Abstract
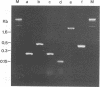
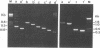
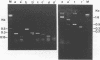
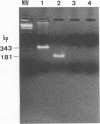
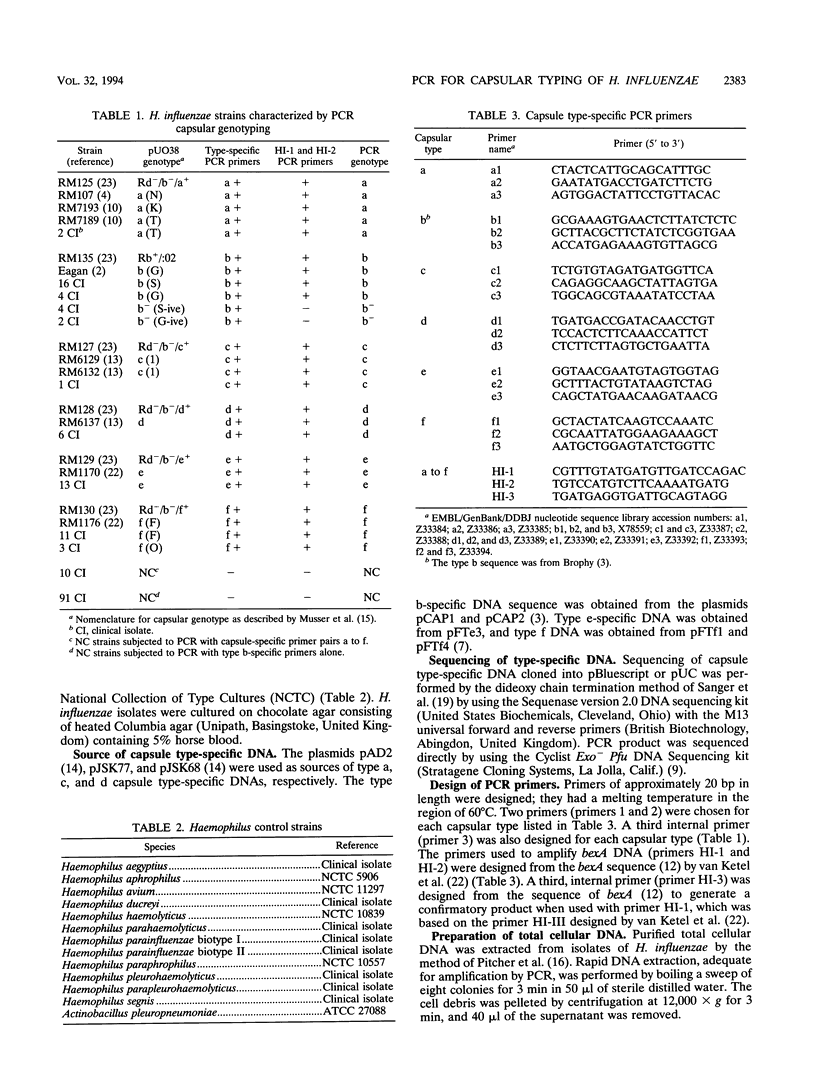
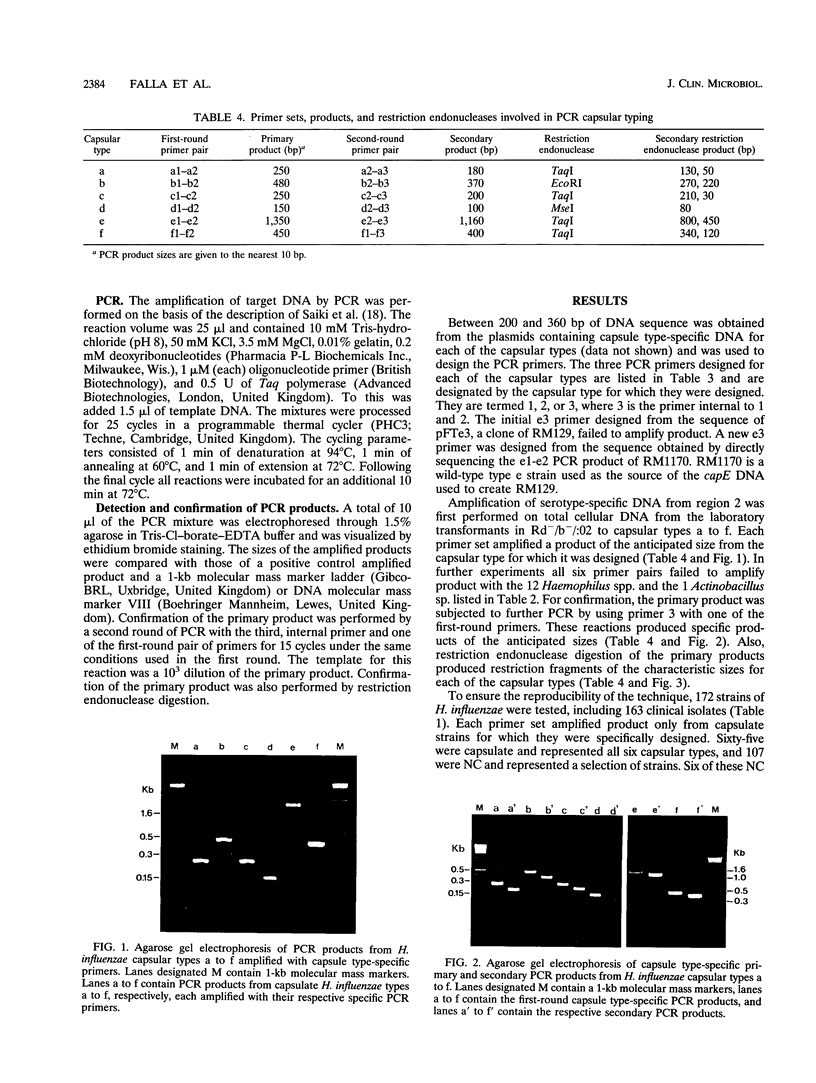
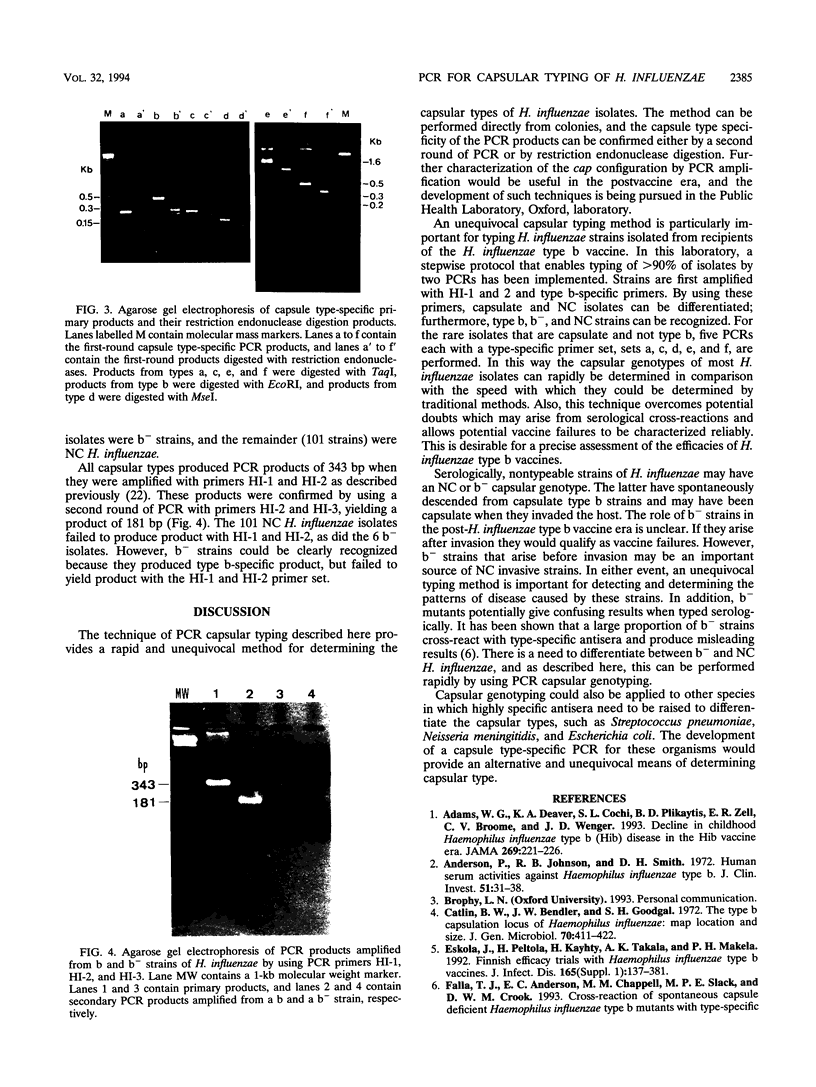
A PCR method for the unequivocal assignment of Haemophilus influenzae capsular type (types a to f) was developed. PCR primers were designed from capsule type-specific DNA sequences cloned from the capsular gene cluster of each of the six capsular types. PCR product was amplified only from the capsular type for which the primers were designed. Product was confirmed by using either an internal oligonucleotide or restriction endonuclease digestion. A total of 172 H. influenzae strains of known capsular type (determined genetically) comprising all capsular types and noncapsulate strains were tested by PCR capsular typing. In all cases the PCR capsular type corresponded to the capsular genotype determined by restriction fragment length polymorphism analysis of the cap region. When used in conjunction with PCR primers derived from the capsular gene bexA, capsulate, noncapsulate, and capsule-deficient type b mutant strains could be differentiated. PCR capsular typing overcomes the problems of cross-reaction and autoagglutination associated with the serotyping of H. influenzae strains. The rapid and unequivocal capsular typing method that is described will be particularly important for typing invasive H. influenzae strains isolated from recipients of H. influenzae type b vaccine.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. G., Deaver K. A., Cochi S. L., Plikaytis B. D., Zell E. R., Broome C. V., Wenger J. D. Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA. 1993 Jan 13;269(2):221–226. [PubMed] [Google Scholar]
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W., Bendler J. W., 3rd, Goodgal S. H. The type b capsulation locus of Haemophilus influenzae: map location and size. J Gen Microbiol. 1972 May;70(3):411–422. doi: 10.1099/00221287-70-3-411. [DOI] [PubMed] [Google Scholar]
- Falla T. J., Dobson S. R., Crook D. W., Kraak W. A., Nichols W. W., Anderson E. C., Jordens J. Z., Slack M. P., Mayon-White D., Moxon E. R. Population-based study of non-typable Haemophilus influenzae invasive disease in children and neonates. Lancet. 1993 Apr 3;341(8849):851–854. doi: 10.1016/0140-6736(93)93059-a. [DOI] [PubMed] [Google Scholar]
- Kroll J. S., Ely S., Moxon E. R. Capsular typing of Haemophilus influenzae with a DNA probe. Mol Cell Probes. 1991 Oct;5(5):375–379. doi: 10.1016/s0890-8508(06)80009-5. [DOI] [PubMed] [Google Scholar]
- Kroll J. S., Hopkins I., Moxon E. R. Capsule loss in H. influenzae type b occurs by recombination-mediated disruption of a gene essential for polysaccharide export. Cell. 1988 May 6;53(3):347–356. doi: 10.1016/0092-8674(88)90155-9. [DOI] [PubMed] [Google Scholar]
- Kroll J. S., Loynds B. M., Moxon E. R. The Haemophilus influenzae capsulation gene cluster: a compound transposon. Mol Microbiol. 1991 Jun;5(6):1549–1560. doi: 10.1111/j.1365-2958.1991.tb00802.x. [DOI] [PubMed] [Google Scholar]
- Kroll J. S., Loynds B., Brophy L. N., Moxon E. R. The bex locus in encapsulated Haemophilus influenzae: a chromosomal region involved in capsule polysaccharide export. Mol Microbiol. 1990 Nov;4(11):1853–1862. doi: 10.1111/j.1365-2958.1990.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Kroll J. S., Zamze S., Loynds B., Moxon E. R. Common organization of chromosomal loci for production of different capsular polysaccharides in Haemophilus influenzae. J Bacteriol. 1989 Jun;171(6):3343–3347. doi: 10.1128/jb.171.6.3343-3347.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Kroll J. S., Granoff D. M., Moxon E. R., Brodeur B. R., Campos J., Dabernat H., Frederiksen W., Hamel J., Hammond G. Global genetic structure and molecular epidemiology of encapsulated Haemophilus influenzae. Rev Infect Dis. 1990 Jan-Feb;12(1):75–111. doi: 10.1093/clinids/12.1.75. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Salvi R. J., Ahroon W., Saunders S. S., Arnold S. A. Evoked potentials: computer-automated threshold-tracking procedure using an objective detection criterion. Ear Hear. 1987 Jun;8(3):151–156. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively R. G., Shigei J. T., Peterson E. M., de la Maza L. M. Typing of Haemophilus influenzae by coagglutination and conventional slide agglutination. J Clin Microbiol. 1981 Dec;14(6):706–708. doi: 10.1128/jcm.14.6.706-708.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk D. C. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1984 Aug;18(1):1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Musher D. M., Septimus E. J., McGowan J. E., Jr, Quinones F. J., Wiss K., Vance P. H., Trier P. A. Haemophilus influenzae infections in adults: characterization of strains by serotypes, biotypes, and beta-lactamase production. J Infect Dis. 1981 Aug;144(2):101–106. doi: 10.1093/infdis/144.2.101. [DOI] [PubMed] [Google Scholar]
- Zwahlen A., Kroll J. S., Rubin L. G., Moxon E. R. The molecular basis of pathogenicity in Haemophilus influenzae: comparative virulence of genetically-related capsular transformants and correlation with changes at the capsulation locus cap. Microb Pathog. 1989 Sep;7(3):225–235. doi: 10.1016/0882-4010(89)90058-2. [DOI] [PubMed] [Google Scholar]
- Zwahlen A., Winkelstein J. A., Moxon E. R. Surface determinants of Haemophilus influenzae pathogenicity: comparative virulence of capsular transformants in normal and complement-depleted rats. J Infect Dis. 1983 Sep;148(3):385–394. doi: 10.1093/infdis/148.3.385. [DOI] [PubMed] [Google Scholar]
- van Ketel R. J., de Wever B., van Alphen L. Detection of Haemophilus influenzae in cerebrospinal fluids by polymerase chain reaction DNA amplification. J Med Microbiol. 1990 Dec;33(4):271–276. doi: 10.1099/00222615-33-4-271. [DOI] [PubMed] [Google Scholar]