatment in rats.
Care, maintenance and testing of the experimental animals were performed in accordance with the policies and recommendations of the International Association for the Study of Pain and the National Institutes of Health guidelines. Adult male Sprague-Dawley rats (200–225g, Harlan Sprague Dawley, Inc., IN) were anesthetized and implanted with 8 cm catheters intrathecally at the level of the lumbar spinal cord, as described previously (Gardell et al, 2002). A total of 6 animals were used in each treatment group. Free flow of fluids through the catheters has been verified by injecting saline prior to further experimentation.
siRNAs stock solutions (100 μM) were prepared in double distilled RNAse free water and stored in aliquots at −80°C. For intrathecal treatment, aliquots of the stock solution (2 μg of the appropriate siRNA) were mixed (1:5 v/v) with i-Fect transfection reagent (Neuromics, Edina, MN). After recovery from the surgery (5–7 days), the animals received intrathecal injections (2ug siRNA/10 ul/rat) of either a lipid encapsulated Raf-1-selective siRNA mixture (Smart pool siRNA, Dharmacon Inc; Chicago, IL, Cat # L-087699-00) (Raf-1 siRNA groups) or i-Fect encapsulated non-targeting dsRNA (Dharmacon, #D-001810-01-20) (control mismatch siRNA groups) or the transfection lipid alone, once daily, for 3 days, as described earlier (Gardell et al., 2002). Intrathecal injections of the siRNAs or the transfection agent alone did not cause any sign of behavioral toxicity. Western blots, using a Raf-1-selective antibody, indicated that intrathecal treatment with the Raf-1-selective siRNA mixture for 3 days significantly reduced Raf-1 protein levels in the dorsal root ganglion and in the dorsal horn of the spinal cord (manuscript in preparation).
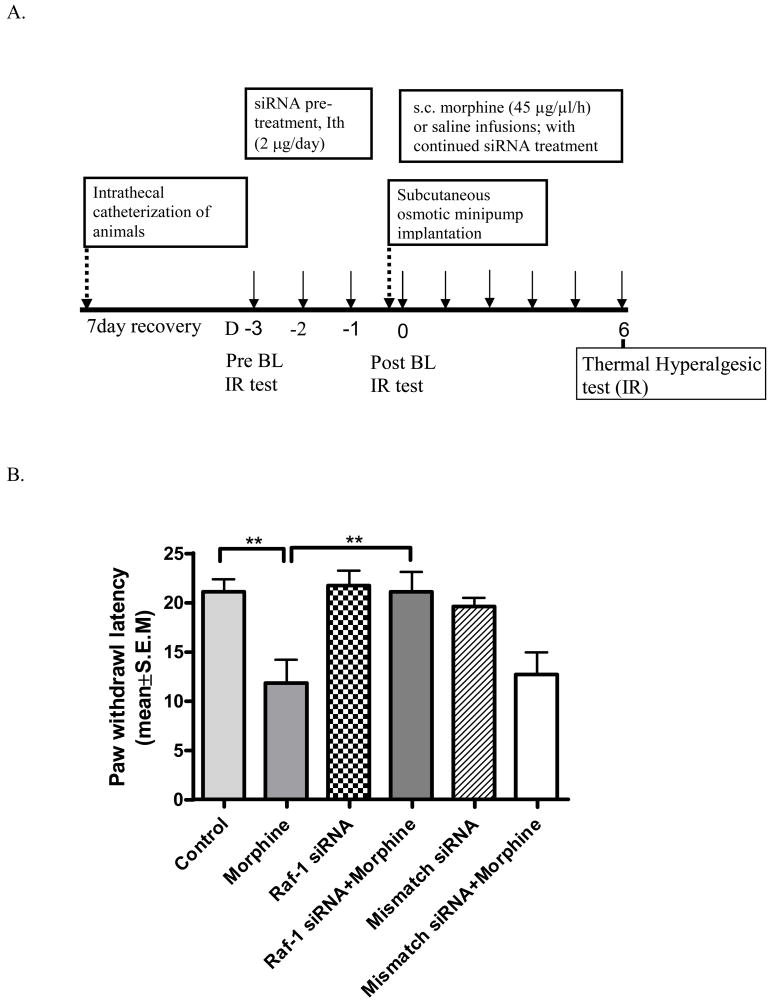
After siRNA pretreatment, osmotic minipumps (Alza, Mountain View, CA) were implanted subcutaneously (s.c.) into each group, and the animals received continuous s.c. morphine (45 μg/μl/h) or saline (control groups) infusions for 7 days. Daily intrathecal treatments with the appropriate siRNA or transfection lipid continued throughout the sustained morphine treatment- and behavioral testing periods (see Fig 1A).
Fig 1.
A. Experimental design. (Ith: Intrathecal; siRNA: Raf-1 siRNA or mismatch siRNA delivery via intrathecal catheter; BL: Baseline; D: Day; IR: Infrared heat). B. Raf-1 siRNA attenuates sustained morphine-mediated thermal hyperalgesia in rats: Male Sprague Dawley rats received intrathecal injections of i-Fect encapsulated Raf-1selective siRNA (Raf-1 siRNA groups); or non-targeting dsRNA (mismatch siRNA groups) or the transfection agent (i-Fect) alone (control) once daily, for 3 days. After pre-treatment, the rats received continous subcutaneous (osmotic minipump) saline (control group, Raf-1 siRNA group and mismatch siRNA group) or morphine (45μg/μl/h) (morphine group, Raf-1 siRNA+morphine group and mismatch siRNA+morphine group) infusions for 6 days. Six animals were included in each treatment group. Thermal hyperalgesia was measured as a decrease in paw withdrawal latencies in a radiant heat paw-withdrawal test.
A modified method of Hargreaves et al. (1988) was used to assess sensitivity of rats to a mildly noxious thermal stimulus, as previously described (Gardell et al., 2002). Briefly, a radiant heat source was focused onto the plantar surface of the hindpaw of rats. Paw withdrawal latencies were measured using a motion detector both before (baseline) and after drug (siRNA, morphine or saline) administration. A maximal cutoff of 33 s was used to prevent tissue damage. One-way ANOVA followed by Newman-Keuls multiple comparison tests (post hoc t-tests) were used to compare mean ± S.E.M among different treatment groups. The differences were considered statistically significant at P<0.05.
Baseline paw withdrawal latency in control, saline-treated animals was 21.1±1.1 s. While acute (6h) morphine treatment increased paw withdrawal latencies, after continuous morphine infusion a time-dependent reduction of paw withdrawal latencies became apparent. Sustained morphine-mediated thermal hyperalgesia reached maximal level after >5 days of morphine treatment. Thus, on day 6 of systemic morphine treatment paw withdrawal latencies were 11.8±2.4 s, which is 46% reduction relative to control (** P<0.01; Fig 1B). Pretreatment of the animals with a Raf-1-selective siRNA mixture (2μg/rat, once daily, 3 days) significantly attenuated the development of thermal hyperalgesia upon sustained morphine treatment. Paw withdrawal latency in the morphine plus Raf-1-selective siRNA-treated group was 21.08±2.0 s on the 6th day of morphine treatment, which equals an 80% reduction relative to the morphine group (** P<0.01; Fig 1B). Treatment with Raf-1 siRNA alone had no effect on the paw withdrawal latencies (21.6±1.6 s, P>0.05 compared with vehicle group; Fig 1B). Animals pre-treated with a control, non-targeting dsRNA construct (mismatch siRNA) showed no change in paw withdrawal latencies relative to the control group, pre-treated with the i-Fect transfection agent alone (19.4±0.9 s, P>0.05; Fig 1B). Similar to the control group, sustained (6 days) morphine treatment reduced paw withdrawal latencies in the mismatch siRNA-treated animals to 12.6±2.3 s; Fig 1B).
Augmentation of excitatory pain neurotransmitter (such as CGRP) release in the dorsal horn of the spinal cord (Gardell et al., 2002) upon sustained morphine treatment may significantly contribute to paradoxical pain sensitization. Earlier, we utilized recombinant cell lines, such as Chinese hamster ovary (CHO) cells stably expressing human opioid receptor types (hDOP/CHO; hMOP/CHO), to investigate the cellular effects of sustained opioid agonist treatment. In these experiments, we have demonstrated that sustained opioid treatment leads to a Raf-1-dependent phosphorylation and superactivation of adenylyl cylcase(s) (Varga et al., 2003, Yue et al., 2008). Subsequently, we demonstrated the physiological role of this molecular mechanism in vitro, in the regulation of pain neurotransmitter (CGRP) release from cultured primary sensory neurons (Yue et al., 2008).
In the present study, we demonstrate the role of this mechanism in vivo, in the development of thermal hyperalgesia upon sustained morphine treatment in rats. Our data indicates that highly selective knock-down of Raf-1 levels in the lumbar region of the spinal cord by Raf-1-selective siRNA pretreatment significantly attenuates sustained morphine induced thermal hyperalgesia in rats, indicating that Raf-1-mediated pathways play an important role in vivo, in sustained morphine-mediated paradoxical pain sensitization.
Acknowledgments
This work was supported by grants from the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Jr, Lai J, Porreca F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci. 2002;22:6747–6755. doi: 10.1523/JNEUROSCI.22-15-06747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Varga EV, Rubenzik MK, Stropova D, Sugiyama M, Grife V, Hruby VJ, Rice KC, Roeske WR, Yamamura HI. Converging protein kinase pathways mediate adenylyl cyclase superactivation upon chronic delta-opioid agonist treatment. J Pharmacol Exp Ther. 2003;306:109–115. doi: 10.1124/jpet.103.049643. [DOI] [PubMed] [Google Scholar]
- Yue X, Tumati S, Navratilova E, Stropova D, St John PA, Roeske WR, Vanderah TW, Yamamura HI, Varga EV. Sustained morphine treatment augments basal CGRP release from cultured primary sensory neurons in a Raf-1 dependent manner. E J Pharmacol. 2008;584:272–277. doi: 10.1016/j.ejphar.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]